

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92520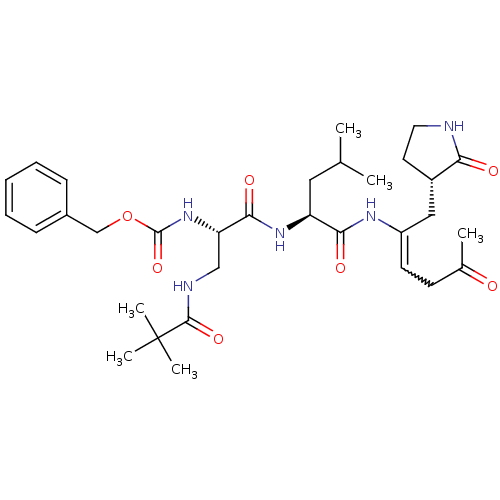 (TG-0204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -42.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92520 (TG-0204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -42.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232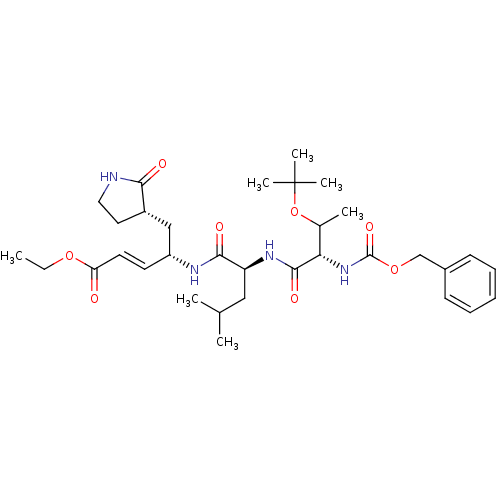 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92521 (TG-0205486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92521 (TG-0205486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231 (N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231 (N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230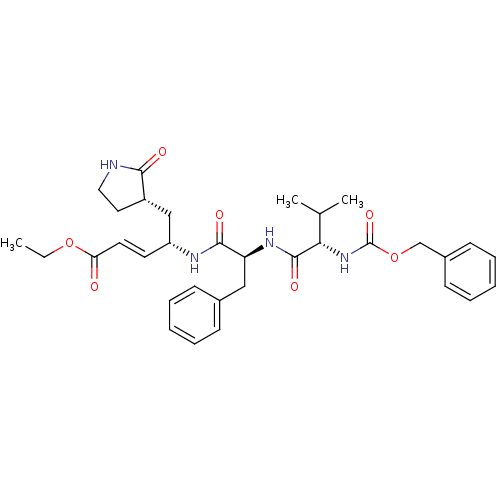 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229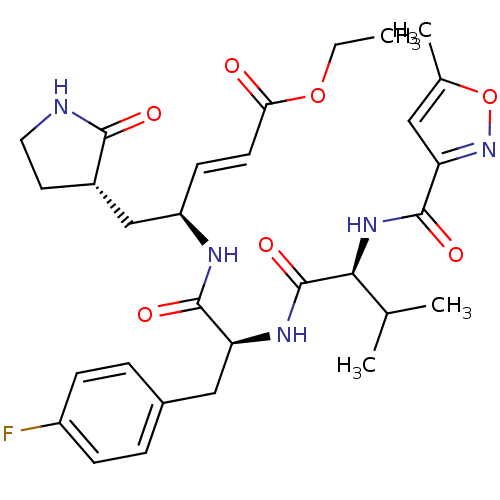 (AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229 (AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666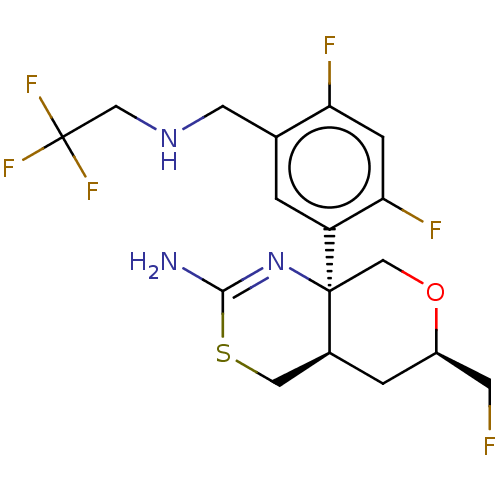 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241905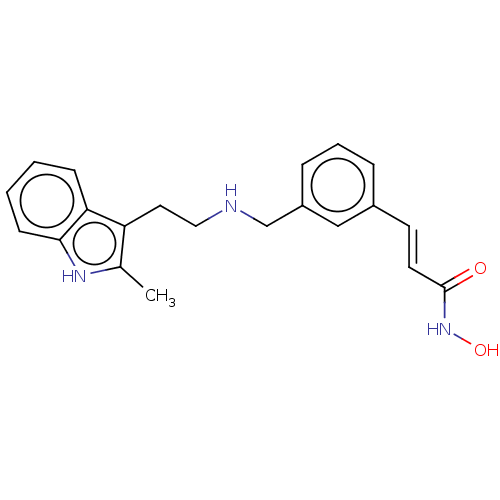 (CHEMBL4080164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241901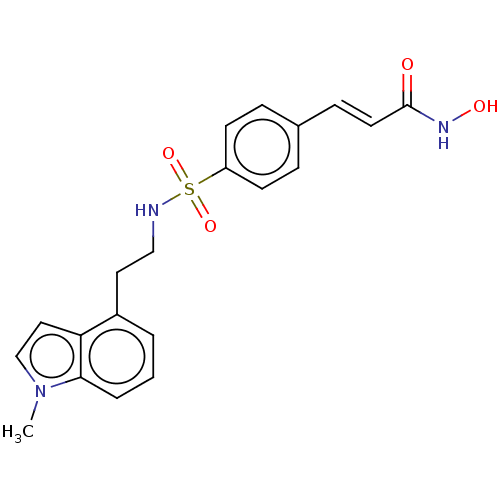 (CHEMBL4065026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241906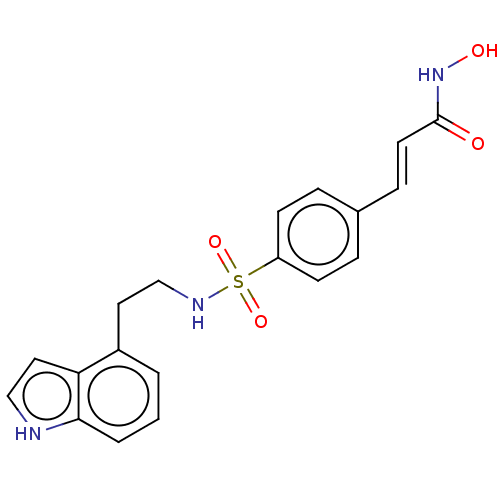 (CHEMBL4069853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350831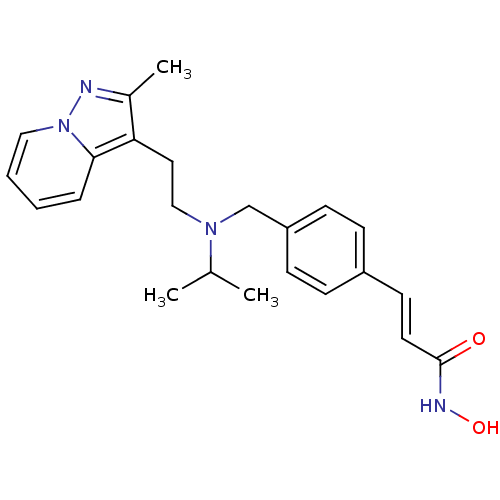 (CHEMBL1819273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350832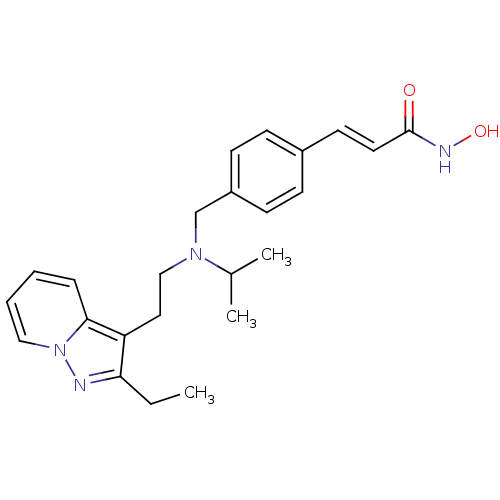 (CHEMBL1819274) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241905 (CHEMBL4080164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241901 (CHEMBL4065026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241906 (CHEMBL4069853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350818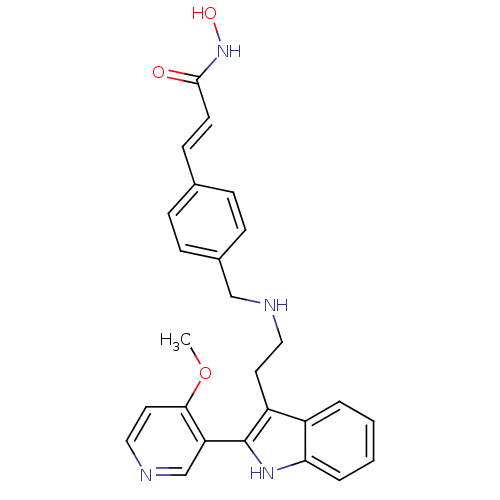 (CHEMBL1819257) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321857 (8-{4-(4-Fluorophenyl)-5-[4-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350827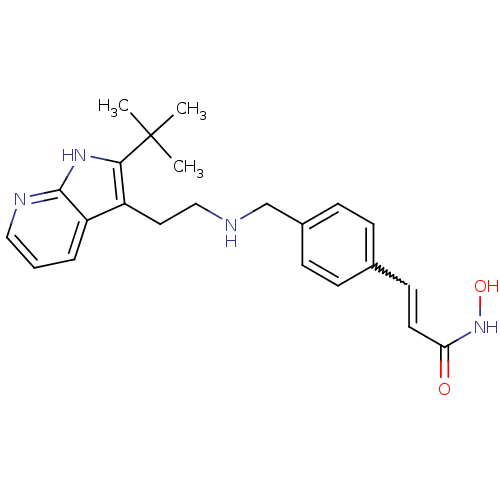 (CHEMBL1819267) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350835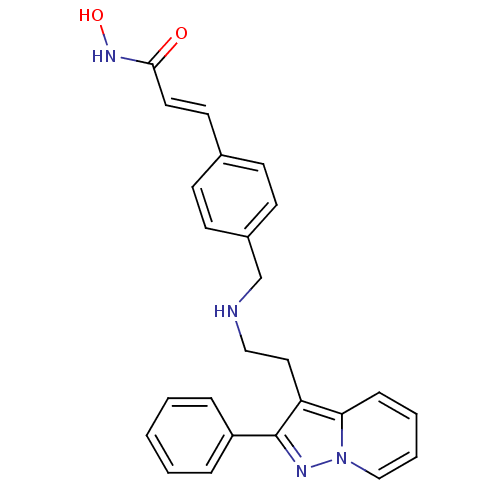 (CHEMBL1819272) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350820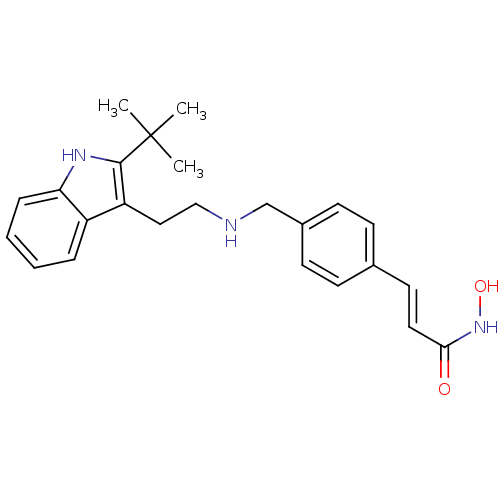 (CHEMBL1819260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321856 (4-{2-[7-Hydroxy-5,6,7,8-tetrahydronaphthalen-1-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259964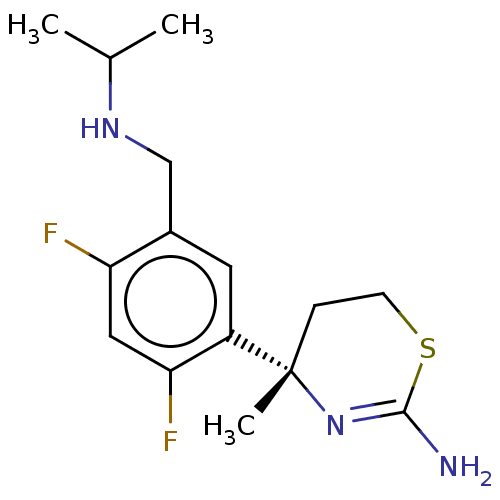 (CHEMBL4083698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321853 (8-{4-Ethyl-5-[4-(trifluoromethyl)phenyl]oxazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499796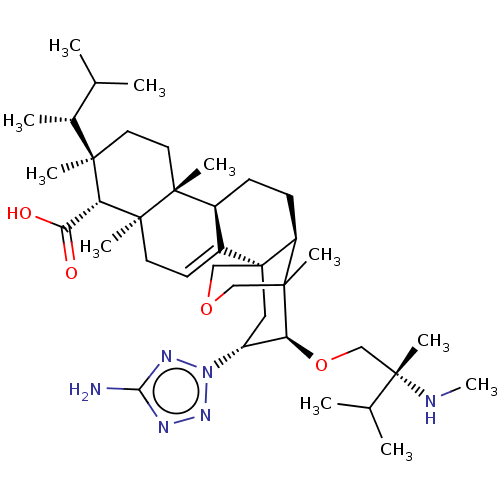 (CHEMBL3742110) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350833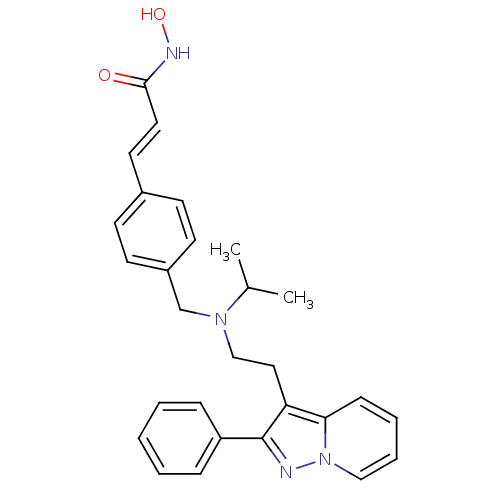 (CHEMBL1819275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350830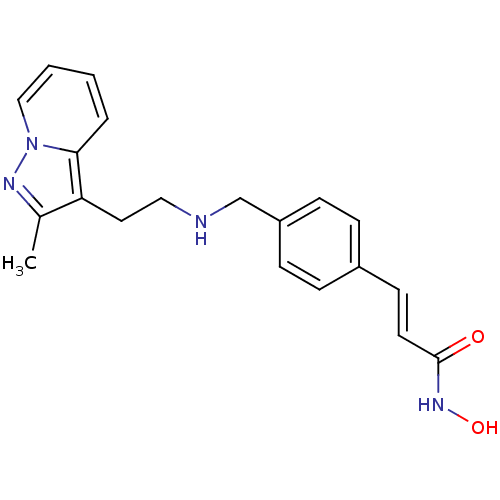 (CHEMBL1819270) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499806 (CHEMBL3740011) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50321850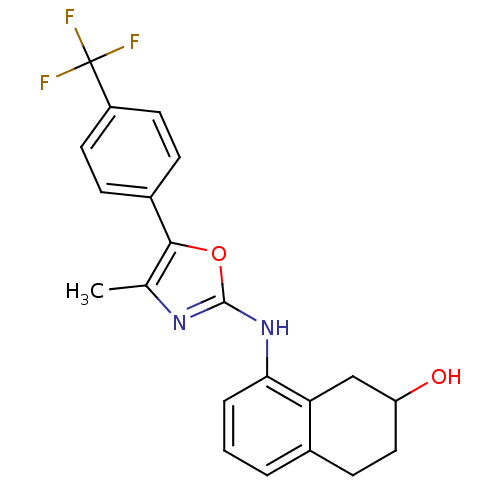 (8-{5-[3-Methyl-4-(trifluoromethyl)phenyl]oxazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca... | Bioorg Med Chem 18: 4821-9 (2011) Article DOI: 10.1016/j.bmc.2010.04.099 BindingDB Entry DOI: 10.7270/Q27H1KJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259974 (CHEMBL4102593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 637 total ) | Next | Last >> |