 Found 859 hits with Last Name = 'mills' and Initial = 'm'
Found 859 hits with Last Name = 'mills' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50612500

(CHEMBL5287761)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Nc1ccc(Br)cc1-c1nnn[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337733
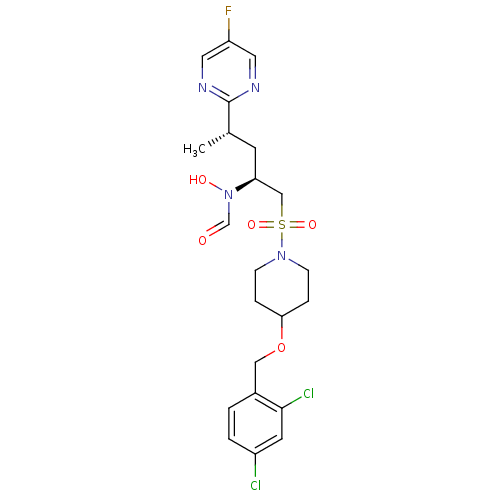
(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337721
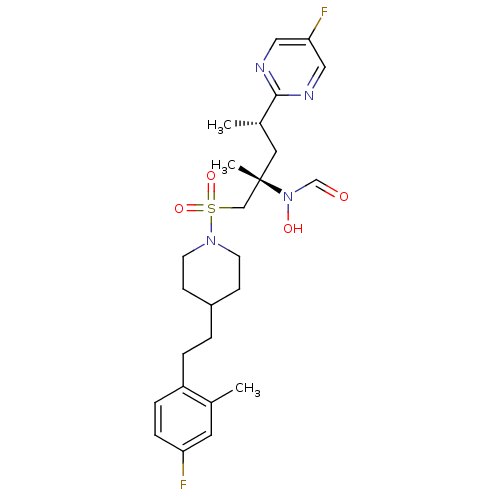
(CHEMBL1683454 | N-((2S,4S)-1-(4-(4-fluoro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34F2N4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337722
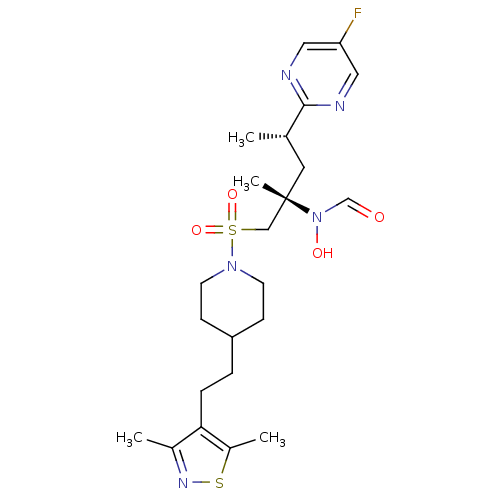
(CHEMBL1683460 | N-((2S,4S)-1-(4-(2-(3,5-dimethylis...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2c(C)nsc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H34FN5O4S2/c1-16(22-25-12-20(24)13-26-22)11-23(4,29(31)15-30)14-35(32,33)28-9-7-19(8-10-28)5-6-21-17(2)27-34-18(21)3/h12-13,15-16,19,31H,5-11,14H2,1-4H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50344193
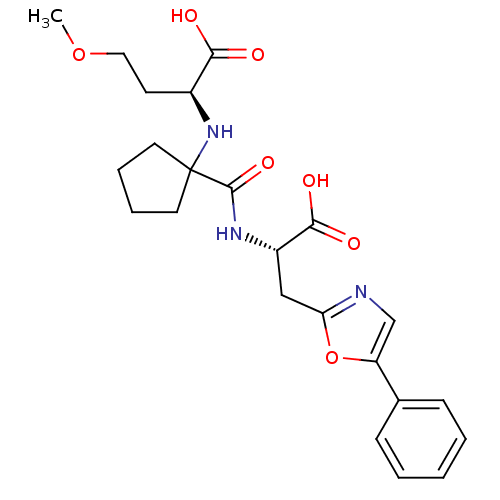
((S)-2-(1-((S)-1-carboxy-2-(5-phenyloxazol-2-yl)eth...)Show SMILES COCC[C@H](NC1(CCCC1)C(=O)N[C@@H](Cc1ncc(o1)-c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H29N3O7/c1-32-12-9-16(20(27)28)26-23(10-5-6-11-23)22(31)25-17(21(29)30)13-19-24-14-18(33-19)15-7-3-2-4-8-15/h2-4,7-8,14,16-17,26H,5-6,9-13H2,1H3,(H,25,31)(H,27,28)(H,29,30)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50344195
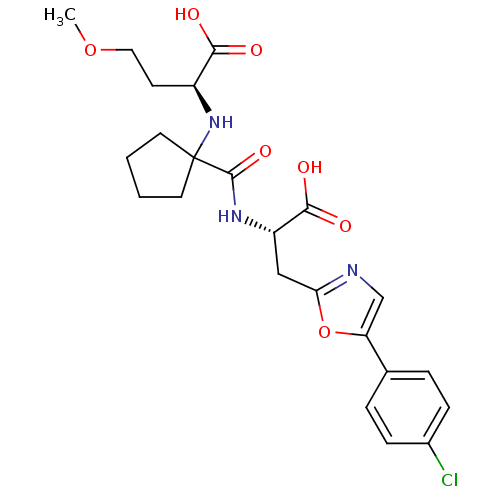
((S)-2-(1-((S)-1-carboxy-2-(5-(4-chlorophenyl)oxazo...)Show SMILES COCC[C@H](NC1(CCCC1)C(=O)N[C@@H](Cc1ncc(o1)-c1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H28ClN3O7/c1-33-11-8-16(20(28)29)27-23(9-2-3-10-23)22(32)26-17(21(30)31)12-19-25-13-18(34-19)14-4-6-15(24)7-5-14/h4-7,13,16-17,27H,2-3,8-12H2,1H3,(H,26,32)(H,28,29)(H,30,31)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337723
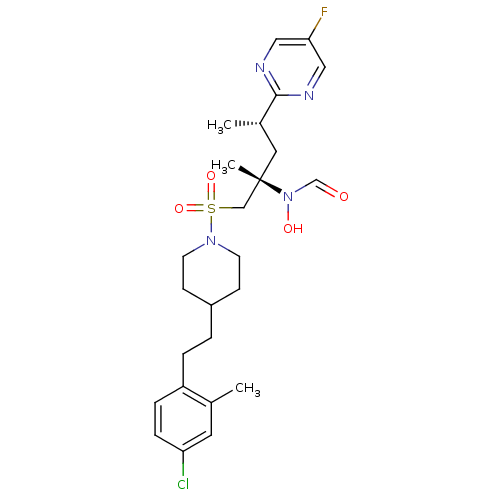
(CHEMBL1683450 | N-((2S,4S)-1-(4-(4-chloro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(Cl)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34ClFN4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337724
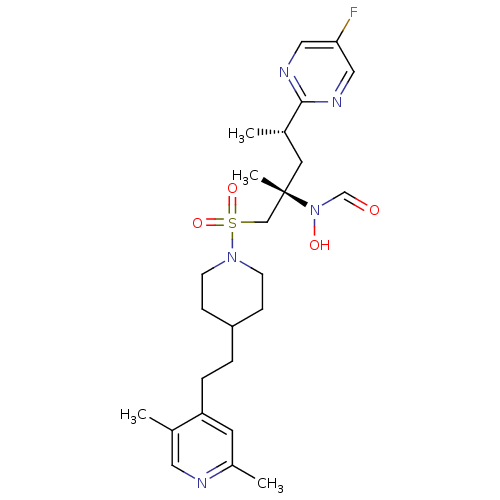
(CHEMBL1683458 | N-((2S,4S)-1-(4-(2-(2,5-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)ncc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18(24-28-14-23(26)15-29-24)12-25(4,31(33)17-32)16-36(34,35)30-9-7-21(8-10-30)5-6-22-11-20(3)27-13-19(22)2/h11,13-15,17-18,21,33H,5-10,12,16H2,1-4H3/t18-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337725

(CHEMBL1683449 | N-((2S,4S)-1-(4-(2-chloro-4-(trifl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2Cl)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H31ClF4N4O4S/c1-17(23-31-13-21(27)14-32-23)12-24(2,34(36)16-35)15-39(37,38)33-9-7-18(8-10-33)3-4-19-5-6-20(11-22(19)26)25(28,29)30/h5-6,11,13-14,16-18,36H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337726
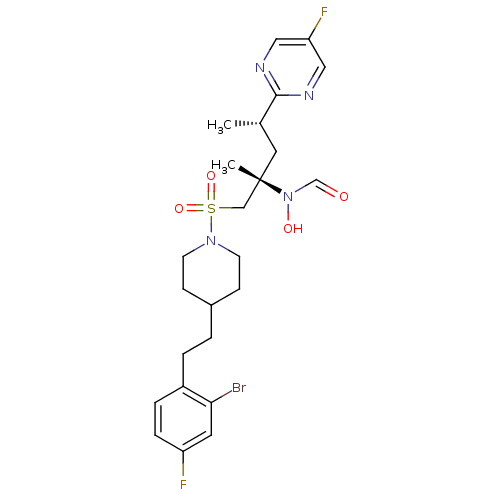
(CHEMBL1683451 | N-((2S,4S)-1-(4-(2-bromo-4-fluorop...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2Br)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31BrF2N4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-5-6-20(26)11-22(19)25/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337727
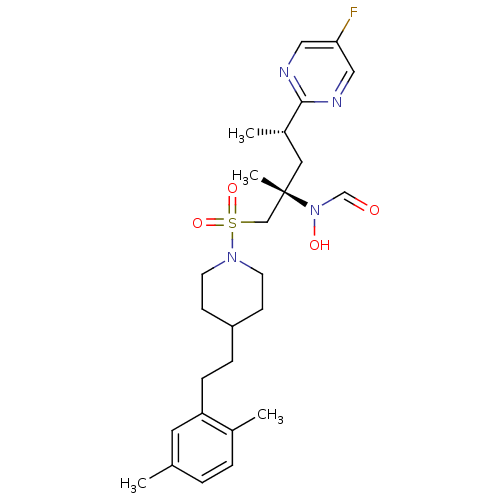
(CHEMBL1683457 | N-((2S,4S)-1-(4-(2,5-dimethylphene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)ccc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C26H37FN4O4S/c1-19-5-6-20(2)23(13-19)8-7-22-9-11-30(12-10-22)36(34,35)17-26(4,31(33)18-32)14-21(3)25-28-15-24(27)16-29-25/h5-6,13,15-16,18,21-22,33H,7-12,14,17H2,1-4H3/t21-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337736
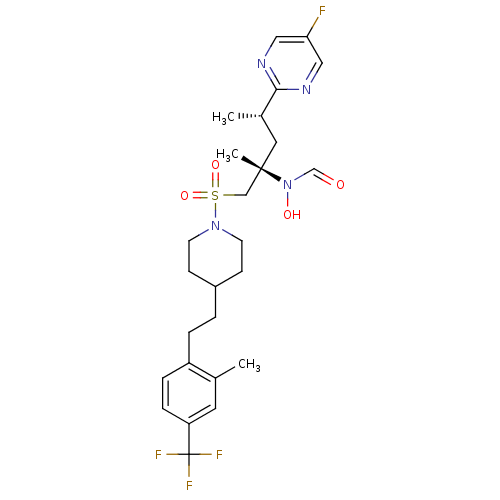
(CHEMBL1683455 | N-((2S,4S)-4-(5-fluoropyrimidin-2-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2C)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C26H34F4N4O4S/c1-18-12-22(26(28,29)30)7-6-21(18)5-4-20-8-10-33(11-9-20)39(37,38)16-25(3,34(36)17-35)13-19(2)24-31-14-23(27)15-32-24/h6-7,12,14-15,17,19-20,36H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337731
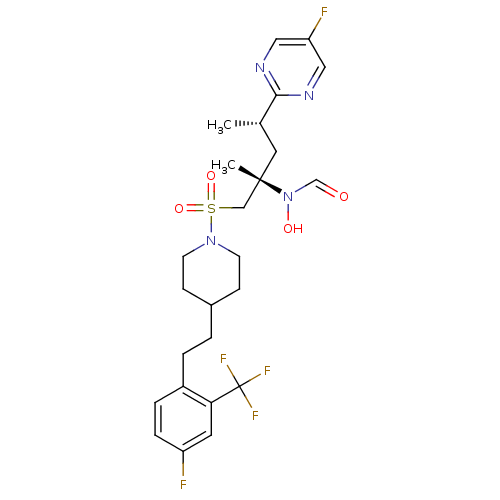
(CHEMBL1683453 | N-((2S,4S)-1-(4-(4-fluoro-2-(trifl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H31F5N4O4S/c1-17(23-31-13-21(27)14-32-23)12-24(2,34(36)16-35)15-39(37,38)33-9-7-18(8-10-33)3-4-19-5-6-20(26)11-22(19)25(28,29)30/h5-6,11,13-14,16-18,36H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337734
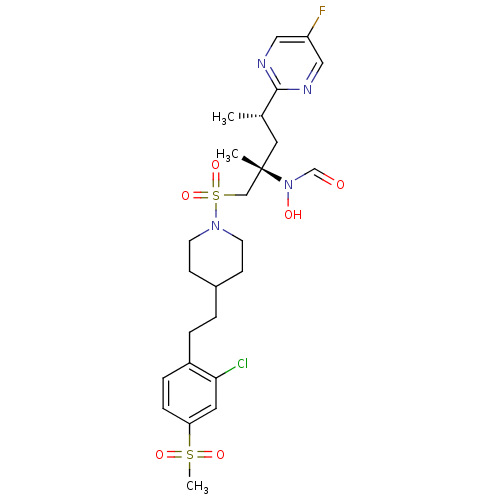
(CHEMBL1683464 | N-((2S,4S)-1-(4-(2-chloro-4-(methy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2Cl)S(C)(=O)=O)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34ClFN4O6S2/c1-18(24-28-14-21(27)15-29-24)13-25(2,31(33)17-32)16-39(36,37)30-10-8-19(9-11-30)4-5-20-6-7-22(12-23(20)26)38(3,34)35/h6-7,12,14-15,17-19,33H,4-5,8-11,13,16H2,1-3H3/t18-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50344191
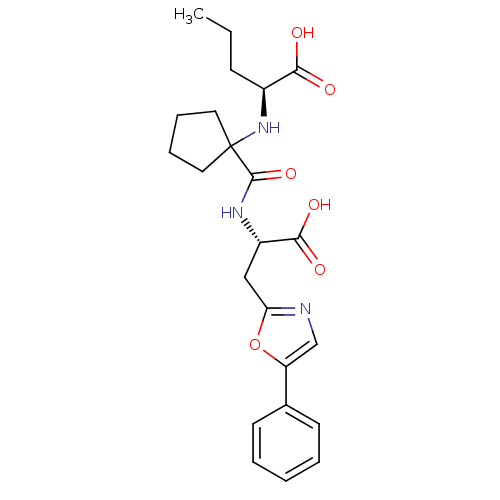
((S)-2-(1-((S)-1-carboxy-2-(5-phenyloxazol-2-yl)eth...)Show SMILES CCC[C@H](NC1(CCCC1)C(=O)N[C@@H](Cc1ncc(o1)-c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H29N3O6/c1-2-8-16(20(27)28)26-23(11-6-7-12-23)22(31)25-17(21(29)30)13-19-24-14-18(32-19)15-9-4-3-5-10-15/h3-5,9-10,14,16-17,26H,2,6-8,11-13H2,1H3,(H,25,31)(H,27,28)(H,29,30)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50085452
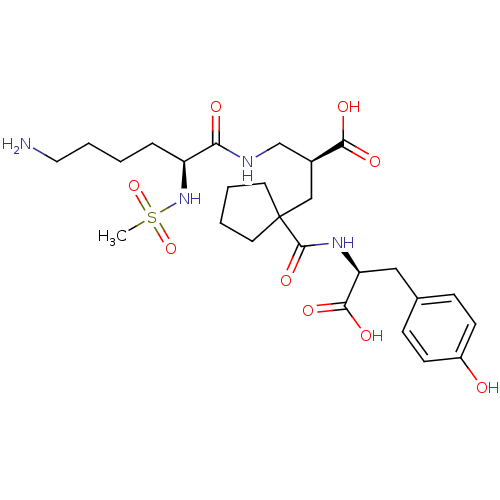
((S)-2-[((S)-6-Amino-2-methanesulfonylamino-hexanoy...)Show SMILES CS(=O)(=O)N[C@@H](CCCCN)C(=O)NC[C@H](CC1(CCCC1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)C(O)=O Show InChI InChI=1S/C26H40N4O9S/c1-40(38,39)30-20(6-2-5-13-27)22(32)28-16-18(23(33)34)15-26(11-3-4-12-26)25(37)29-21(24(35)36)14-17-7-9-19(31)10-8-17/h7-10,18,20-21,30-31H,2-6,11-16,27H2,1H3,(H,28,32)(H,29,37)(H,33,34)(H,35,36)/t18-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337739

(CHEMBL1683461 | N-((2S,4S)-1-(4-(2-(4,6-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cnc(C)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18-11-20(3)27-13-22(18)6-5-21-7-9-30(10-8-21)36(34,35)16-25(4,31(33)17-32)12-19(2)24-28-14-23(26)15-29-24/h11,13-15,17,19,21,33H,5-10,12,16H2,1-4H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337735
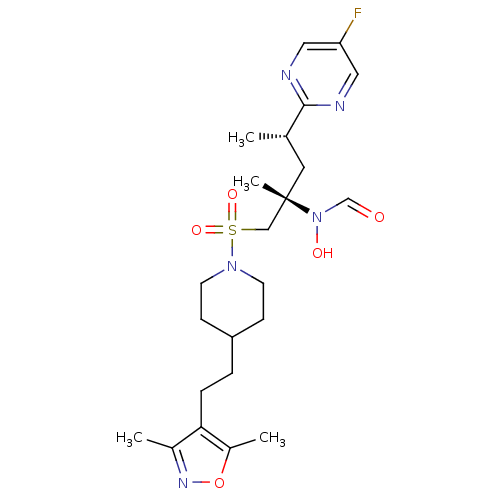
(CHEMBL1615187 | N-[(2S,4S)-1-({4-[2-(3,5-dimethyl-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2c(C)noc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H34FN5O5S/c1-16(22-25-12-20(24)13-26-22)11-23(4,29(31)15-30)14-35(32,33)28-9-7-19(8-10-28)5-6-21-17(2)27-34-18(21)3/h12-13,15-16,19,31H,5-11,14H2,1-4H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337743
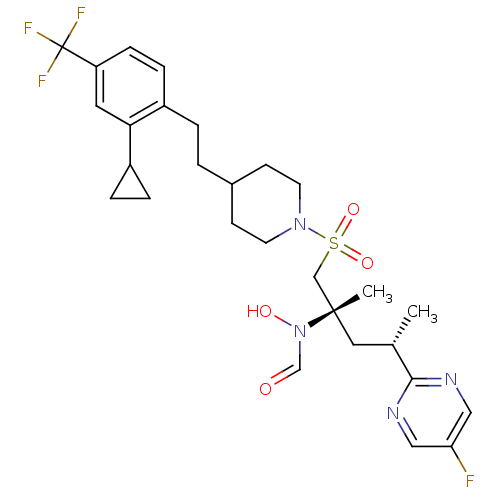
(CHEMBL1683456 | N-((2S,4S)-1-(4-(2-cyclopropyl-4-(...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2C2CC2)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C28H36F4N4O4S/c1-19(26-33-15-24(29)16-34-26)14-27(2,36(38)18-37)17-41(39,40)35-11-9-20(10-12-35)3-4-21-7-8-23(28(30,31)32)13-25(21)22-5-6-22/h7-8,13,15-16,18-20,22,38H,3-6,9-12,14,17H2,1-2H3/t19-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337733

(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337744
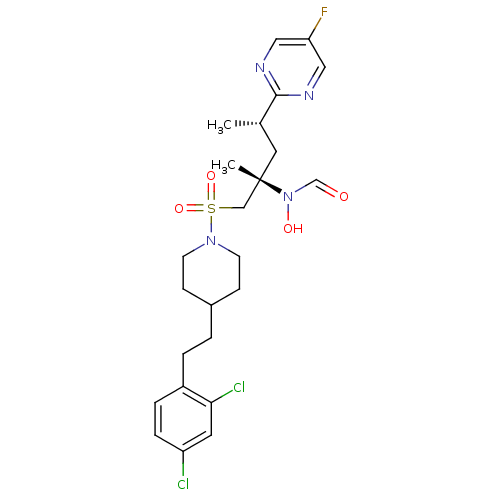
(CHEMBL1683447 | N-((2S,4S)-1-(4-(2,4-dichlorophene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(Cl)cc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31Cl2FN4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-5-6-20(25)11-22(19)26/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337730
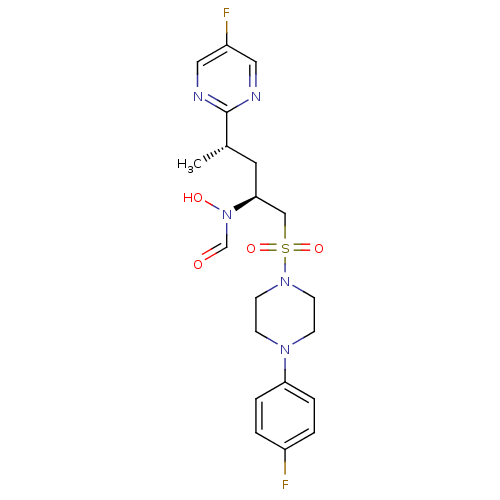
(CHEMBL1683443 | N-((2S,4S)-1-(4-(4-fluorophenyl)pi...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccc(F)cc1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N5O4S/c1-15(20-23-11-17(22)12-24-20)10-19(27(29)14-28)13-32(30,31)26-8-6-25(7-9-26)18-4-2-16(21)3-5-18/h2-5,11-12,14-15,19,29H,6-10,13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337730

(CHEMBL1683443 | N-((2S,4S)-1-(4-(4-fluorophenyl)pi...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccc(F)cc1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N5O4S/c1-15(20-23-11-17(22)12-24-20)10-19(27(29)14-28)13-32(30,31)26-8-6-25(7-9-26)18-4-2-16(21)3-5-18/h2-5,11-12,14-15,19,29H,6-10,13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Natriuretic peptides A
(Homo sapiens (Human)) | BDBM50344187
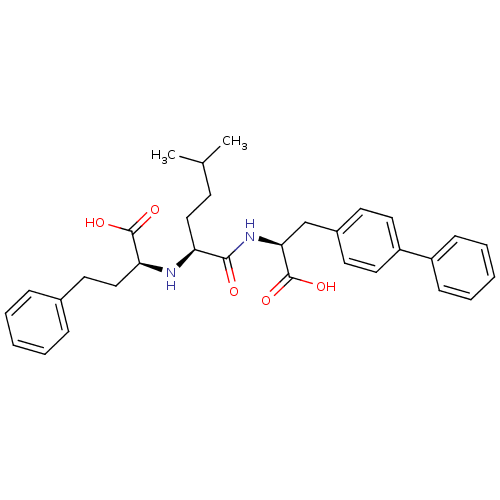
((S)-2-((S)-1-((S)-2-(biphenyl-4-yl)-1-carboxyethyl...)Show SMILES CC(C)CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C32H38N2O5/c1-22(2)13-19-27(33-28(31(36)37)20-16-23-9-5-3-6-10-23)30(35)34-29(32(38)39)21-24-14-17-26(18-15-24)25-11-7-4-8-12-25/h3-12,14-15,17-18,22,27-29,33H,13,16,19-21H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)/t27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of atrial natriuretic peptide |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337737
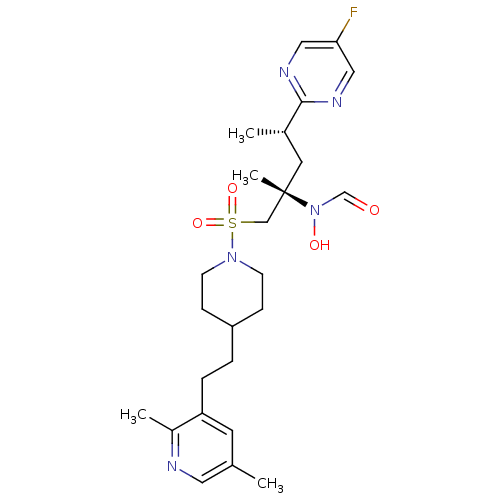
(CHEMBL1683459 | N-((2S,4S)-1-(4-(2-(2,5-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)cnc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18-11-22(20(3)27-13-18)6-5-21-7-9-30(10-8-21)36(34,35)16-25(4,31(33)17-32)12-19(2)24-28-14-23(26)15-29-24/h11,13-15,17,19,21,33H,5-10,12,16H2,1-4H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50344197
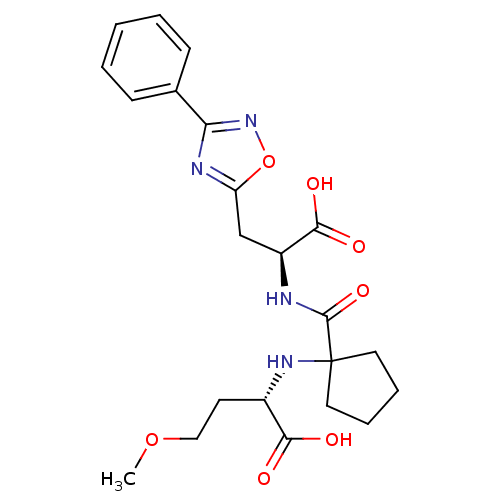
((S)-2-(1-((S)-1-carboxy-2-(3-phenyl-1,2,4-oxadiazo...)Show SMILES COCC[C@H](NC1(CCCC1)C(=O)N[C@@H](Cc1nc(no1)-c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H28N4O7/c1-32-12-9-15(19(27)28)25-22(10-5-6-11-22)21(31)23-16(20(29)30)13-17-24-18(26-33-17)14-7-3-2-4-8-14/h2-4,7-8,15-16,25H,5-6,9-13H2,1H3,(H,23,31)(H,27,28)(H,29,30)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626369

((S)—N-(1-(1-(4-fluorophenyl)-6-methyl-1H-inda...)Show SMILES CCCn1cc(cn1)S(=O)(=O)N[C@H]1CCN(C1)c1cc2cnn(-c3ccc(F)cc3)c2cc1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50437788
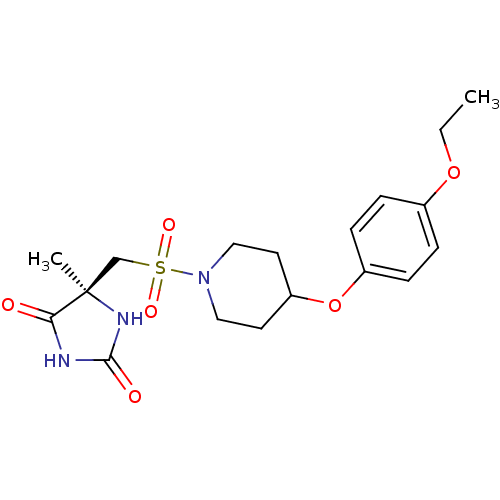
(CHEMBL2409699)Show SMILES CCOc1ccc(OC2CCN(CC2)S(=O)(=O)C[C@@]2(C)NC(=O)NC2=O)cc1 |r| Show InChI InChI=1S/C18H25N3O6S/c1-3-26-13-4-6-14(7-5-13)27-15-8-10-21(11-9-15)28(24,25)12-18(2)16(22)19-17(23)20-18/h4-7,15H,3,8-12H2,1-2H3,(H2,19,20,22,23)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 4705-12 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.089
BindingDB Entry DOI: 10.7270/Q2WS8VNH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337740
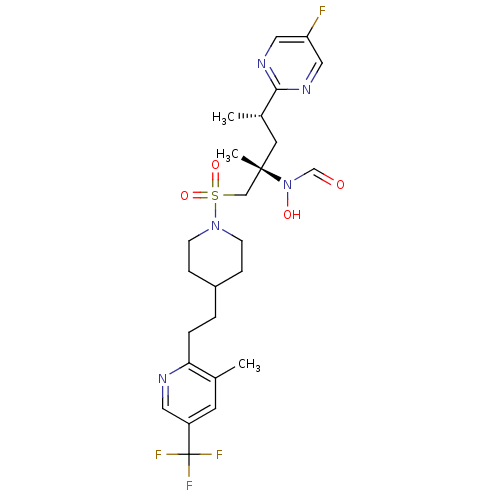
(CHEMBL1683463 | N-((2S,4S)-4-(5-fluoropyrimidin-2-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ncc(cc2C)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H33F4N5O4S/c1-17-10-20(25(27,28)29)12-30-22(17)5-4-19-6-8-33(9-7-19)39(37,38)15-24(3,34(36)16-35)11-18(2)23-31-13-21(26)14-32-23/h10,12-14,16,18-19,36H,4-9,11,15H2,1-3H3/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337732
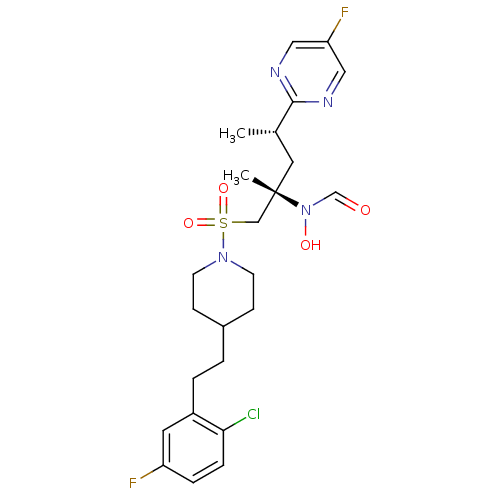
(CHEMBL1683452 | N-((2S,4S)-1-(4-(2-chloro-5-fluoro...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(F)ccc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31ClF2N4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-11-20(26)5-6-22(19)25/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626566
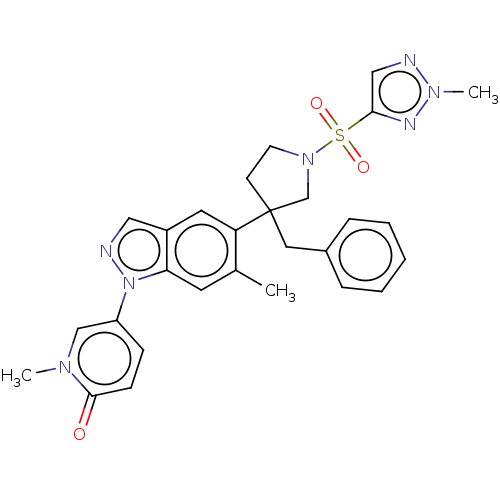
(US11787780, Example 261 | US11787780, Example 266 ...)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cnn(C)n1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626603

(US11787780, Example 311)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cnccc1Cl)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626597

(US11787780, Example 305)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1ccc(Cl)cc1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626472

(US11787780, Example 120)Show SMILES CCCn1cc(cn1)S(=O)(=O)NC1(C)CCN(C1)c1cc2cnn(-c3ccc(F)cc3)c2cc1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626465

(US11787780, Example 111)Show SMILES Cc1cc2n(ncc2cc1N1CC[C@@H](C1)NS(=O)(=O)c1cnn(C)c1)-c1ccc(F)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626461

(US11787780, Example 107)Show SMILES CCCn1cc(cn1)S(=O)(=O)NC1CCN(CC1)c1cc2cnn(-c3ccc(F)cc3)c2cc1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50437787

(CHEMBL2409700)Show SMILES C[C@]1(CS(=O)(=O)N2CCC(CC2)Oc2ccc(OC(F)(F)F)cc2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H20F3N3O6S/c1-16(14(24)21-15(25)22-16)10-30(26,27)23-8-6-12(7-9-23)28-11-2-4-13(5-3-11)29-17(18,19)20/h2-5,12H,6-10H2,1H3,(H2,21,22,24,25)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 4705-12 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.089
BindingDB Entry DOI: 10.7270/Q2WS8VNH |
More data for this
Ligand-Target Pair | |
Natriuretic peptides A
(Homo sapiens (Human)) | BDBM50085452

((S)-2-[((S)-6-Amino-2-methanesulfonylamino-hexanoy...)Show SMILES CS(=O)(=O)N[C@@H](CCCCN)C(=O)NC[C@H](CC1(CCCC1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)C(O)=O Show InChI InChI=1S/C26H40N4O9S/c1-40(38,39)30-20(6-2-5-13-27)22(32)28-16-18(23(33)34)15-26(11-3-4-12-26)25(37)29-21(24(35)36)14-17-7-9-19(31)10-8-17/h7-10,18,20-21,30-31H,2-6,11-16,27H2,1H3,(H,28,32)(H,29,37)(H,33,34)(H,35,36)/t18-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of atrial natriuretic peptide |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337729
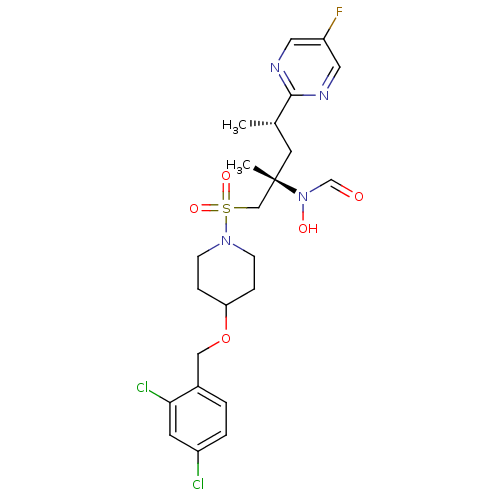
(CHEMBL1615186 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H29Cl2FN4O5S/c1-16(22-27-11-19(26)12-28-22)10-23(2,30(32)15-31)14-36(33,34)29-7-5-20(6-8-29)35-13-17-3-4-18(24)9-21(17)25/h3-4,9,11-12,15-16,20,32H,5-8,10,13-14H2,1-2H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626590

(US11787780, Example 298)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1ccc(F)cc1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337745
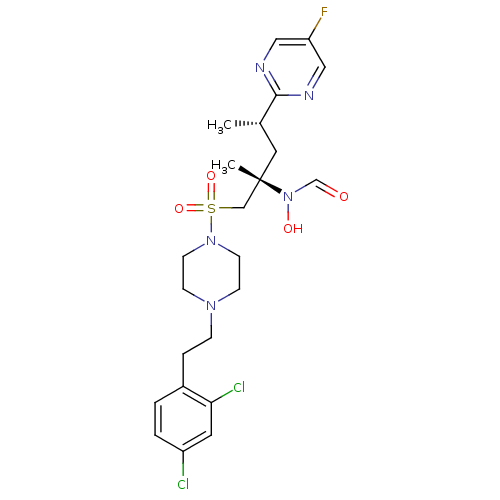
(CHEMBL1683446 | N-((2S,4S)-1-(4-(2,4-dichlorophene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCN(CCc2ccc(Cl)cc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H30Cl2FN5O4S/c1-17(22-27-13-20(26)14-28-22)12-23(2,31(33)16-32)15-36(34,35)30-9-7-29(8-10-30)6-5-18-3-4-19(24)11-21(18)25/h3-4,11,13-14,16-17,33H,5-10,12,15H2,1-2H3/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626483

(US11787780, Example 131)Show SMILES Cc1cc2n(ncc2cc1N1CCC(C)(CC1)NS(=O)(=O)c1cnn(C)c1)-c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626586

(US11787780, Example 294)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1ccccc1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626554

(US11787780, Example 249 | US11787780, Example 250)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cnn(C)n1)-c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337741
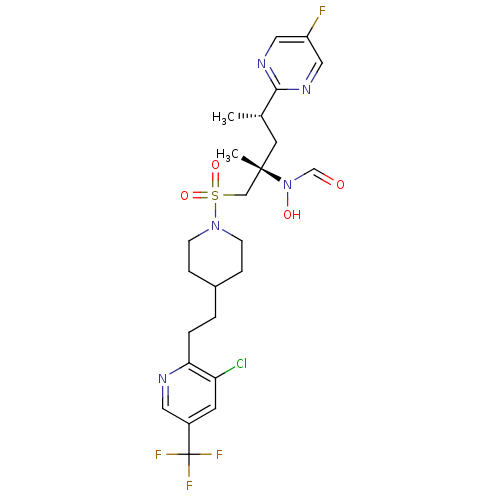
(CHEMBL1683462 | N-((2S,4S)-1-(4-(2-(3-chloro-5-(tr...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ncc(cc2Cl)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H30ClF4N5O4S/c1-16(22-31-12-19(26)13-32-22)10-23(2,34(36)15-35)14-39(37,38)33-7-5-17(6-8-33)3-4-21-20(25)9-18(11-30-21)24(27,28)29/h9,11-13,15-17,36H,3-8,10,14H2,1-2H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626595

(US11787780, Example 303)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cccc(n1)C(F)(F)F)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626592

(US11787780, Example 300)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cncc(F)c1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626566

(US11787780, Example 261 | US11787780, Example 266 ...)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cnn(C)n1)-c1ccc(=O)n(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50344192
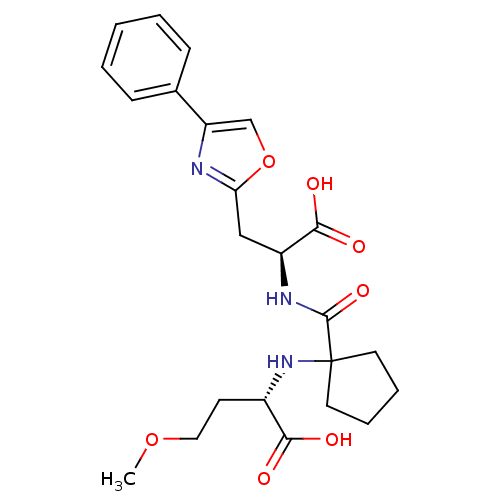
((S)-2-(1-((S)-1-carboxy-2-(4-phenyloxazol-2-yl)eth...)Show SMILES COCC[C@H](NC1(CCCC1)C(=O)N[C@@H](Cc1nc(co1)-c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H29N3O7/c1-32-12-9-16(20(27)28)26-23(10-5-6-11-23)22(31)25-17(21(29)30)13-19-24-18(14-33-19)15-7-3-2-4-8-15/h2-4,7-8,14,16-17,26H,5-6,9-13H2,1H3,(H,25,31)(H,27,28)(H,29,30)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 21: 3404-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.109
BindingDB Entry DOI: 10.7270/Q2PG1S2F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM626554

(US11787780, Example 249 | US11787780, Example 250)Show SMILES Cc1cc2n(ncc2cc1C1(Cc2ccccc2)CCN(C1)S(=O)(=O)c1cnn(C)n1)-c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data