 Found 362 hits with Last Name = 'iqbal' and Initial = 'm'
Found 362 hits with Last Name = 'iqbal' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM87059
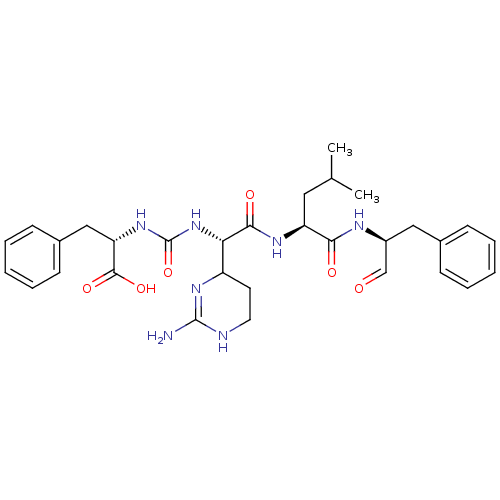
(CHEMBL247767 | Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(N)=N1)C(=O)N[C@@H](Cc1ccccc1)C=O |c:30| Show InChI InChI=1S/C31H41N7O6/c1-19(2)15-24(27(40)34-22(18-39)16-20-9-5-3-6-10-20)35-28(41)26(23-13-14-33-30(32)36-23)38-31(44)37-25(29(42)43)17-21-11-7-4-8-12-21/h3-12,18-19,22-26H,13-17H2,1-2H3,(H,34,40)(H,35,41)(H,42,43)(H3,32,33,36)(H2,37,38,44)/t22-,23?,24-,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.24E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi
| Assay Description
Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. |
J Enzyme Inhib Med Chem 23: 400-5 (2008)
Article DOI: 10.1080/14756360701584653
BindingDB Entry DOI: 10.7270/Q2JS9P23 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM87058
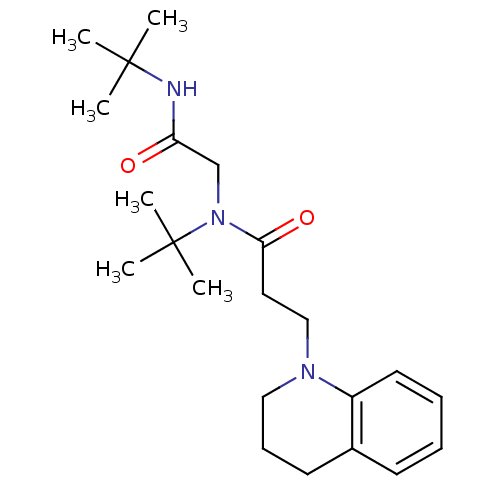
(Lignan, 3 | MLS000034632 | N-tert-Butyl-N-(tert-bu...)Show SMILES CC(C)(C)NC(=O)CN(C(=O)CCN1CCCc2ccccc12)C(C)(C)C Show InChI InChI=1S/C22H35N3O2/c1-21(2,3)23-19(26)16-25(22(4,5)6)20(27)13-15-24-14-9-11-17-10-7-8-12-18(17)24/h7-8,10,12H,9,11,13-16H2,1-6H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.18E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi
| Assay Description
Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. |
J Enzyme Inhib Med Chem 23: 400-5 (2008)
Article DOI: 10.1080/14756360701584653
BindingDB Entry DOI: 10.7270/Q2JS9P23 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM87060
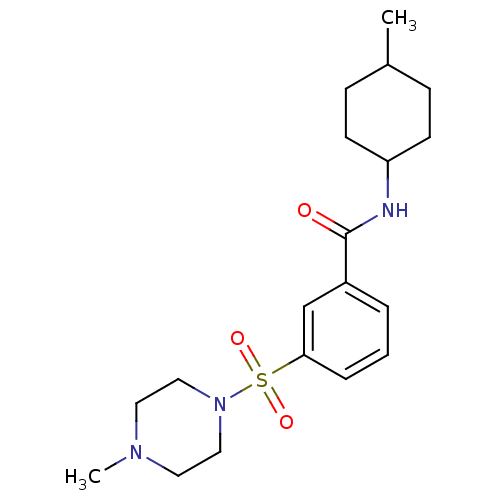
(Lignan, 4 | MLS001138823 | N-(4-methylcyclohexyl)-...)Show SMILES CC1CCC(CC1)NC(=O)c1cccc(c1)S(=O)(=O)N1CCN(C)CC1 |(3.08,-7.7,;4.41,-6.93,;5.75,-7.7,;7.08,-6.93,;7.08,-5.39,;5.75,-4.62,;4.41,-5.39,;8.41,-4.62,;8.41,-3.08,;7.08,-2.31,;9.75,-2.31,;11.08,-3.08,;12.42,-2.31,;12.42,-.77,;11.08,,;9.75,-.77,;11.08,1.54,;12.62,1.54,;9.54,1.54,;11.08,3.08,;12.42,3.85,;12.42,5.39,;11.08,6.16,;11.08,7.7,;9.75,5.39,;9.75,3.85,)| Show InChI InChI=1S/C19H29N3O3S/c1-15-6-8-17(9-7-15)20-19(23)16-4-3-5-18(14-16)26(24,25)22-12-10-21(2)11-13-22/h3-5,14-15,17H,6-13H2,1-2H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.71E+4 | -24.7 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi
| Assay Description
Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. |
J Enzyme Inhib Med Chem 23: 400-5 (2008)
Article DOI: 10.1080/14756360701584653
BindingDB Entry DOI: 10.7270/Q2JS9P23 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50305146
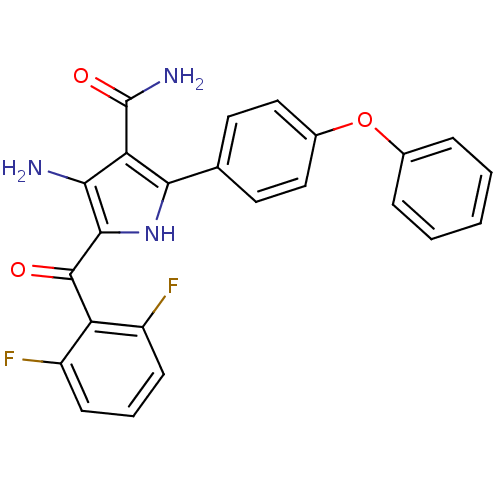
(4-amino-5-(2,6-difluorobenzoyl)-2-(4-phenoxyphenyl...)Show SMILES NC(=O)c1c(N)c([nH]c1-c1ccc(Oc2ccccc2)cc1)C(=O)c1c(F)cccc1F Show InChI InChI=1S/C24H17F2N3O3/c25-16-7-4-8-17(26)18(16)23(30)22-20(27)19(24(28)31)21(29-22)13-9-11-15(12-10-13)32-14-5-2-1-3-6-14/h1-12,29H,27H2,(H2,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50136008
(12-(3-hydroxypropyl)-9-isopropoxymethyl-6,7,12,13-...)Show SMILES CC(C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C28H28N2O3/c1-16(2)33-15-17-8-9-23-20(12-17)25-22-14-29-28(32)26(22)24-19-7-4-3-6-18(19)13-21(24)27(25)30(23)10-5-11-31/h3-4,6-9,12,16,31H,5,10-11,13-15H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosine-protein kinase receptor FLT3 (fms-related tyrosine kinase 3) receptor |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50031442
(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCN1C(=O)c2ccccc2C1=O)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C35H53N7O7/c1-24(2)22-26(23-43)38-32(45)30(19-13-20-37-35(36)40-42(48)49)39-31(44)27(25-14-8-9-15-25)16-7-5-3-4-6-12-21-41-33(46)28-17-10-11-18-29(28)34(41)47/h10-11,17-18,23-27,30H,3-9,12-16,19-22H2,1-2H3,(H,38,45)(H,39,44)(H3,36,37,40) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50031442

(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCN1C(=O)c2ccccc2C1=O)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C35H53N7O7/c1-24(2)22-26(23-43)38-32(45)30(19-13-20-37-35(36)40-42(48)49)39-31(44)27(25-14-8-9-15-25)16-7-5-3-4-6-12-21-41-33(46)28-17-10-11-18-29(28)34(41)47/h10-11,17-18,23-27,30H,3-9,12-16,19-22H2,1-2H3,(H,38,45)(H,39,44)(H3,36,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50611818
(CHEMBL5275393)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2ccc(cc2OC)C2CCN(CC2)C(=O)CO)n1)C(F)(F)F)C1CCN(CC1)C(=O)CO | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50611818

(CHEMBL5275393)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2ccc(cc2OC)C2CCN(CC2)C(=O)CO)n1)C(F)(F)F)C1CCN(CC1)C(=O)CO | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50136019
(9-ethoxymethyl-12-(3-hydroxypropyl)-6,7,12,13-tetr...)Show SMILES CCOCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C27H26N2O3/c1-2-32-15-16-8-9-22-19(12-16)24-21-14-28-27(31)25(21)23-18-7-4-3-6-17(18)13-20(23)26(24)29(22)10-5-11-30/h3-4,6-9,12,30H,2,5,10-11,13-15H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human Vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50136008

(12-(3-hydroxypropyl)-9-isopropoxymethyl-6,7,12,13-...)Show SMILES CC(C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C28H28N2O3/c1-16(2)33-15-17-8-9-23-20(12-17)25-22-14-29-28(32)26(22)24-19-7-4-3-6-18(19)13-21(24)27(25)30(23)10-5-11-31/h3-4,6-9,12,16,31H,5,10-11,13-15H2,1-2H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 3 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291234
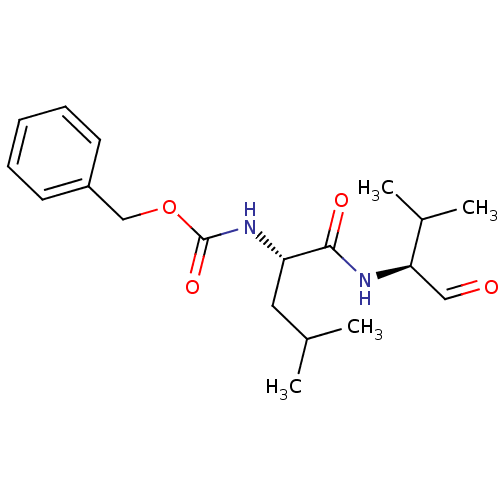
(CHEMBL150600 | [(S)-1-((S)-1-Formyl-2-methyl-propy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](C=O)C(C)C Show InChI InChI=1S/C19H28N2O4/c1-13(2)10-16(18(23)20-17(11-22)14(3)4)21-19(24)25-12-15-8-6-5-7-9-15/h5-9,11,13-14,16-17H,10,12H2,1-4H3,(H,20,23)(H,21,24)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291219
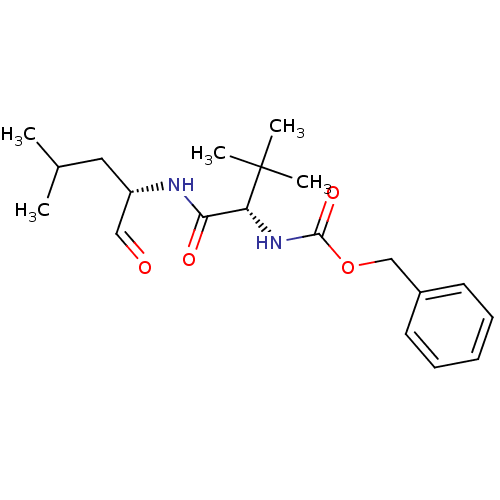
(CHEMBL151521 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)C)C=O Show InChI InChI=1S/C20H30N2O4/c1-14(2)11-16(12-23)21-18(24)17(20(3,4)5)22-19(25)26-13-15-9-7-6-8-10-15/h6-10,12,14,16-17H,11,13H2,1-5H3,(H,21,24)(H,22,25)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... |
Eur J Med Chem 143: 1373-1386 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.036
BindingDB Entry DOI: 10.7270/Q21V5HHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291225
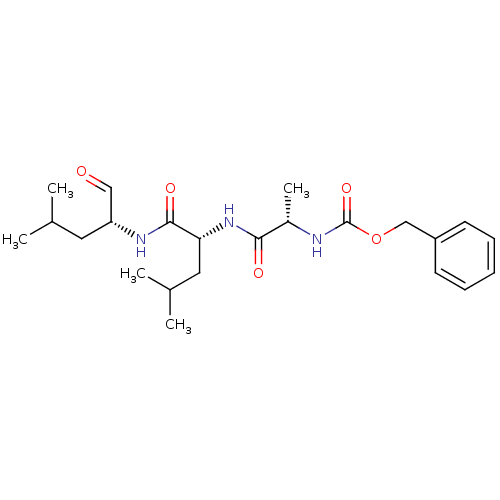
(CHEMBL357595 | {(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C23H35N3O5/c1-15(2)11-19(13-27)25-22(29)20(12-16(3)4)26-21(28)17(5)24-23(30)31-14-18-9-7-6-8-10-18/h6-10,13,15-17,19-20H,11-12,14H2,1-5H3,(H,24,30)(H,25,29)(H,26,28)/t17-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50084655
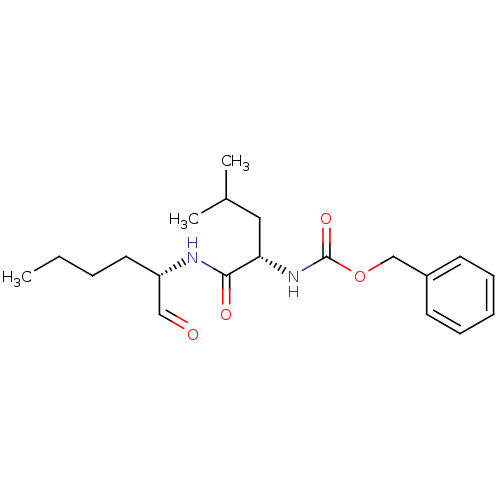
(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50031440
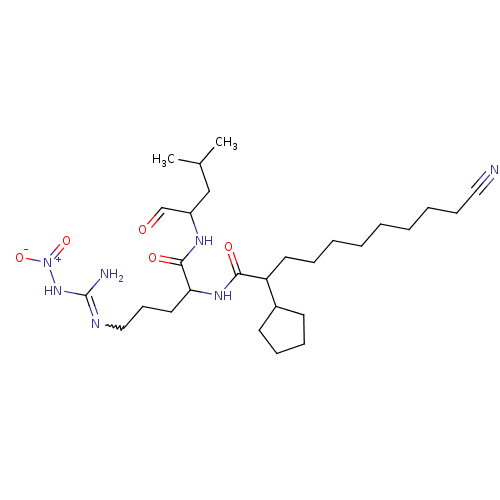
(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C28H49N7O5/c1-21(2)19-23(20-36)32-27(38)25(16-12-18-31-28(30)34-35(39)40)33-26(37)24(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-29/h20-25H,3-16,18-19H2,1-2H3,(H,32,38)(H,33,37)(H3,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50182149
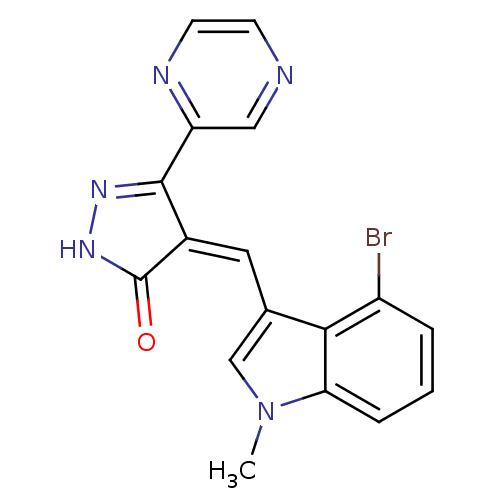
(4-((4-bromo-1-methyl-1H-indol-3-yl)methylene)-3-(p...)Show SMILES Cn1cc(\C=C2/C(=O)NN=C2c2cnccn2)c2c(Br)cccc12 |c:9| Show InChI InChI=1S/C17H12BrN5O/c1-23-9-10(15-12(18)3-2-4-14(15)23)7-11-16(21-22-17(11)24)13-8-19-5-6-20-13/h2-9H,1H3,(H,22,24)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 16: 2158-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.063
BindingDB Entry DOI: 10.7270/Q25T3K2H |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50031440

(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C28H49N7O5/c1-21(2)19-23(20-36)32-27(38)25(16-12-18-31-28(30)34-35(39)40)33-26(37)24(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-29/h20-25H,3-16,18-19H2,1-2H3,(H,32,38)(H,33,37)(H3,30,31,34) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291188

(((S)-1-{(R)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylc...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H46N4O6/c1-18(2)13-23(16-34)31-27(36)24(14-19(3)4)33-28(37)25(15-20(5)6)32-26(35)21(7)30-29(38)39-17-22-11-9-8-10-12-22/h8-12,16,18-21,23-25H,13-15,17H2,1-7H3,(H,30,38)(H,31,36)(H,32,35)(H,33,37)/t21-,23-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291222
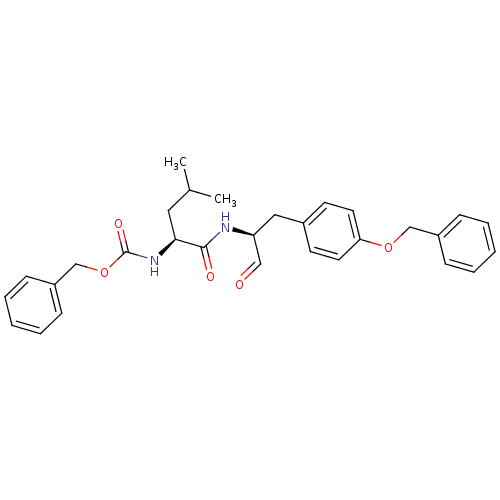
(CHEMBL423222 | {(S)-1-[(S)-1-(4-Benzyloxy-benzyl)-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C=O Show InChI InChI=1S/C30H34N2O5/c1-22(2)17-28(32-30(35)37-21-25-11-7-4-8-12-25)29(34)31-26(19-33)18-23-13-15-27(16-14-23)36-20-24-9-5-3-6-10-24/h3-16,19,22,26,28H,17-18,20-21H2,1-2H3,(H,31,34)(H,32,35)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291192
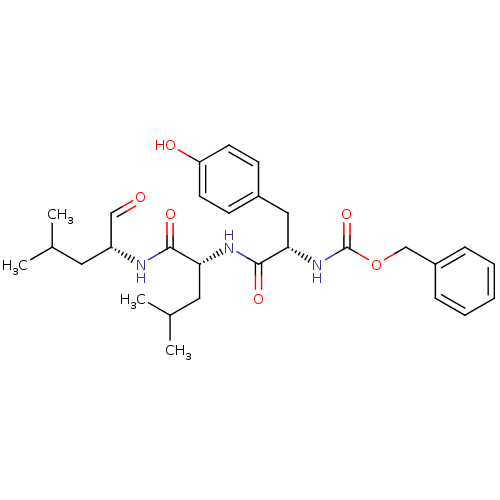
(CHEMBL345337 | [(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H39N3O6/c1-19(2)14-23(17-33)30-27(35)25(15-20(3)4)31-28(36)26(16-21-10-12-24(34)13-11-21)32-29(37)38-18-22-8-6-5-7-9-22/h5-13,17,19-20,23,25-26,34H,14-16,18H2,1-4H3,(H,30,35)(H,31,36)(H,32,37)/t23-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291236
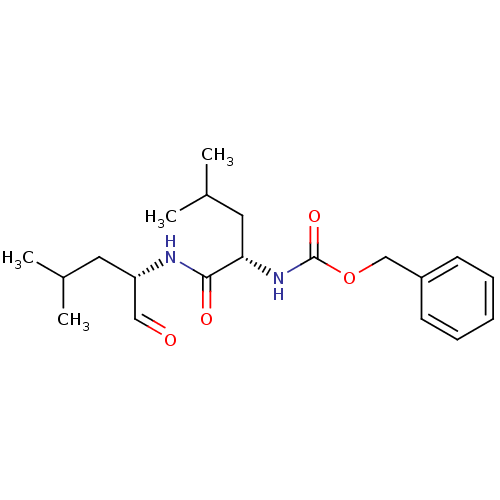
(CHEMBL356841 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C20H30N2O4/c1-14(2)10-17(12-23)21-19(24)18(11-15(3)4)22-20(25)26-13-16-8-6-5-7-9-16/h5-9,12,14-15,17-18H,10-11,13H2,1-4H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291228
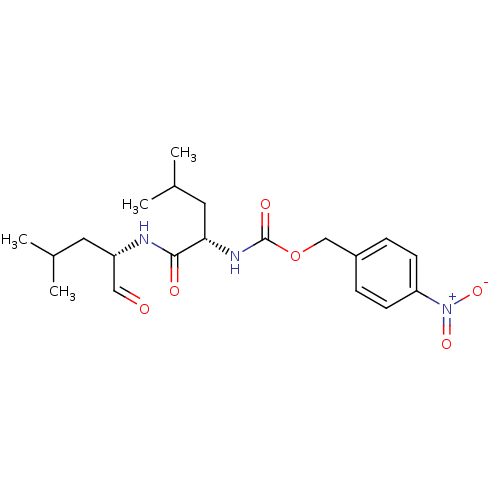
(CHEMBL347742 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccc(cc1)[N+]([O-])=O)C=O Show InChI InChI=1S/C20H29N3O6/c1-13(2)9-16(11-24)21-19(25)18(10-14(3)4)22-20(26)29-12-15-5-7-17(8-6-15)23(27)28/h5-8,11,13-14,16,18H,9-10,12H2,1-4H3,(H,21,25)(H,22,26)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50136008

(12-(3-hydroxypropyl)-9-isopropoxymethyl-6,7,12,13-...)Show SMILES CC(C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C28H28N2O3/c1-16(2)33-15-17-8-9-23-20(12-17)25-22-14-29-28(32)26(22)24-19-7-4-3-6-18(19)13-21(24)27(25)30(23)10-5-11-31/h3-4,6-9,12,16,31H,5,10-11,13-15H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human Vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288611
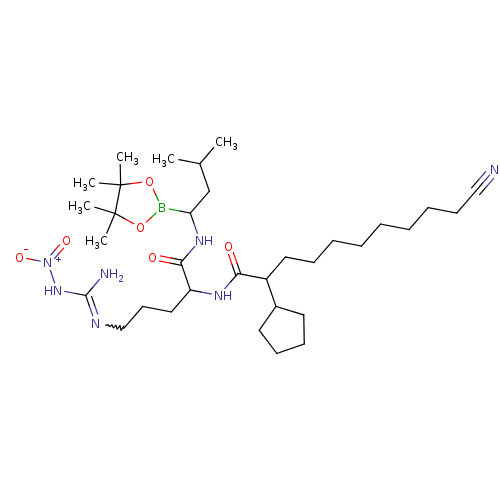
(Alpha-ketocarbonyl boronic ester derivative | CHEM...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)B1OC(C)(C)C(C)(C)O1 |w:12.11| Show InChI InChI=1S/C33H60BN7O6/c1-24(2)23-28(34-46-32(3,4)33(5,6)47-34)39-30(43)27(20-16-22-37-31(36)40-41(44)45)38-29(42)26(25-17-13-14-18-25)19-12-10-8-7-9-11-15-21-35/h24-28H,7-20,22-23H2,1-6H3,(H,38,42)(H,39,43)(H3,36,37,40) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50218973
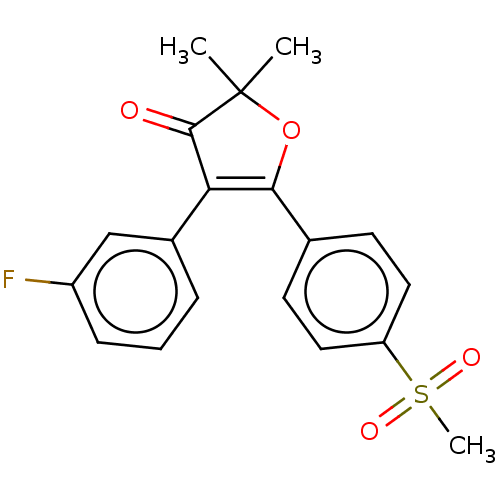
(CHEMBL44001)Show SMILES CC1(C)OC(=C(C1=O)c1cccc(F)c1)c1ccc(cc1)S(C)(=O)=O |c:4| Show InChI InChI=1S/C19H17FO4S/c1-19(2)18(21)16(13-5-4-6-14(20)11-13)17(24-19)12-7-9-15(10-8-12)25(3,22)23/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX-2 in LPS-induced mouse peritoneal macrophage assessed as reduction in PGE2 release incubated for 2 hrs by ELISA |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50182139
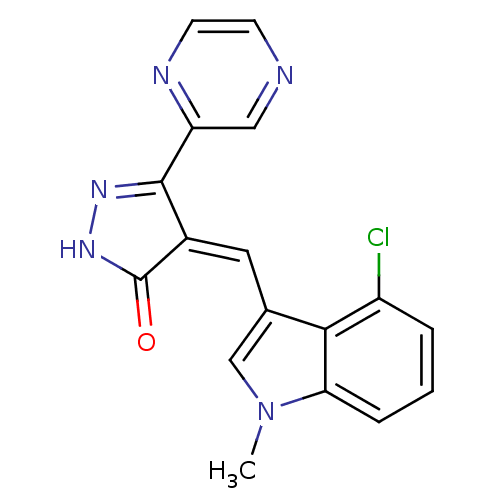
(4-((4-chloro-1-methyl-1H-indol-3-yl)methylene)-3-(...)Show SMILES Cn1cc(\C=C2/C(=O)NN=C2c2cnccn2)c2c(Cl)cccc12 |c:9| Show InChI InChI=1S/C17H12ClN5O/c1-23-9-10(15-12(18)3-2-4-14(15)23)7-11-16(21-22-17(11)24)13-8-19-5-6-20-13/h2-9H,1H3,(H,22,24)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 16: 2158-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.063
BindingDB Entry DOI: 10.7270/Q25T3K2H |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50137397
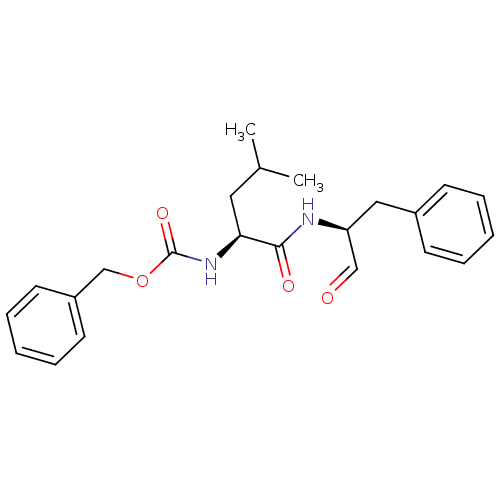
(CHEMBL341014 | [(S)-1-((S)-1-Benzyl-2-oxo-ethylcar...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O4/c1-17(2)13-21(25-23(28)29-16-19-11-7-4-8-12-19)22(27)24-20(15-26)14-18-9-5-3-6-10-18/h3-12,15,17,20-21H,13-14,16H2,1-2H3,(H,24,27)(H,25,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50182144

(4-((5-chloro-1-methyl-1H-indol-3-yl)methylene)-3-(...)Show SMILES Cn1cc(\C=C2/C(=O)NN=C2c2cnccn2)c2cc(Cl)ccc12 |c:9| Show InChI InChI=1S/C17H12ClN5O/c1-23-9-10(12-7-11(18)2-3-15(12)23)6-13-16(21-22-17(13)24)14-8-19-4-5-20-14/h2-9H,1H3,(H,22,24)/b13-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 16: 2158-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.063
BindingDB Entry DOI: 10.7270/Q25T3K2H |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291223
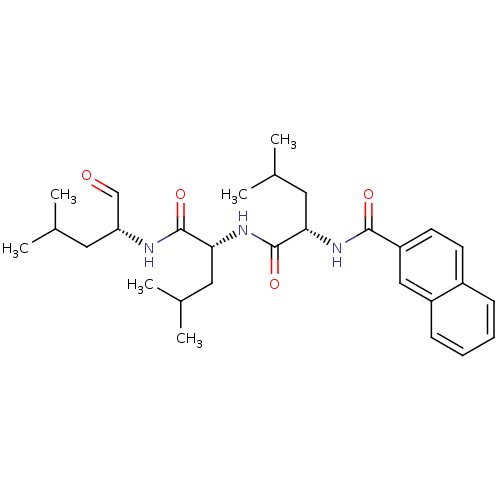
(CHEMBL150134 | Naphthalene-2-carboxylic acid {(S)-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C29H41N3O4/c1-18(2)13-24(17-33)30-28(35)25(14-19(3)4)32-29(36)26(15-20(5)6)31-27(34)23-12-11-21-9-7-8-10-22(21)16-23/h7-12,16-20,24-26H,13-15H2,1-6H3,(H,30,35)(H,31,34)(H,32,36)/t24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospholipase A2
(Apis mellifera) | BDBM50524139
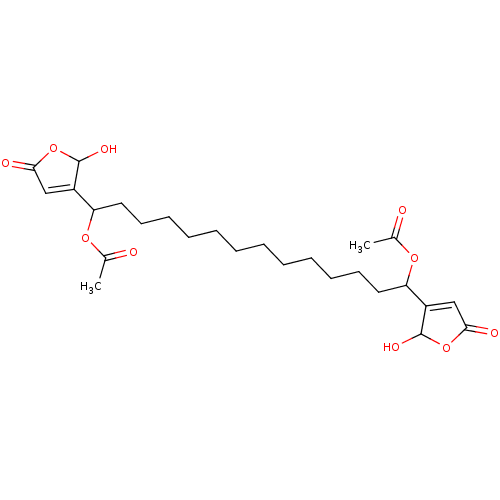
(CHEMBL4467602)Show SMILES CC(=O)OC(CCCCCCCCCCCCC(OC(C)=O)C1=CC(=O)OC1O)C1=CC(=O)OC1O |t:22,30| Show InChI InChI=1S/C26H38O10/c1-17(27)33-21(19-15-23(29)35-25(19)31)13-11-9-7-5-3-4-6-8-10-12-14-22(34-18(2)28)20-16-24(30)36-26(20)32/h15-16,21-22,25-26,31-32H,3-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 preincubated for 1 hr followed by addition of phosphotidylcholine and 1-palmitoyl-2-(1-14C) palmitoyl furthe... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291194
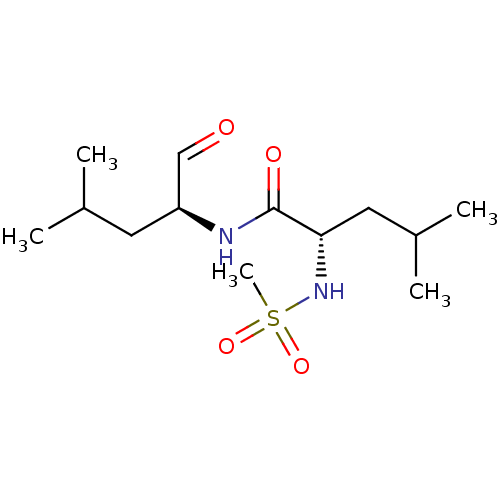
((S)-2-Methanesulfonylamino-4-methyl-pentanoic acid...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NS(C)(=O)=O)C=O Show InChI InChI=1S/C13H26N2O4S/c1-9(2)6-11(8-16)14-13(17)12(7-10(3)4)15-20(5,18)19/h8-12,15H,6-7H2,1-5H3,(H,14,17)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291218
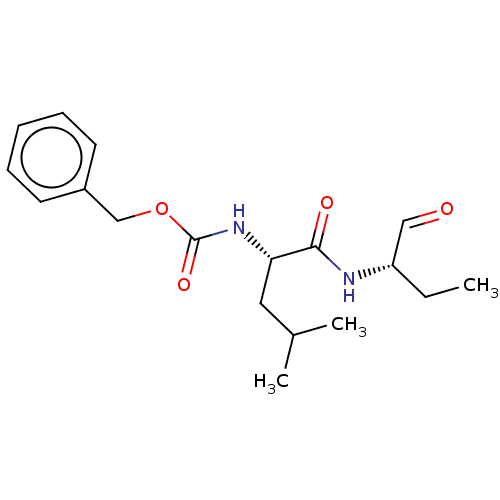
(CHEMBL2371036 | [(S)-1-(1-Formyl-propylcarbamoyl)-...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C18H26N2O4/c1-4-15(11-21)19-17(22)16(10-13(2)3)20-18(23)24-12-14-8-6-5-7-9-14/h5-9,11,13,15-16H,4,10,12H2,1-3H3,(H,19,22)(H,20,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291197
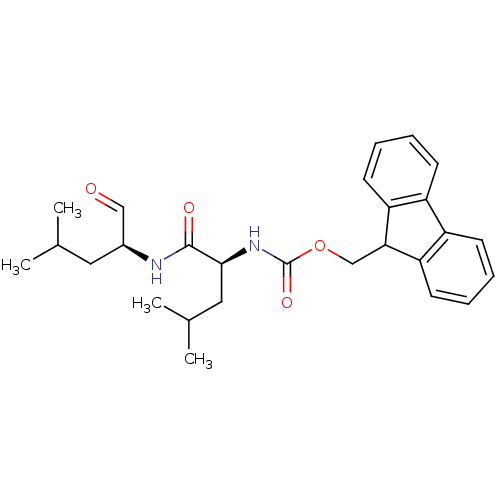
(CHEMBL149754 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCC1c2ccccc2-c2ccccc12)C=O Show InChI InChI=1S/C27H34N2O4/c1-17(2)13-19(15-30)28-26(31)25(14-18(3)4)29-27(32)33-16-24-22-11-7-5-9-20(22)21-10-6-8-12-23(21)24/h5-12,15,17-19,24-25H,13-14,16H2,1-4H3,(H,28,31)(H,29,32)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291226
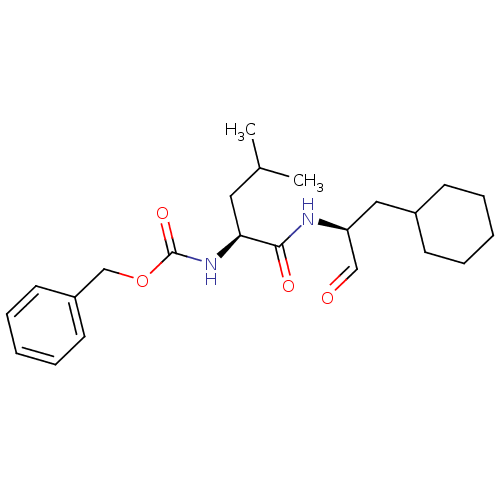
(CHEMBL151829 | [(S)-1-((S)-1-Cyclohexylmethyl-2-ox...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C=O Show InChI InChI=1S/C23H34N2O4/c1-17(2)13-21(25-23(28)29-16-19-11-7-4-8-12-19)22(27)24-20(15-26)14-18-9-5-3-6-10-18/h4,7-8,11-12,15,17-18,20-21H,3,5-6,9-10,13-14,16H2,1-2H3,(H,24,27)(H,25,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50142287
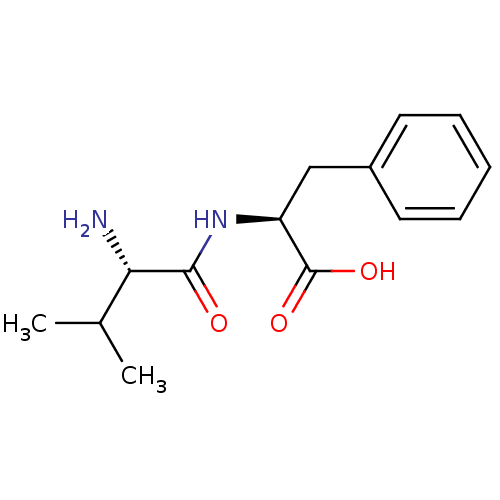
((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-phenyl...)Show InChI InChI=1S/C14H20N2O3/c1-9(2)12(15)13(17)16-11(14(18)19)8-10-6-4-3-5-7-10/h3-7,9,11-12H,8,15H2,1-2H3,(H,16,17)(H,18,19)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was evaluated against recombinant human calpain I |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50136018
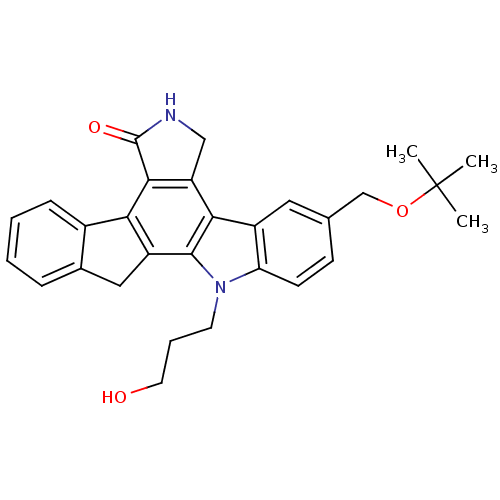
(9-(tert-butoxymethyl)-12-(3-hydroxypropyl)-6,7,12,...)Show SMILES CC(C)(C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C29H30N2O3/c1-29(2,3)34-16-17-9-10-23-20(13-17)25-22-15-30-28(33)26(22)24-19-8-5-4-7-18(19)14-21(24)27(25)31(23)11-6-12-32/h4-5,7-10,13,32H,6,11-12,14-16H2,1-3H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human Vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291187
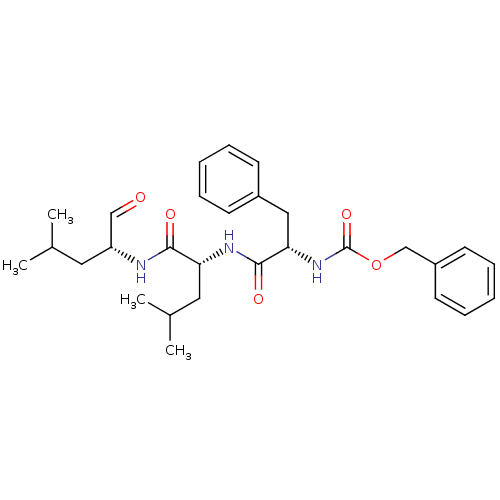
(CHEMBL151370 | {(S)-1-[(R)-1-((R)-1-Formyl-3-methy...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H39N3O5/c1-20(2)15-24(18-33)30-27(34)25(16-21(3)4)31-28(35)26(17-22-11-7-5-8-12-22)32-29(36)37-19-23-13-9-6-10-14-23/h5-14,18,20-21,24-26H,15-17,19H2,1-4H3,(H,30,34)(H,31,35)(H,32,36)/t24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50136013
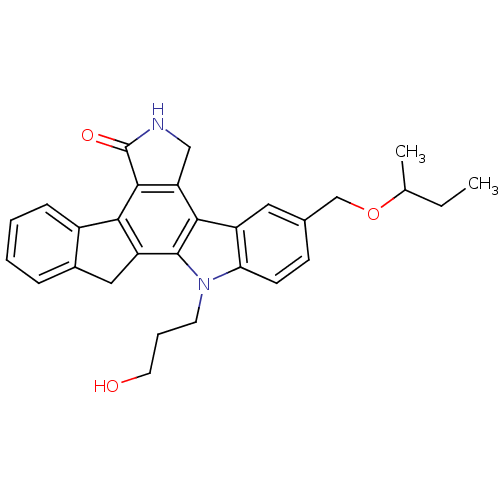
(9-(sec-butoxymethyl)-12-(3-hydroxypropyl)-6,7,12,1...)Show SMILES CCC(C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C29H30N2O3/c1-3-17(2)34-16-18-9-10-24-21(13-18)26-23-15-30-29(33)27(23)25-20-8-5-4-7-19(20)14-22(25)28(26)31(24)11-6-12-32/h4-5,7-10,13,17,32H,3,6,11-12,14-16H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human Vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50024274
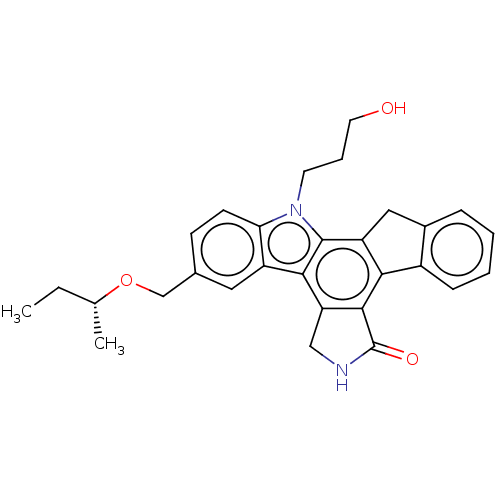
(CHEMBL2448133)Show SMILES CC[C@@H](C)OCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 |r| Show InChI InChI=1S/C29H30N2O3/c1-3-17(2)34-16-18-9-10-24-21(13-18)26-23-15-30-29(33)27(23)25-20-8-5-4-7-19(20)14-22(25)28(26)31(24)11-6-12-32/h4-5,7-10,13,17,32H,3,6,11-12,14-16H2,1-2H3,(H,30,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291177

((S)-2-Acetylamino-4-methyl-pentanoic acid [(R)-1-(...)Show SMILES CCCC[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O)C=O Show InChI InChI=1S/C20H37N3O4/c1-7-8-9-16(12-24)22-19(26)18(11-14(4)5)23-20(27)17(10-13(2)3)21-15(6)25/h12-14,16-18H,7-11H2,1-6H3,(H,21,25)(H,22,26)(H,23,27)/t16-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291205
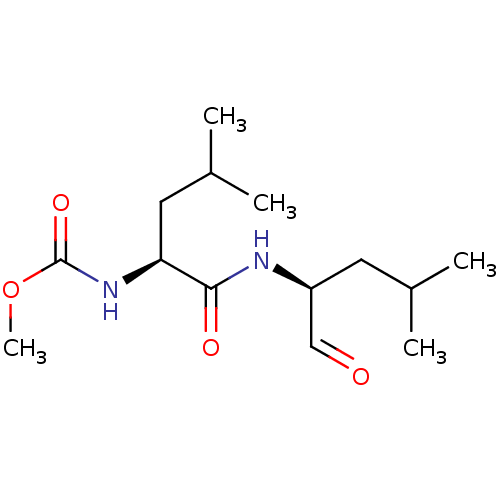
(CHEMBL358404 | [(S)-1-((S)-1-Formyl-3-methyl-butyl...)Show SMILES COC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C=O Show InChI InChI=1S/C14H26N2O4/c1-9(2)6-11(8-17)15-13(18)12(7-10(3)4)16-14(19)20-5/h8-12H,6-7H2,1-5H3,(H,15,18)(H,16,19)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM193425
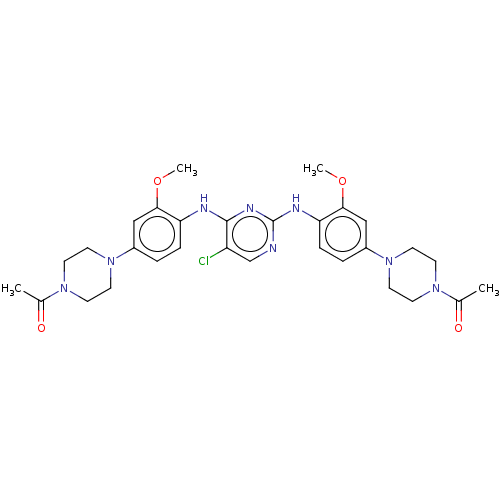
(US9199944, 7)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccc(cc2OC)N2CCN(CC2)C(C)=O)n1)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C30H37ClN8O4/c1-20(40)36-9-13-38(14-10-36)22-5-7-25(27(17-22)42-3)33-29-24(31)19-32-30(35-29)34-26-8-6-23(18-28(26)43-4)39-15-11-37(12-16-39)21(2)41/h5-8,17-19H,9-16H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291181
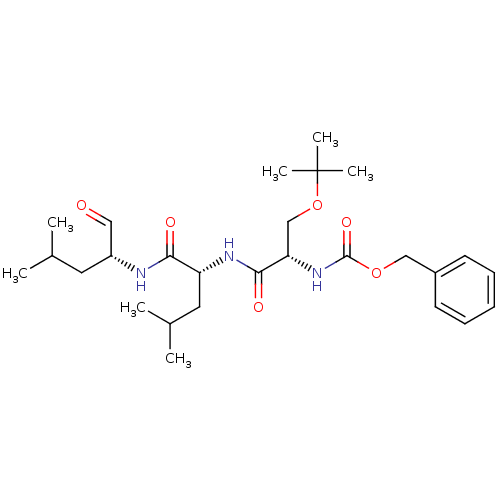
(CHEMBL150976 | {(S)-2-tert-Butoxy-1-[(R)-1-((R)-1-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](COC(C)(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C27H43N3O6/c1-18(2)13-21(15-31)28-24(32)22(14-19(3)4)29-25(33)23(17-36-27(5,6)7)30-26(34)35-16-20-11-9-8-10-12-20/h8-12,15,18-19,21-23H,13-14,16-17H2,1-7H3,(H,28,32)(H,29,33)(H,30,34)/t21-,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50136021
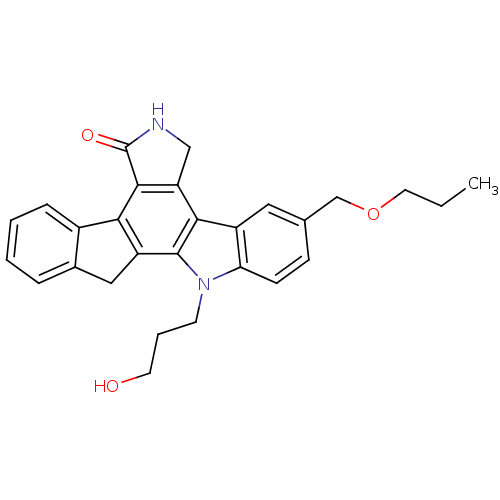
(12-(3-hydroxypropyl)-9-propoxymethyl-6,7,12,13-tet...)Show SMILES CCCOCc1ccc2n(CCCO)c3c4Cc5ccccc5-c4c4C(=O)NCc4c3c2c1 Show InChI InChI=1S/C28H28N2O3/c1-2-12-33-16-17-8-9-23-20(13-17)25-22-15-29-28(32)26(22)24-19-7-4-3-6-18(19)14-21(24)27(25)30(23)10-5-11-31/h3-4,6-9,13,31H,2,5,10-12,14-16H2,1H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human Vascular endothelial growth factor receptor 2 |
J Med Chem 46: 5375-88 (2003)
Article DOI: 10.1021/jm0301641
BindingDB Entry DOI: 10.7270/Q2V69J1X |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291176
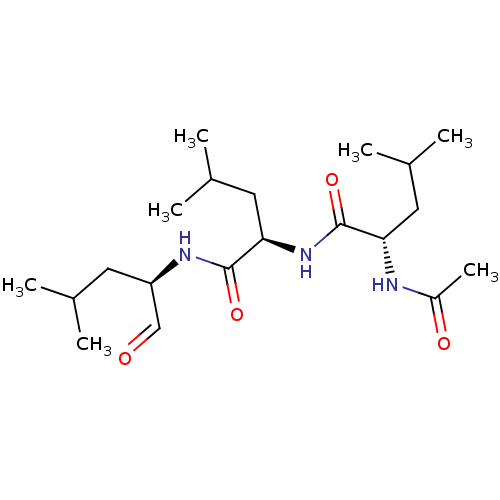
((S)-2-Acetylamino-4-methyl-pentanoic acid [(R)-1-(...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O)C=O Show InChI InChI=1S/C20H37N3O4/c1-12(2)8-16(11-24)22-19(26)18(10-14(5)6)23-20(27)17(9-13(3)4)21-15(7)25/h11-14,16-18H,8-10H2,1-7H3,(H,21,25)(H,22,26)(H,23,27)/t16-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291207
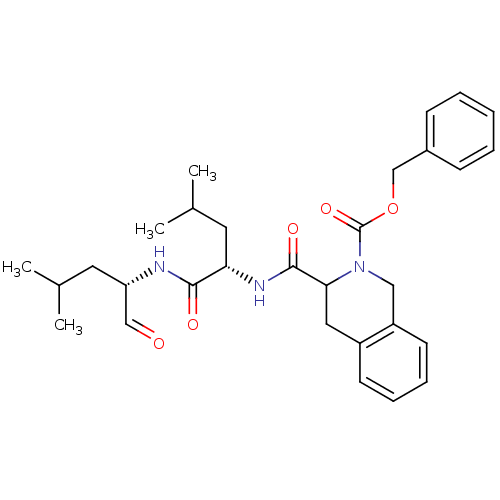
(3-[(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)C1Cc2ccccc2CN1C(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C30H39N3O5/c1-20(2)14-25(18-34)31-28(35)26(15-21(3)4)32-29(36)27-16-23-12-8-9-13-24(23)17-33(27)30(37)38-19-22-10-6-5-7-11-22/h5-13,18,20-21,25-27H,14-17,19H2,1-4H3,(H,31,35)(H,32,36)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50291224
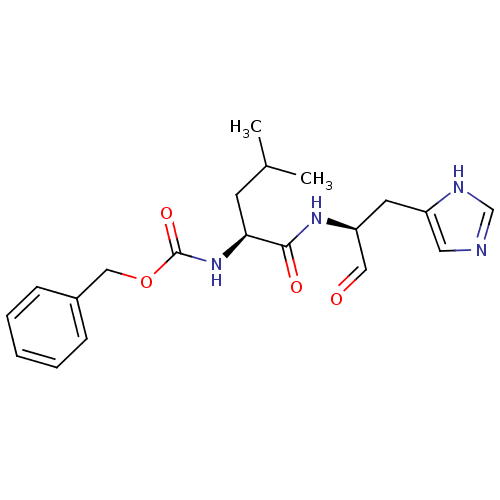
(CHEMBL424142 | {(S)-1-[(S)-1-Formyl-2-(3H-imidazol...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C=O Show InChI InChI=1S/C20H26N4O4/c1-14(2)8-18(24-20(27)28-12-15-6-4-3-5-7-15)19(26)23-17(11-25)9-16-10-21-13-22-16/h3-7,10-11,13-14,17-18H,8-9,12H2,1-2H3,(H,21,22)(H,23,26)(H,24,27)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against recombinant human calpain 1 |
Bioorg Med Chem Lett 7: 539-544 (1997)
Article DOI: 10.1016/S0960-894X(97)00063-2
BindingDB Entry DOI: 10.7270/Q2W37WBM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data