

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM26193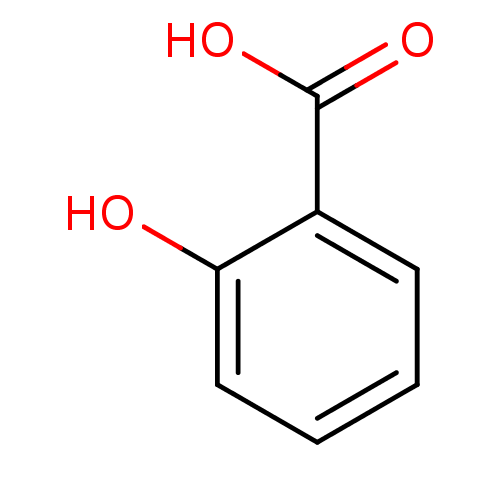 (2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Reversible inhibition of bovine xanthine oxidase | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449101 (CHEMBL3127130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive reversible inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 ... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449100 (CHEMBL3127135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive reversible inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 ... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141376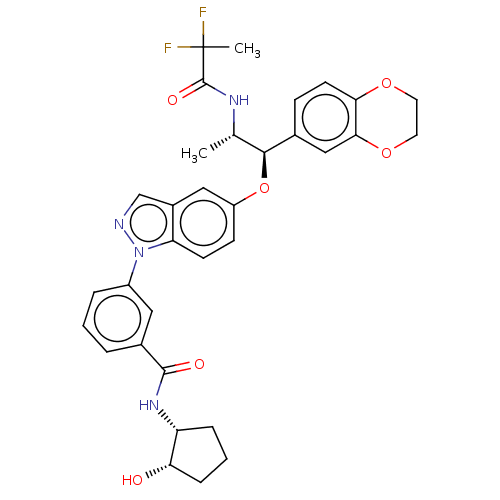 (US8916600, 10 | US9738632, Example 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.647 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141385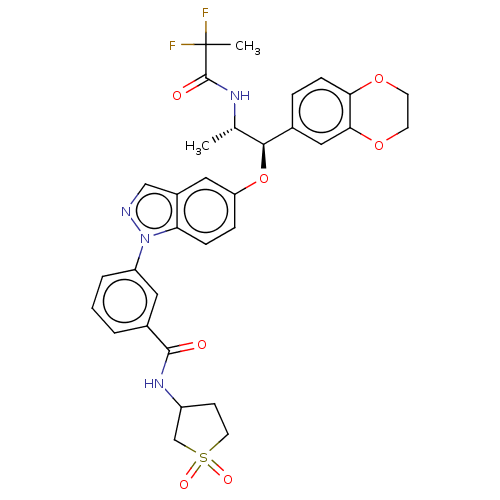 (US8916600, 19 | US9738632, Example 19) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.663 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141378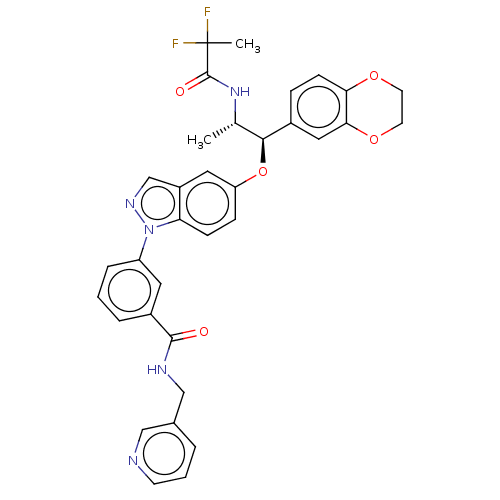 (US8916600, 12 | US9738632, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.767 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141374 (US8916600, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.796 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379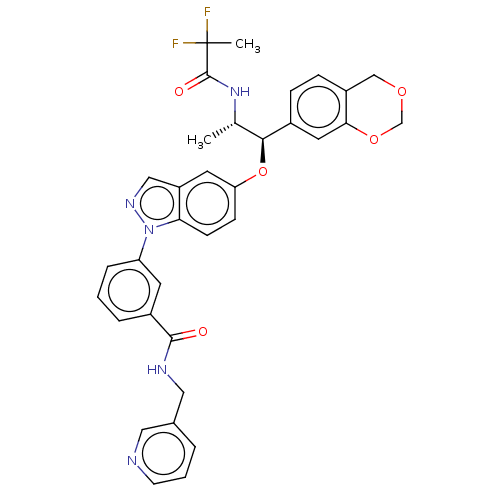 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.817 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141372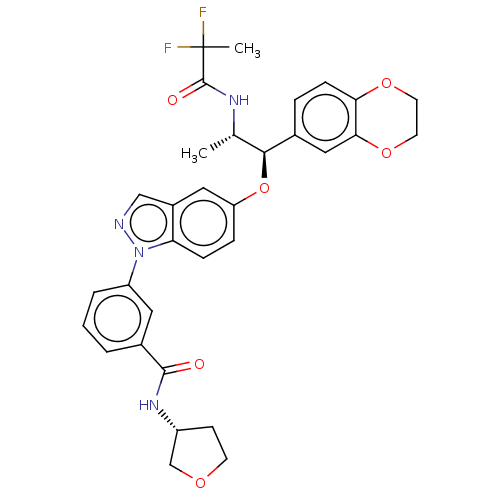 (US8916600, 6 | US9738632, Example 6) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.913 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141383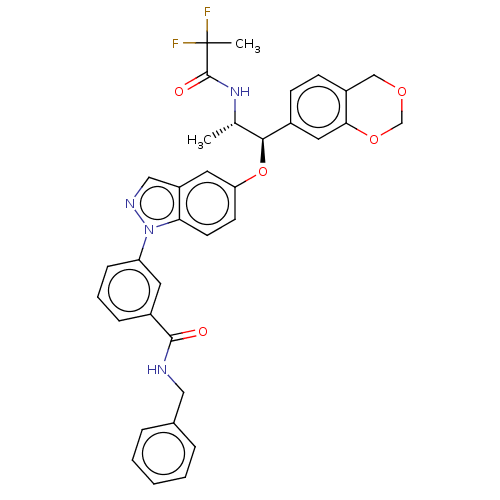 (US8916600, 17 | US9738632, Example 17) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.996 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449104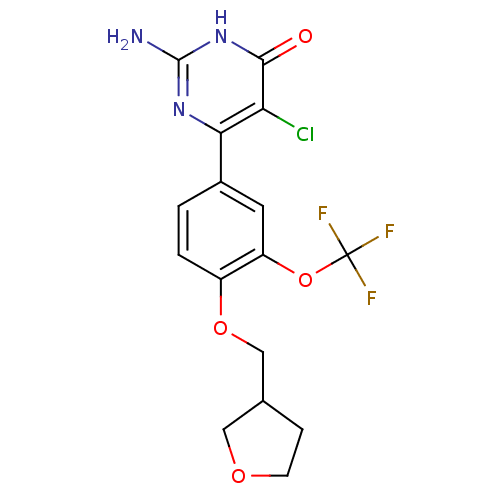 (CHEMBL3127117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141371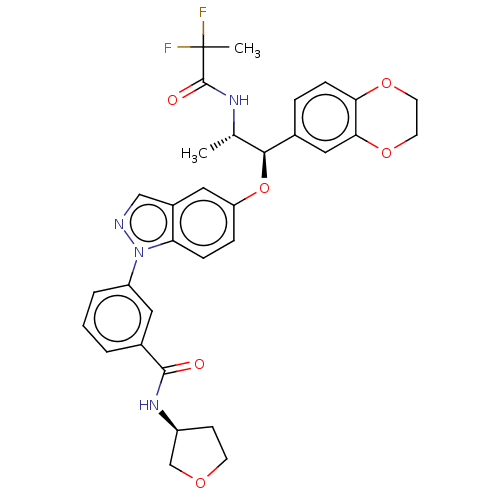 (US8916600, 5 | US9738632, Example 5) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141375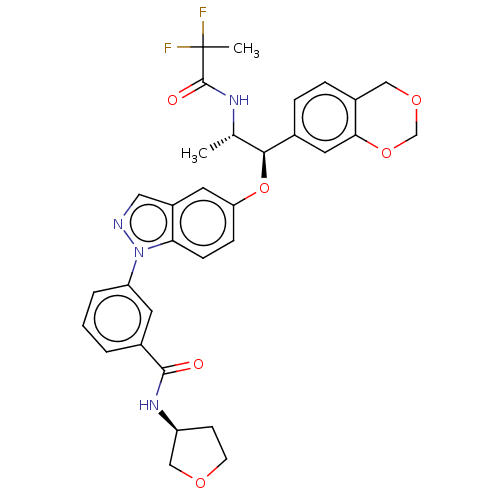 (US8916600, 9 | US9738632, Example 9) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449106 (CHEMBL3127137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141373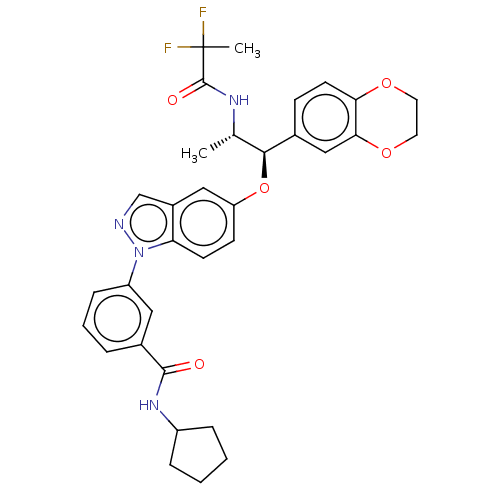 (US8916600, 7 | US9738632, Example 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449108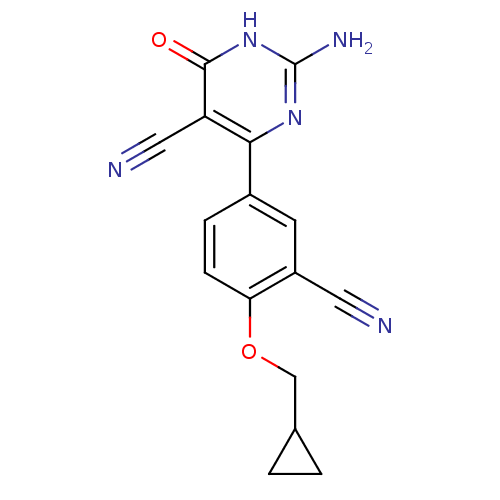 (CHEMBL3127134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50320491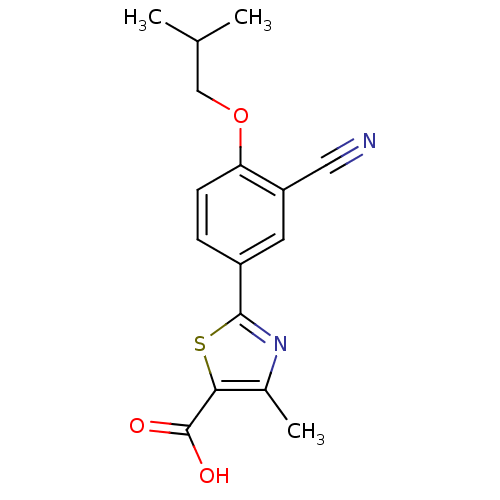 (2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141367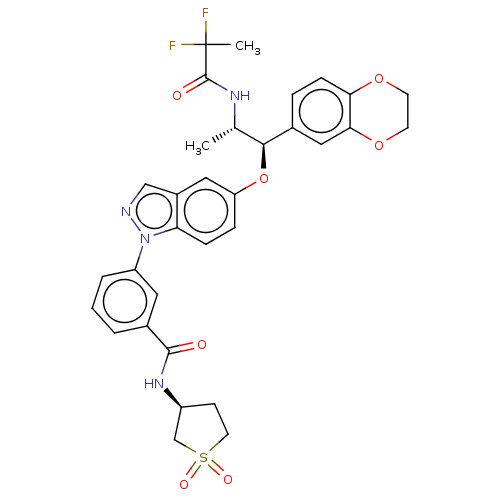 (US8916600, 1 | US8916600, 4 | US9738632, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141368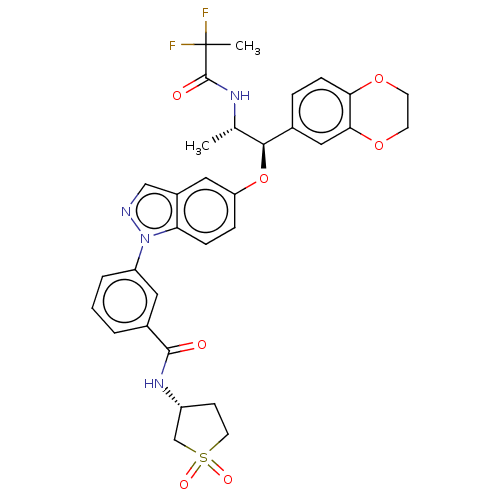 (US8916600, 2 | US8916600, 3 | US9738632, Example 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141367 (US8916600, 1 | US8916600, 4 | US9738632, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332676 (US10196374, Example 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449099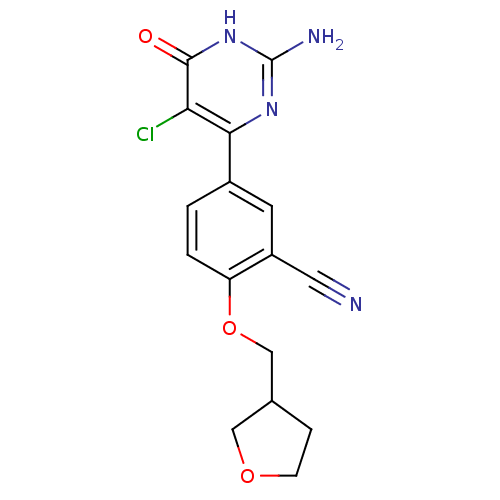 (CHEMBL3127114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive reversible inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 ... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141368 (US8916600, 2 | US8916600, 3 | US9738632, Example 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449111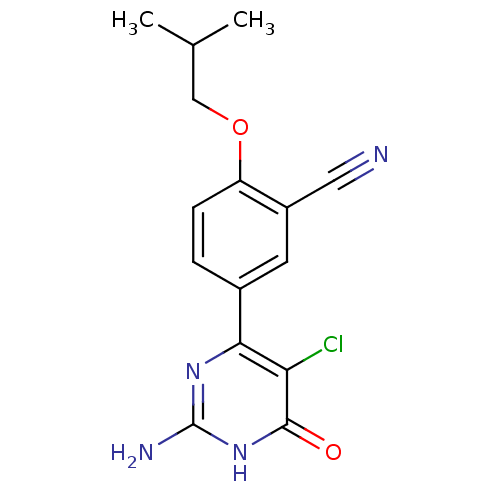 (CHEMBL3127131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141380 (US8916600, 14 | US9738632, Example 14) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141386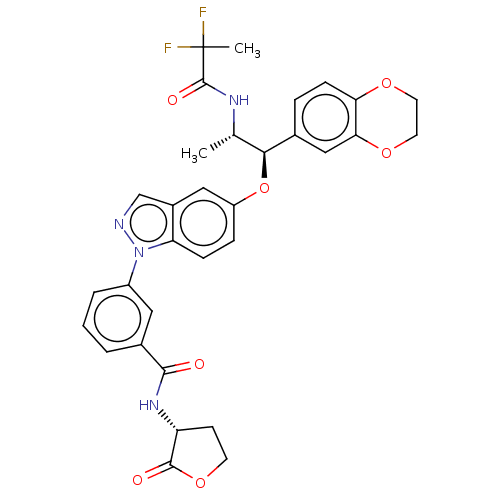 (US8916600, 20 | US9738632, Example 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141377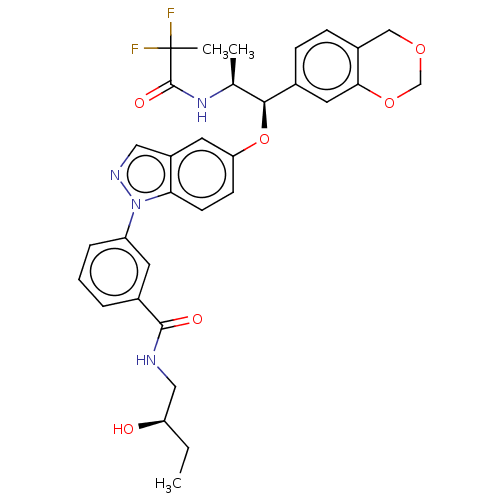 (US8916600, 11 | US9738632, Example 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141384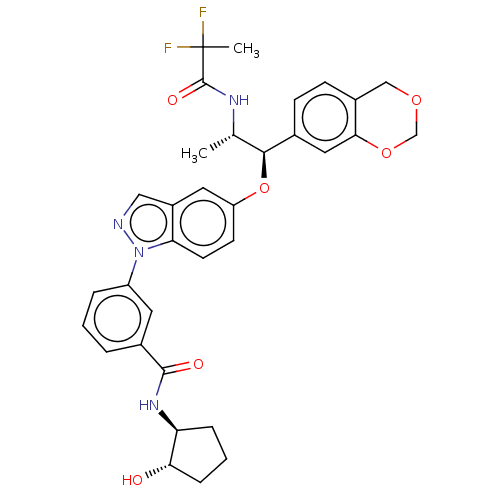 (US8916600, 18 | US9738632, Example 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.44 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141381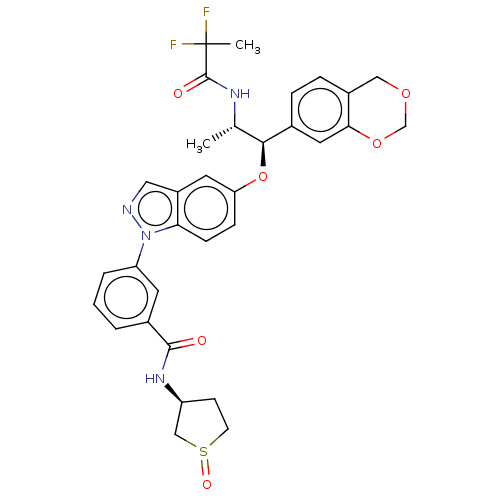 (US8916600, 15 | US8916600, 16 | US9738632, Example...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449109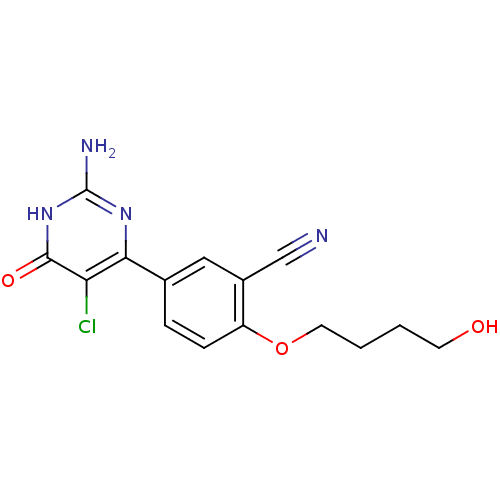 (CHEMBL3127133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50320491 (2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of rat xanthine oxidase | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141381 (US8916600, 15 | US8916600, 16 | US9738632, Example...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449105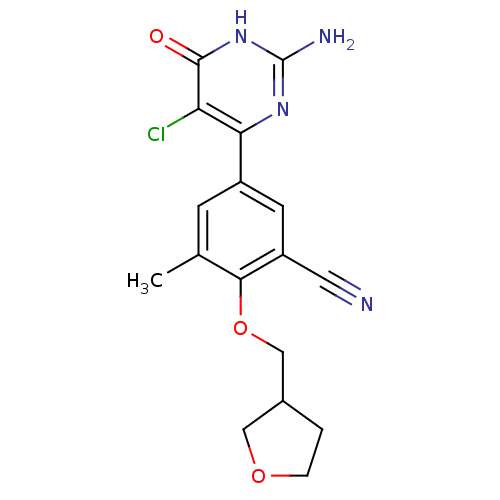 (CHEMBL3127115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449098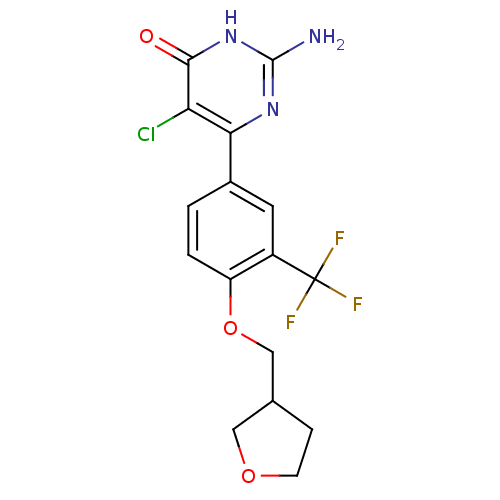 (CHEMBL3127116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive reversible inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 ... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50449104 (CHEMBL3127117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of rat xanthine oxidase | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50449107 (CHEMBL3127136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50402272 (CHEMBL2205509) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminally FLAG-tagged human xanthine oxidase (amino acid 1 to 1333) expressed in baculovirus system after 15 mins by spectrophotomet... | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332690 (US10196374, Example 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332691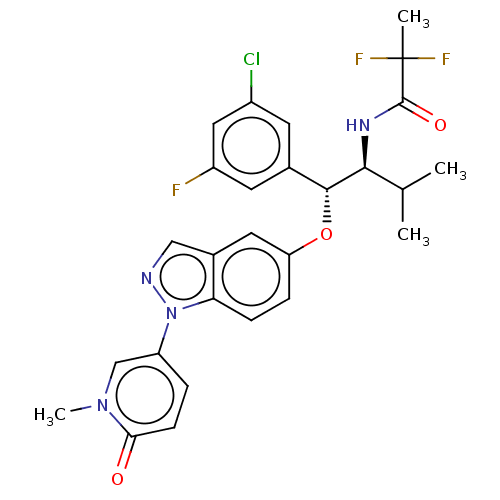 (US10196374, Example 19) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332669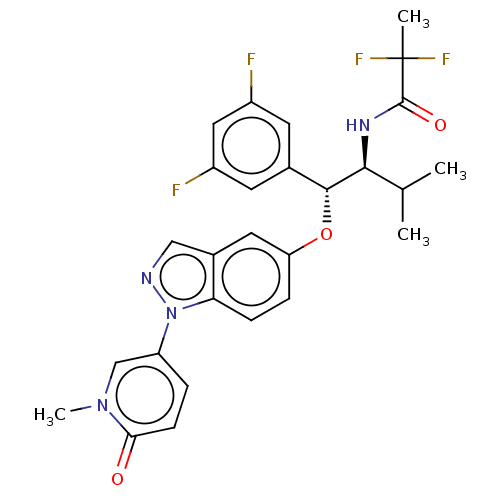 (US10196374, Example 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332689 (US10196374, Example 17) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332687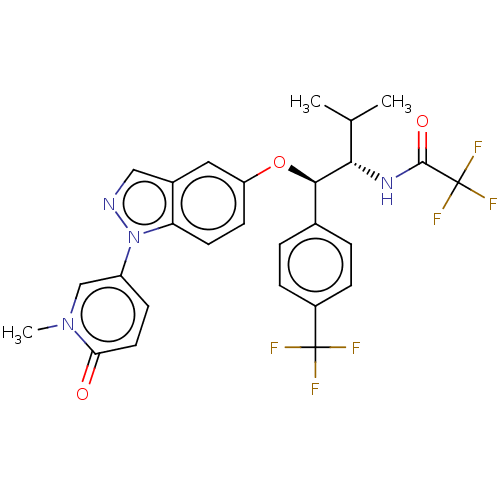 (US10196374, Example 15) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332678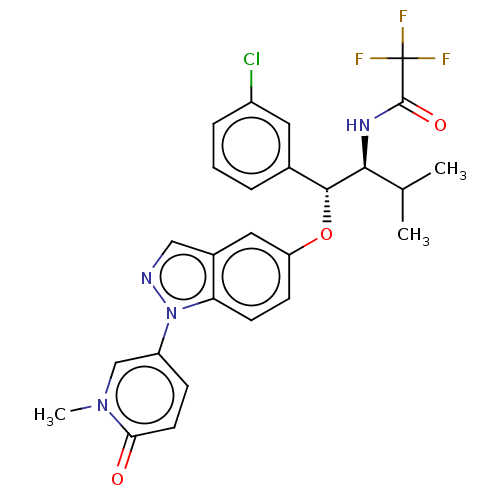 (US10196374, Example 6) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332693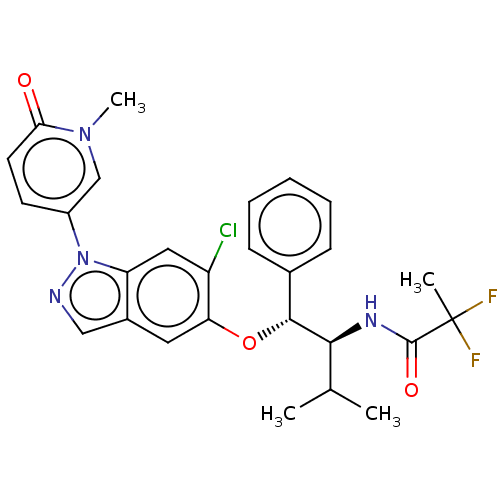 (US10196374, Example 21) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332696 (US10196374, Example 22) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332692 (US10196374, Example 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50449133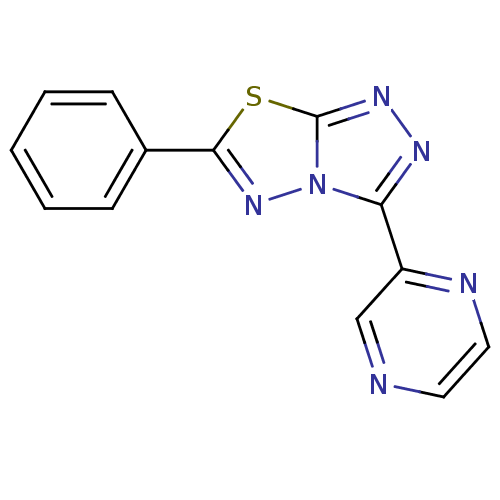 (CHEMBL3127139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase | Bioorg Med Chem Lett 24: 1315-21 (2014) Article DOI: 10.1016/j.bmcl.2014.01.050 BindingDB Entry DOI: 10.7270/Q2V69M3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332679 (US10196374, Example 7) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332684 (US10196374, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332682 (US10196374, Example 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A reporter cell line (ChagoK1 18:7:2 s4/GRE) was established by stable transfection of the human bronchogenic carcinoma celline, ChaGo K1 (ATCC: HTB ... | US Patent US10196374 (2019) BindingDB Entry DOI: 10.7270/Q2377BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |