 Found 3012 hits with Last Name = 'khan' and Initial = 'm'
Found 3012 hits with Last Name = 'khan' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22916
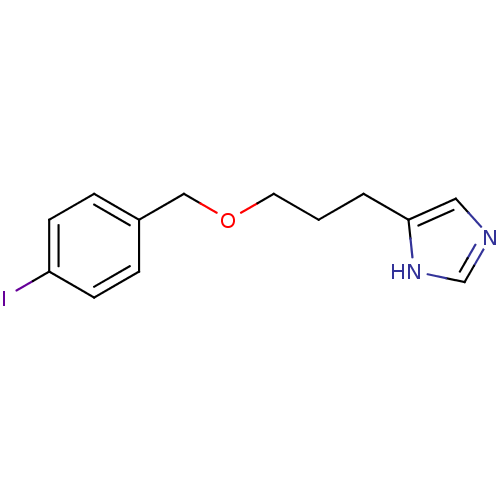
(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
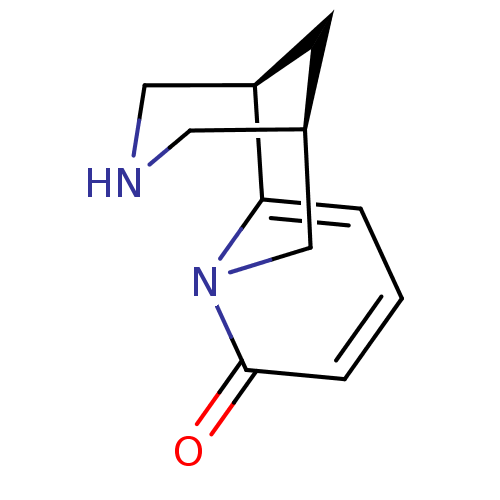
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863

((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50070214
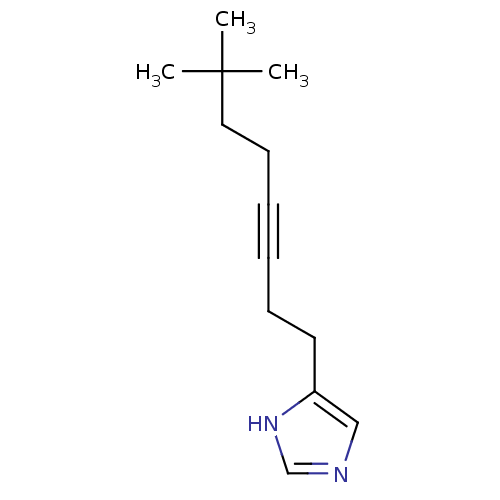
(4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...)Show InChI InChI=1S/C13H20N2/c1-13(2,3)9-7-5-4-6-8-12-10-14-11-15-12/h10-11H,6-9H2,1-3H3,(H,14,15) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50070220
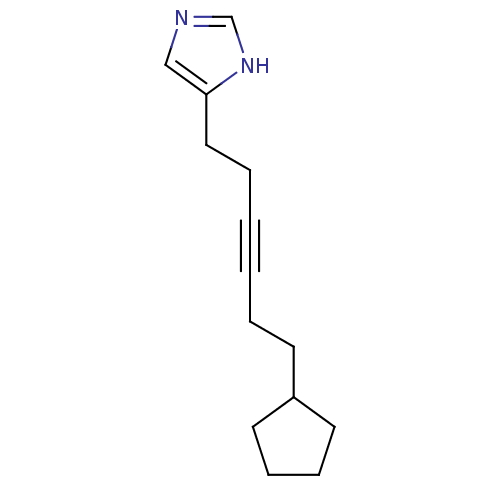
(4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...)Show InChI InChI=1S/C14H20N2/c1(3-7-13-8-5-6-9-13)2-4-10-14-11-15-12-16-14/h11-13H,3-10H2,(H,15,16) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85407
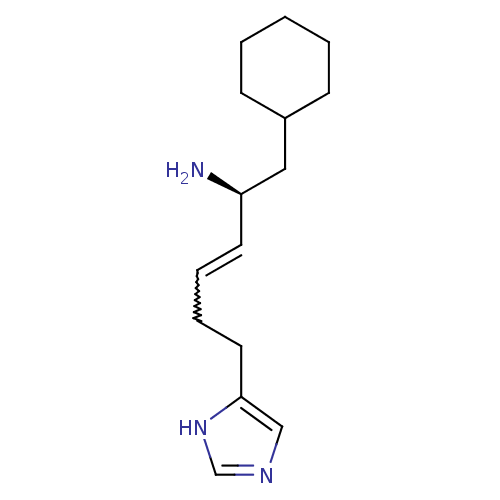
(GT 2231)Show SMILES N[C@@H](CC1CCCCC1)C=CCCc1cnc[nH]1 |r,w:10.11| Show InChI InChI=1S/C15H25N3/c16-14(10-13-6-2-1-3-7-13)8-4-5-9-15-11-17-12-18-15/h4,8,11-14H,1-3,5-7,9-10,16H2,(H,17,18)/b8-4+/t14-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114658
BindingDB Entry DOI: 10.7270/Q2NP28CD |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 275: 598-604 (1995)
BindingDB Entry DOI: 10.7270/Q21G0JSP |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070213
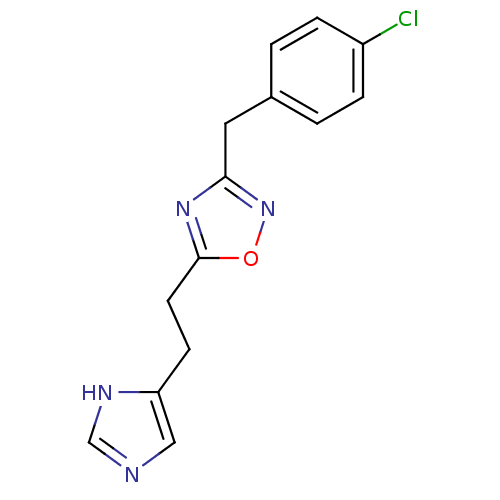
(3-(4-Chloro-benzyl)-5-[2-(1H-imidazol-4-yl)-ethyl]...)Show InChI InChI=1S/C14H13ClN4O/c15-11-3-1-10(2-4-11)7-13-18-14(20-19-13)6-5-12-8-16-9-17-12/h1-4,8-9H,5-7H2,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H](R)-alpha-methylhistamine from histamine H3 receptor in rat brain membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462785
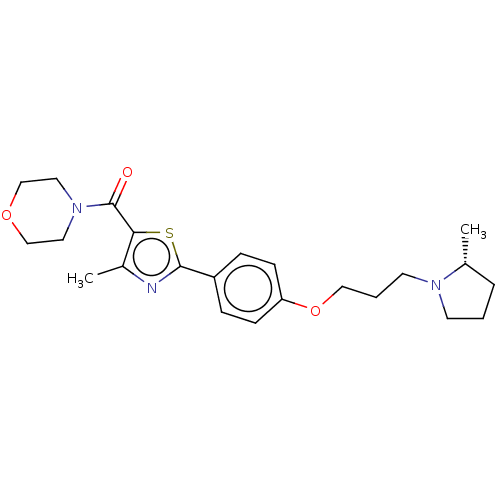
(CHEMBL4245973)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1nc(C)c(s1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C23H31N3O3S/c1-17-5-3-10-25(17)11-4-14-29-20-8-6-19(7-9-20)22-24-18(2)21(30-22)23(27)26-12-15-28-16-13-26/h6-9,17H,3-5,10-16H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in CHO cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50070211
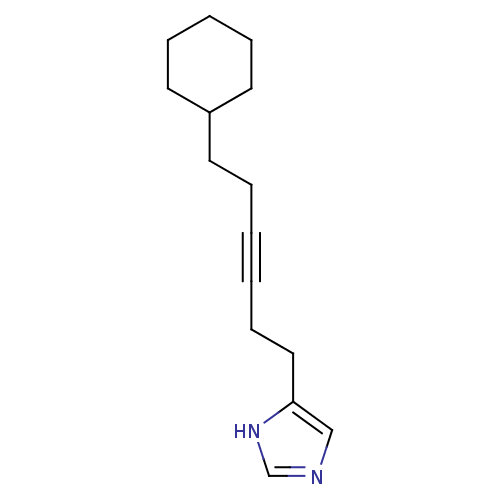
(4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...)Show InChI InChI=1S/C15H22N2/c1(2-7-11-15-12-16-13-17-15)4-8-14-9-5-3-6-10-14/h12-14H,3-11H2,(H,16,17) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50413824
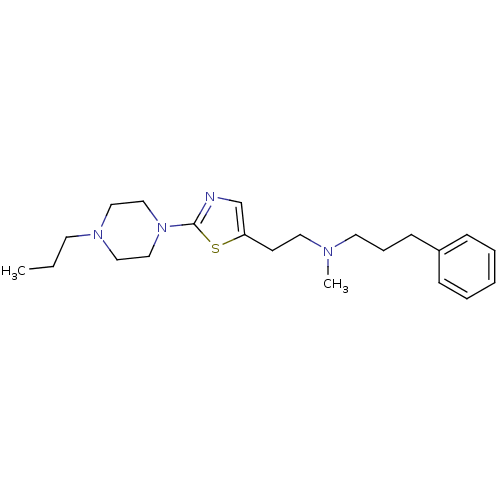
(CHEMBL473093)Show InChI InChI=1S/C22H34N4S/c1-3-12-25-15-17-26(18-16-25)22-23-19-21(27-22)11-14-24(2)13-7-10-20-8-5-4-6-9-20/h4-6,8-9,19H,3,7,10-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293T cell membranes after 60 mins by liquid scintillatio... |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85405
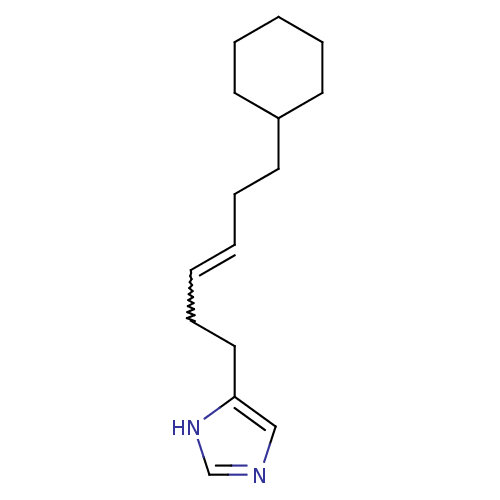
(GT 2227 | GT 2228)Show InChI InChI=1S/C15H24N2/c1(2-7-11-15-12-16-13-17-15)4-8-14-9-5-3-6-10-14/h1-2,12-14H,3-11H2,(H,16,17)/b2-1- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85412
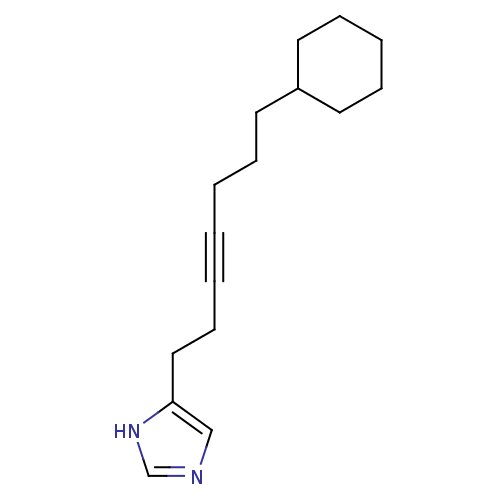
(GT 2287)Show InChI InChI=1S/C16H24N2/c1(3-8-12-16-13-17-14-18-16)2-5-9-15-10-6-4-7-11-15/h13-15H,2,4-12H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85405

(GT 2227 | GT 2228)Show InChI InChI=1S/C15H24N2/c1(2-7-11-15-12-16-13-17-15)4-8-14-9-5-3-6-10-14/h1-2,12-14H,3-11H2,(H,16,17)/b2-1- | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462811
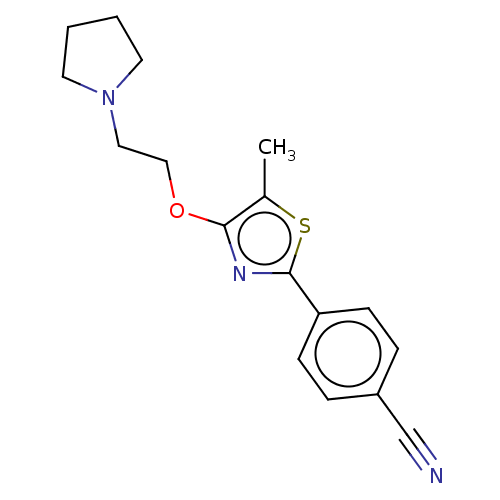
(CHEMBL4240664)Show InChI InChI=1S/C17H19N3OS/c1-13-16(21-11-10-20-8-2-3-9-20)19-17(22-13)15-6-4-14(12-18)5-7-15/h4-7H,2-3,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM82546
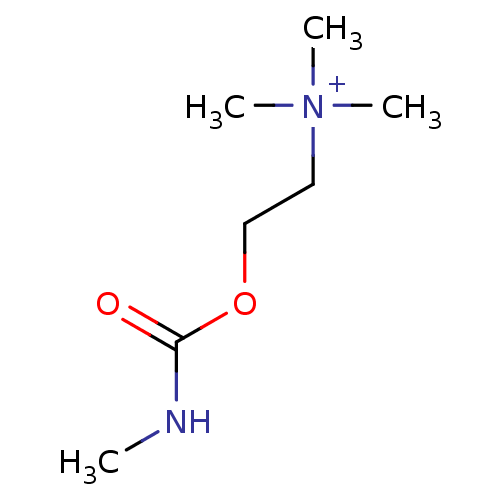
(CAS_14721-69-8 | MCC | N-methylcarbamylcholine | N...)Show InChI InChI=1S/C7H16N2O2/c1-8-7(10)11-6-5-9(2,3)4/h5-6H2,1-4H3/p+1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462808
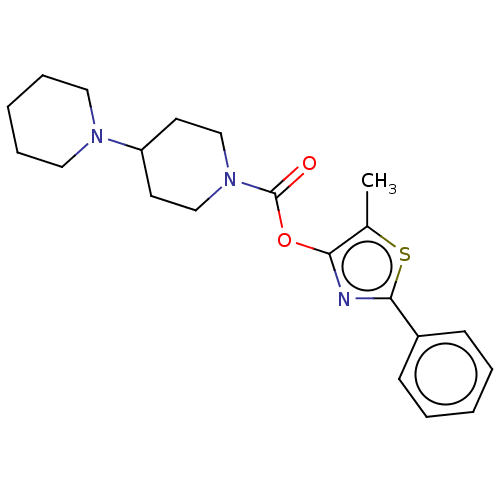
(CHEMBL4240159)Show SMILES Cc1sc(nc1OC(=O)N1CCC(CC1)N1CCCCC1)-c1ccccc1 Show InChI InChI=1S/C21H27N3O2S/c1-16-19(22-20(27-16)17-8-4-2-5-9-17)26-21(25)24-14-10-18(11-15-24)23-12-6-3-7-13-23/h2,4-5,8-9,18H,3,6-7,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85402
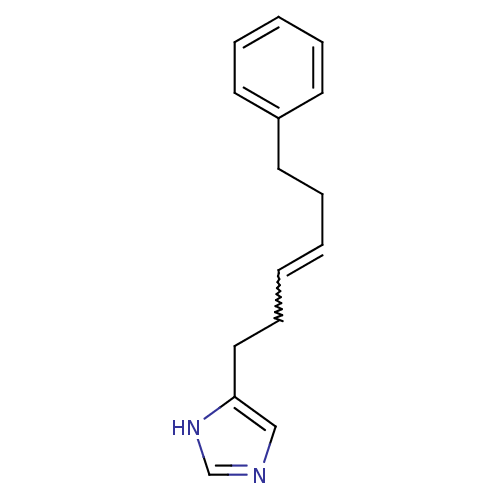
(GT 2327)Show InChI InChI=1S/C15H18N2/c1(2-7-11-15-12-16-13-17-15)4-8-14-9-5-3-6-10-14/h1-3,5-6,9-10,12-13H,4,7-8,11H2,(H,16,17)/b2-1- | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50061562

((12R,13aR)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...)Show SMILES CO[C@@H]1CC=C2CCN3CCC4=C(CC(=O)OC4)[C@@]23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3/t13-,16-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462821
(CHEMBL4247219)Show InChI InChI=1S/C21H24N4OS/c1-16-20(24-21(27-16)19-13-22-9-10-23-19)26-15-18-7-5-17(6-8-18)14-25-11-3-2-4-12-25/h5-10,13H,2-4,11-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50054820
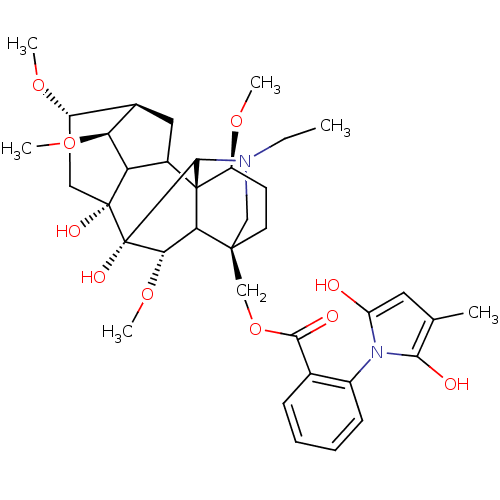
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
G-protein coupled estrogen receptor 1
(Homo sapiens (Human)) | BDBM50590843

(CHEMBL5170710)Show SMILES [H][C@]12CC=C[C@@]1([H])c1cc(ccc1NC2c1cc2OCOc2cc1Br)C(C)=O |r,c:3| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114658
BindingDB Entry DOI: 10.7270/Q2NP28CD |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85399
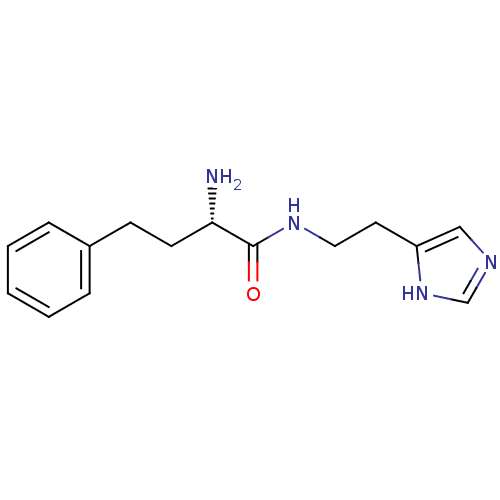
(GT 2148)Show InChI InChI=1S/C15H20N4O/c16-14(7-6-12-4-2-1-3-5-12)15(20)18-9-8-13-10-17-11-19-13/h1-5,10-11,14H,6-9,16H2,(H,17,19)(H,18,20)/t14-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Thermolysin
(Bacillus thermoproteolyticus) | BDBM50321113
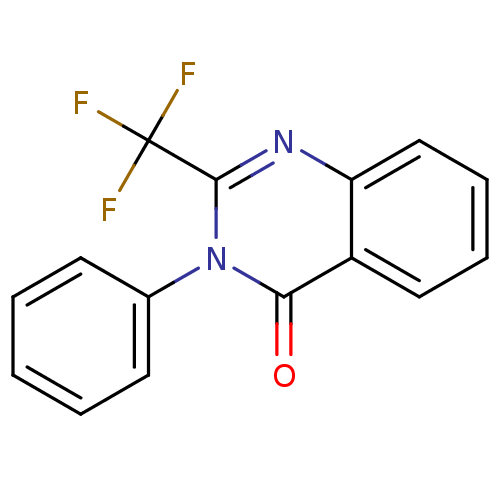
(3-Phenyl-2-(trifluoromethyl)quinazolin-4(3H)-one |...)Show InChI InChI=1S/C15H9F3N2O/c16-15(17,18)14-19-12-9-5-4-8-11(12)13(21)20(14)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa |
Bioorg Med Chem 18: 4317-27 (2010)
Article DOI: 10.1016/j.bmc.2010.04.083
BindingDB Entry DOI: 10.7270/Q2QV3MP9 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85405

(GT 2227 | GT 2228)Show InChI InChI=1S/C15H24N2/c1(2-7-11-15-12-16-13-17-15)4-8-14-9-5-3-6-10-14/h1-2,12-14H,3-11H2,(H,16,17)/b2-1- | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50047021
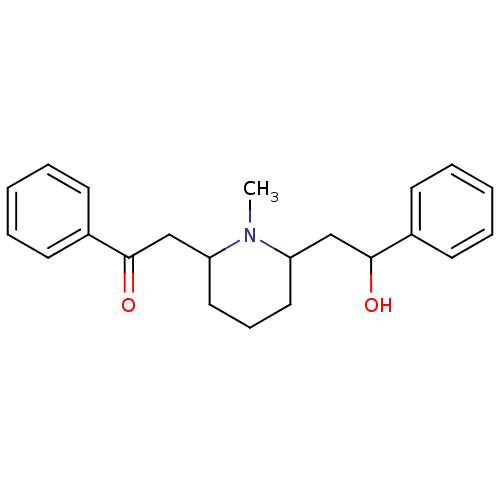
(2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2...)Show InChI InChI=1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85411
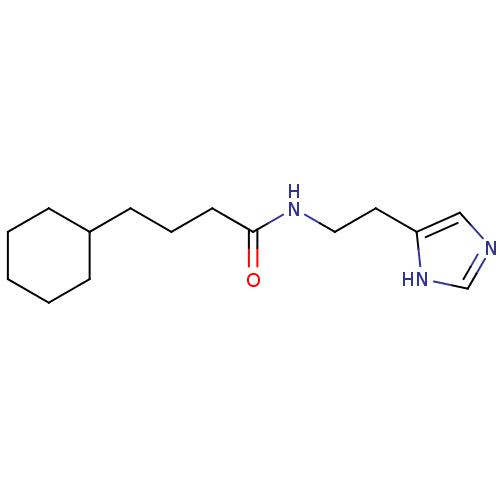
(GT 2174)Show InChI InChI=1S/C15H25N3O/c19-15(17-10-9-14-11-16-12-18-14)8-4-7-13-5-2-1-3-6-13/h11-13H,1-10H2,(H,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50510681
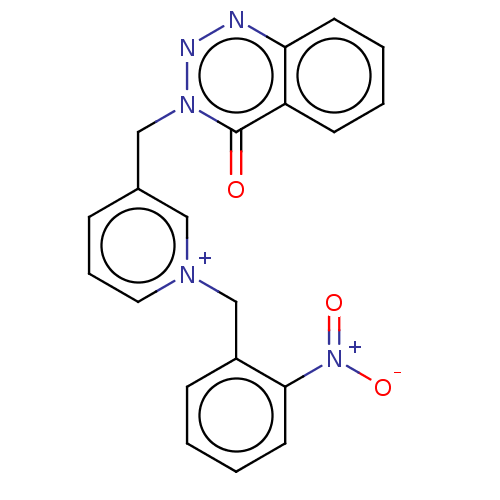
(CHEMBL4451509)Show SMILES [O-][N+](=O)c1ccccc1C[n+]1cccc(Cn2nnc3ccccc3c2=O)c1 Show InChI InChI=1S/C20H16N5O3/c26-20-17-8-2-3-9-18(17)21-22-24(20)13-15-6-5-11-23(12-15)14-16-7-1-4-10-19(16)25(27)28/h1-12H,13-14H2/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zanjan
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition and measured after 15 mins by ... |
Bioorg Med Chem 27: 2914-2922 (2019)
Article DOI: 10.1016/j.bmc.2019.05.023
BindingDB Entry DOI: 10.7270/Q22F7RRB |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50136199
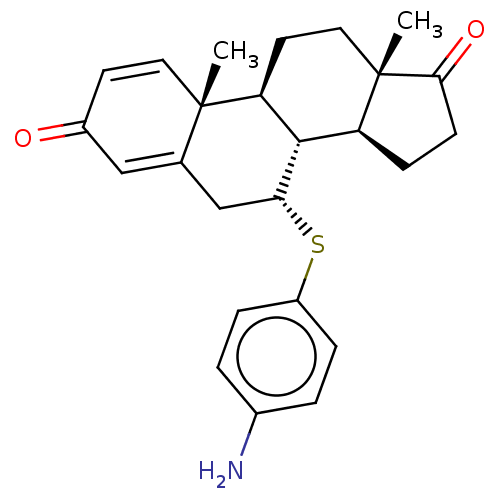
(CHEMBL3349548)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)C=C[C@]12C)Sc1ccc(N)cc1 |c:22,t:18| Show InChI InChI=1S/C25H29NO2S/c1-24-11-9-17(27)13-15(24)14-21(29-18-5-3-16(26)4-6-18)23-19-7-8-22(28)25(19,2)12-10-20(23)24/h3-6,9,11,13,19-21,23H,7-8,10,12,14,26H2,1-2H3/t19-,20-,21+,23-,24-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114658
BindingDB Entry DOI: 10.7270/Q2NP28CD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462792
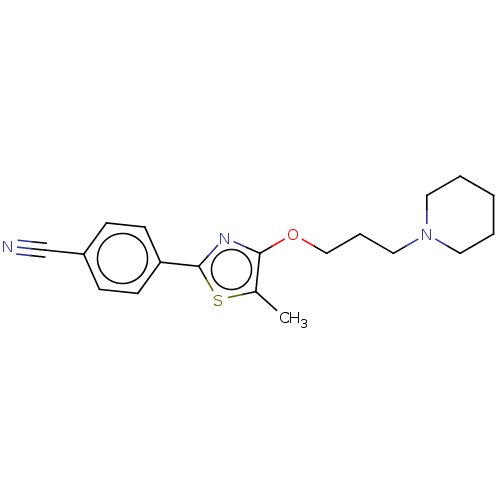
(CHEMBL4248654)Show InChI InChI=1S/C19H23N3OS/c1-15-18(23-13-5-12-22-10-3-2-4-11-22)21-19(24-15)17-8-6-16(14-20)7-9-17/h6-9H,2-5,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462791
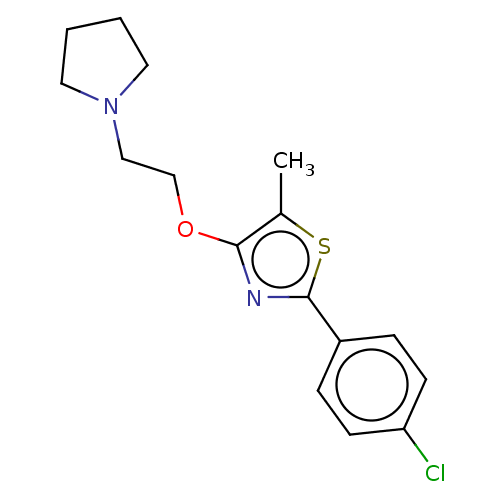
(CHEMBL4243928)Show InChI InChI=1S/C16H19ClN2OS/c1-12-15(20-11-10-19-8-2-3-9-19)18-16(21-12)13-4-6-14(17)7-5-13/h4-7H,2-3,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 25 | -44.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi
| Assay Description
AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... |
J Enzyme Inhib Med Chem 21: 703-10 (2006)
Article DOI: 10.1080/14756360600889708
BindingDB Entry DOI: 10.7270/Q26D5RJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinic acetylcholine receptor
(RAT) | BDBM50061562

((12R,13aR)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...)Show SMILES CO[C@@H]1CC=C2CCN3CCC4=C(CC(=O)OC4)[C@@]23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3/t13-,16-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50061567
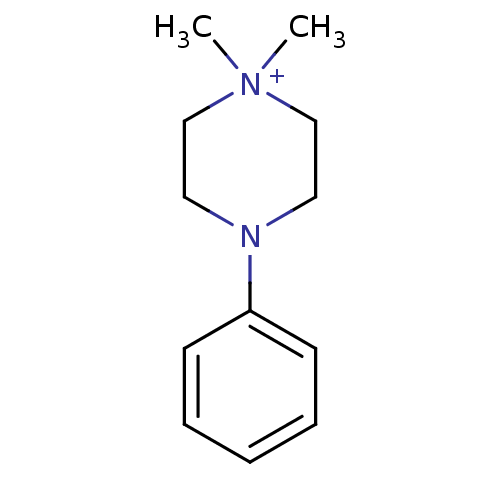
(1,1-Dimethyl-4-phenyl-piperazin-1-ium | CHEMBL1347...)Show InChI InChI=1S/C12H19N2/c1-14(2)10-8-13(9-11-14)12-6-4-3-5-7-12/h3-7H,8-11H2,1-2H3/q+1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85401
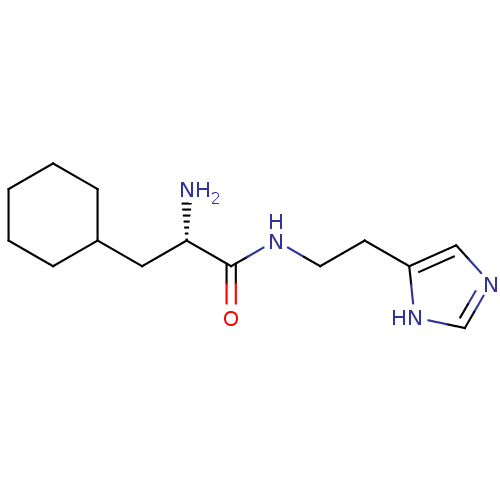
(GT 2140)Show InChI InChI=1S/C14H24N4O/c15-13(8-11-4-2-1-3-5-11)14(19)17-7-6-12-9-16-10-18-12/h9-11,13H,1-8,15H2,(H,16,18)(H,17,19)/t13-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM82068

(CHEMBL9732 | Nicotine-D salicylate | Nicotine-L sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 159-66 (1994)
BindingDB Entry DOI: 10.7270/Q22Z141C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM86490
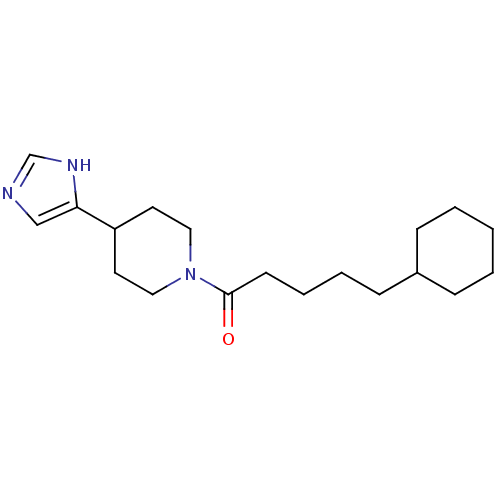
(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462781
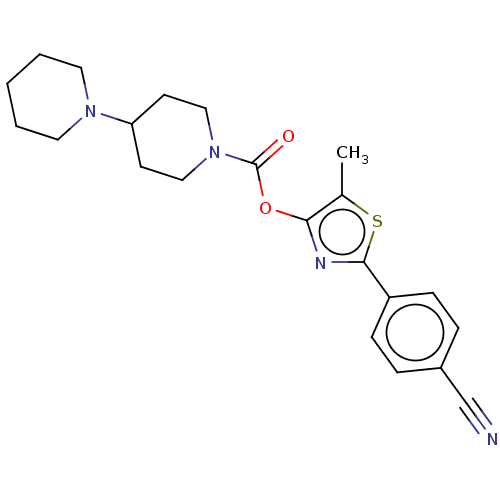
(CHEMBL4244388)Show SMILES Cc1sc(nc1OC(=O)N1CCC(CC1)N1CCCCC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2S/c1-16-20(24-21(29-16)18-7-5-17(15-23)6-8-18)28-22(27)26-13-9-19(10-14-26)25-11-3-2-4-12-25/h5-8,19H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
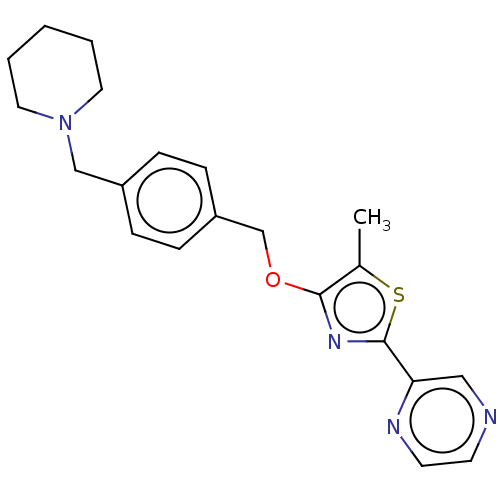
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462809
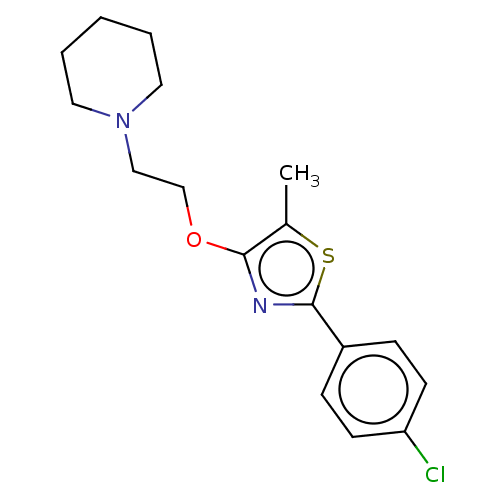
(CHEMBL4248174)Show InChI InChI=1S/C17H21ClN2OS/c1-13-16(21-12-11-20-9-3-2-4-10-20)19-17(22-13)14-5-7-15(18)8-6-14/h5-8H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462805
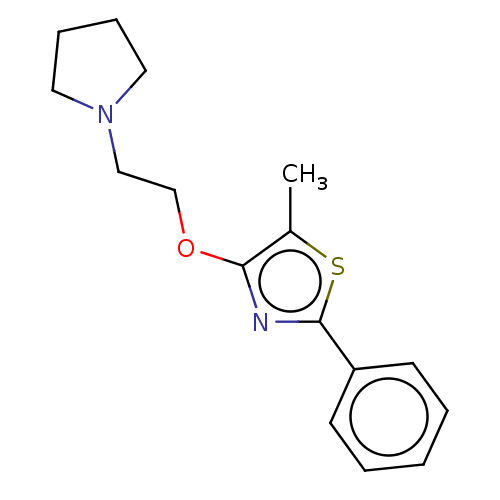
(CHEMBL4238115)Show InChI InChI=1S/C16H20N2OS/c1-13-15(19-12-11-18-9-5-6-10-18)17-16(20-13)14-7-3-2-4-8-14/h2-4,7-8H,5-6,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462810
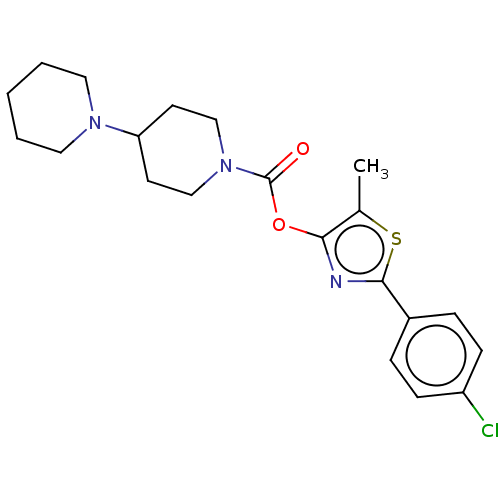
(CHEMBL4240564)Show SMILES Cc1sc(nc1OC(=O)N1CCC(CC1)N1CCCCC1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H26ClN3O2S/c1-15-19(23-20(28-15)16-5-7-17(22)8-6-16)27-21(26)25-13-9-18(10-14-25)24-11-3-2-4-12-24/h5-8,18H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462816
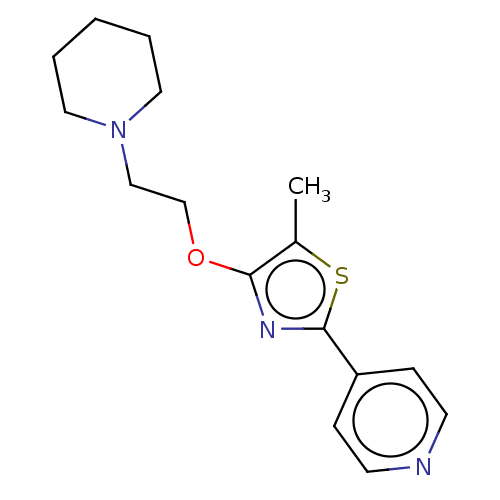
(CHEMBL4246071)Show InChI InChI=1S/C16H21N3OS/c1-13-15(20-12-11-19-9-3-2-4-10-19)18-16(21-13)14-5-7-17-8-6-14/h5-8H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50462796

(CHEMBL4237493)Show InChI InChI=1S/C20H22N4OS/c1-15-19(23-20(26-15)18-12-21-8-9-22-18)25-14-17-6-4-16(5-7-17)13-24-10-2-3-11-24/h4-9,12H,2-3,10-11,13-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 26: 4034-4046 (2018)
Article DOI: 10.1016/j.bmc.2018.06.028
BindingDB Entry DOI: 10.7270/Q29026FG |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50511123
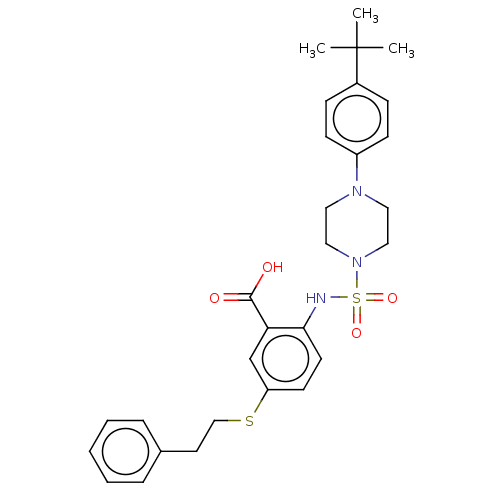
(CHEMBL4522193)Show SMILES CC(C)(C)c1ccc(cc1)N1CCN(CC1)S(=O)(=O)Nc1ccc(SCCc2ccccc2)cc1C(O)=O Show InChI InChI=1S/C29H35N3O4S2/c1-29(2,3)23-9-11-24(12-10-23)31-16-18-32(19-17-31)38(35,36)30-27-14-13-25(21-26(27)28(33)34)37-20-15-22-7-5-4-6-8-22/h4-14,21,30H,15-20H2,1-3H3,(H,33,34) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human Mcl1 expressed in Escherichia coli Rosetta2 DE3 by fluorescent labeled Flu-Bid peptide based fluorescence ... |
J Med Chem 63: 2489-2510 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01442
BindingDB Entry DOI: 10.7270/Q2542RW2 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50511124

(CHEMBL4533559)Show SMILES COc1cccc(CCSc2ccc(NS(=O)(=O)N3CCN(CC3)c3ccc(cc3)C(C)(C)C)c(c2)C(O)=O)c1 Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)23-8-10-24(11-9-23)32-15-17-33(18-16-32)40(36,37)31-28-13-12-26(21-27(28)29(34)35)39-19-14-22-6-5-7-25(20-22)38-4/h5-13,20-21,31H,14-19H2,1-4H3,(H,34,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human Mcl1 expressed in Escherichia coli Rosetta2 DE3 by fluorescent labeled Flu-Bid peptide based fluorescence ... |
J Med Chem 63: 2489-2510 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01442
BindingDB Entry DOI: 10.7270/Q2542RW2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data