

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50430584 (CHEMBL2337806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50469035 (CHEMBL4282693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586357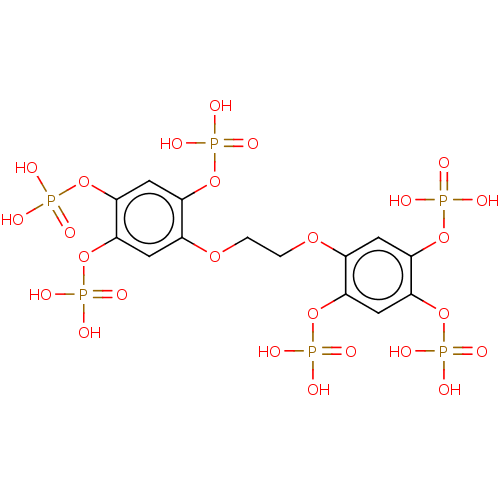 (CHEMBL5080660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040619 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3-nitro-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040622 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(9,10-diox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholine esterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040626 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(5-nitro-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040657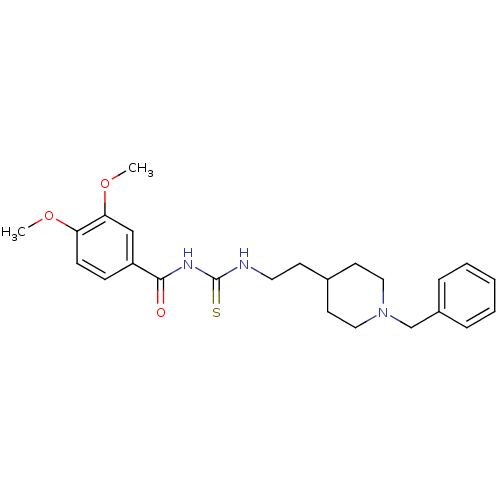 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3,4-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032395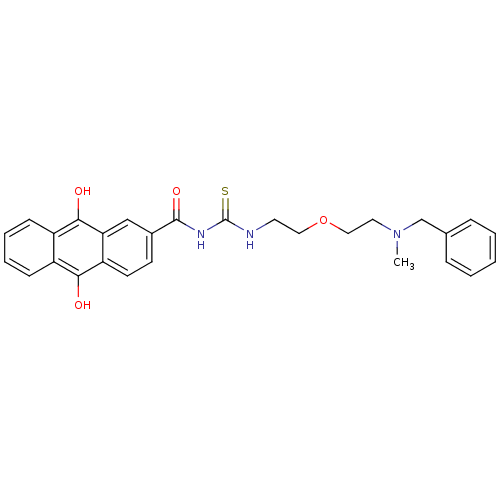 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(9,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040643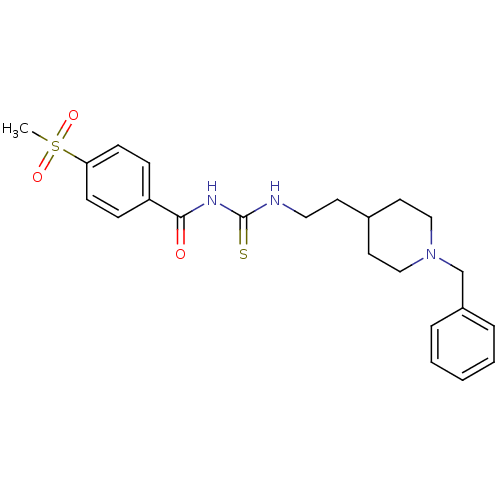 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(4-methane...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040618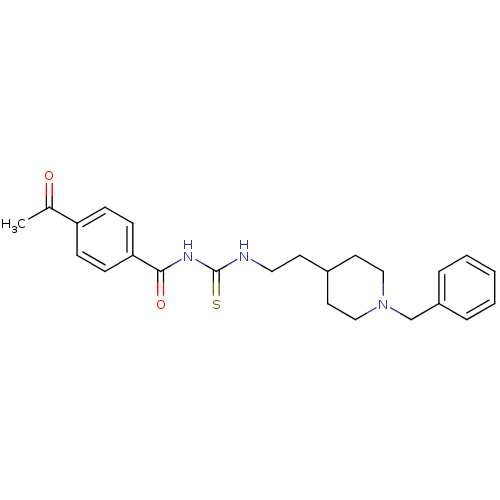 (1-(4-Acetyl-benzoyl)-3-[2-(1-benzyl-piperidin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040646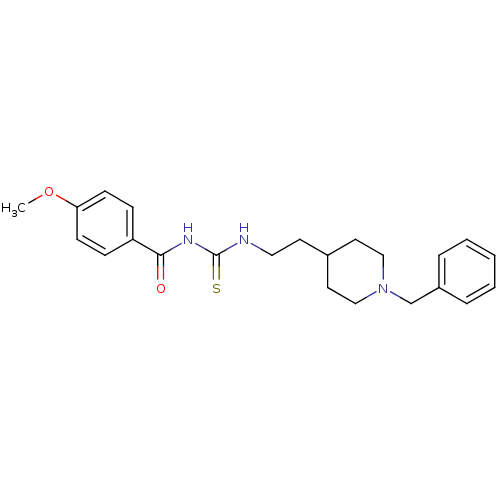 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(4-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040637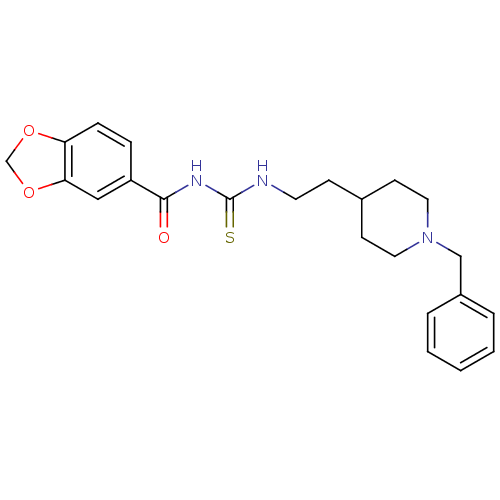 (1-(Benzo[1,3]dioxole-5-carbonyl)-3-[2-(1-benzyl-pi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040632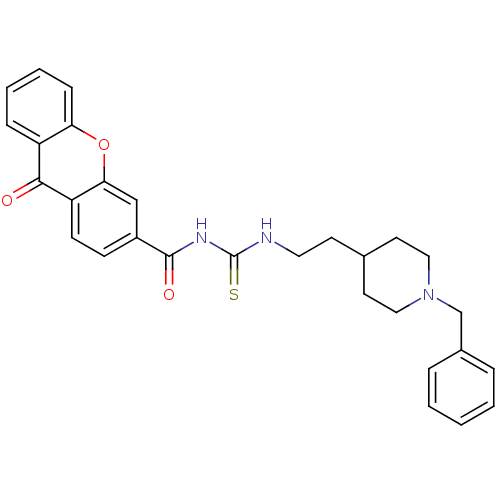 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(9-oxo-9H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032384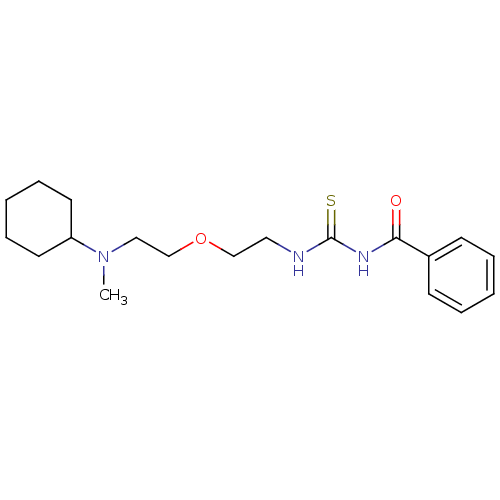 (1-Benzoyl-3-{2-[2-(cyclohexyl-methyl-amino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032391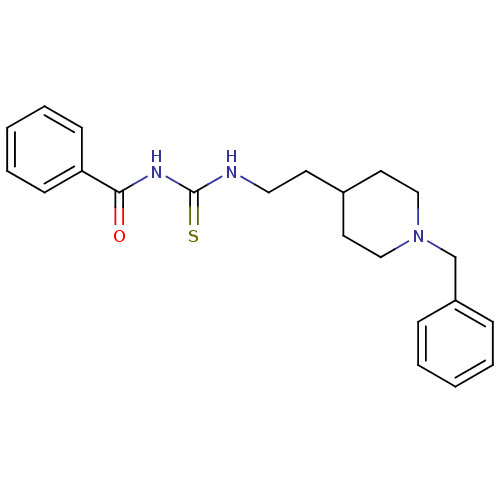 (1-Benzoyl-3-[2-(1-benzyl-piperidin-4-yl)-ethyl]-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032391 (1-Benzoyl-3-[2-(1-benzyl-piperidin-4-yl)-ethyl]-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040655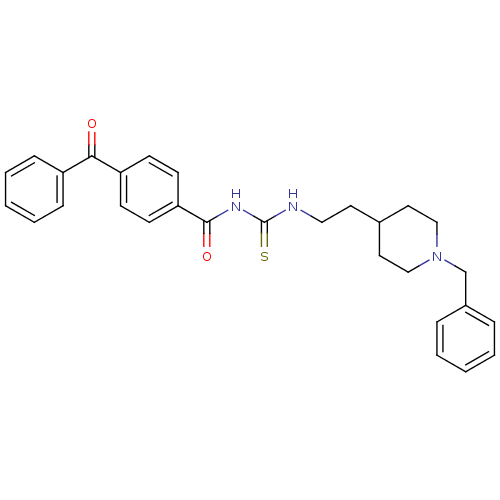 (1-(4-Benzoyl-benzoyl)-3-[2-(1-benzyl-piperidin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040631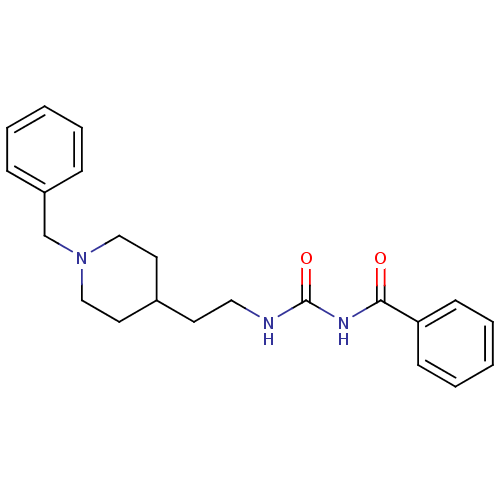 (1-Benzoyl-3-[2-(1-benzyl-piperidin-4-yl)-ethyl]-ur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032390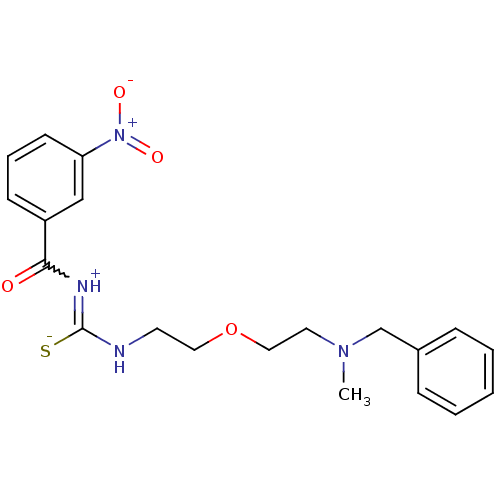 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040620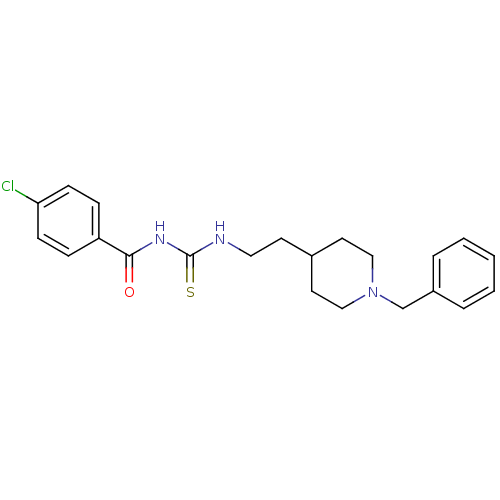 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(4-chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040633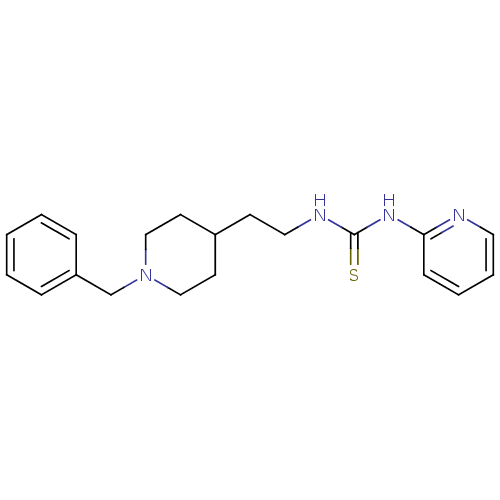 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-pyridin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586355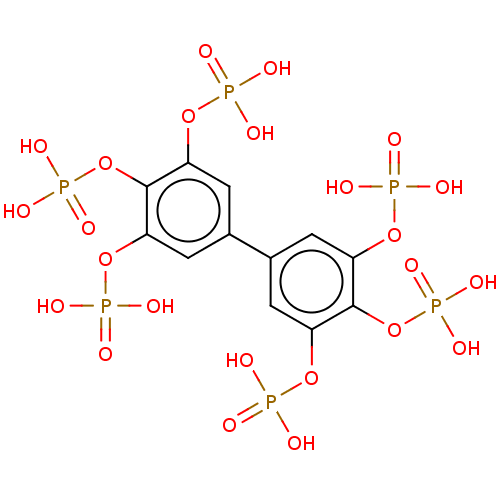 (CHEMBL5087243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032398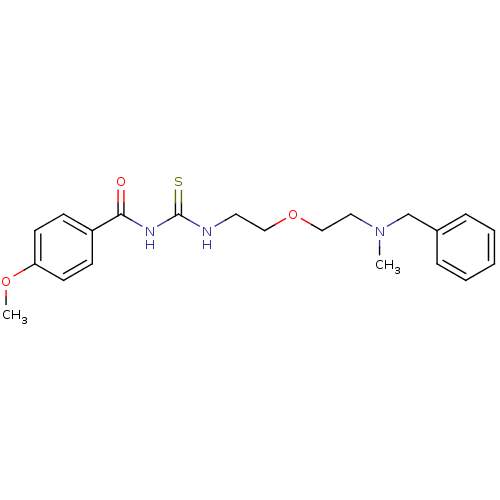 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586354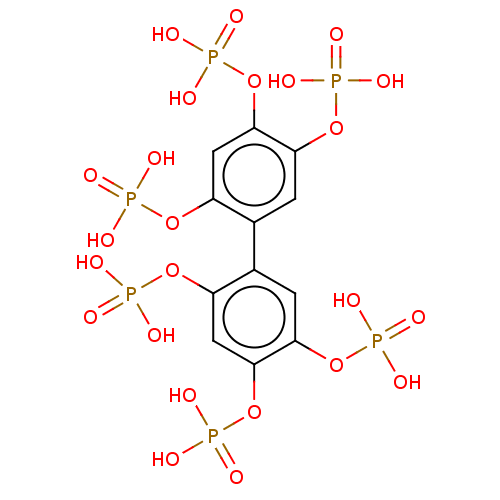 (CHEMBL5078323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inactive phospholipase C-like protein 1 (Rattus norvegicus) | BDBM50287772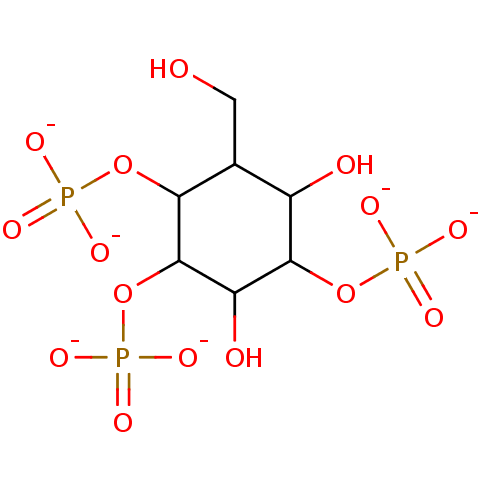 (Phosphoric acid mono-((3S,5R,6R)-3,5-dihydroxy-2-h...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [3H]Ins(1,4,5)P3 binding to Inositol 1,4,5-trisphosphate receptor in rat cerebellar membranes. | Bioorg Med Chem Lett 6: 2197-2200 (1996) Article DOI: 10.1016/0960-894X(96)00399-X BindingDB Entry DOI: 10.7270/Q25B030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586353 (CHEMBL5092991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032394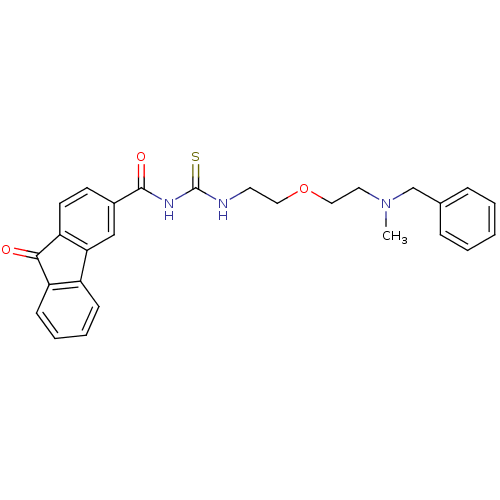 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(9-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032393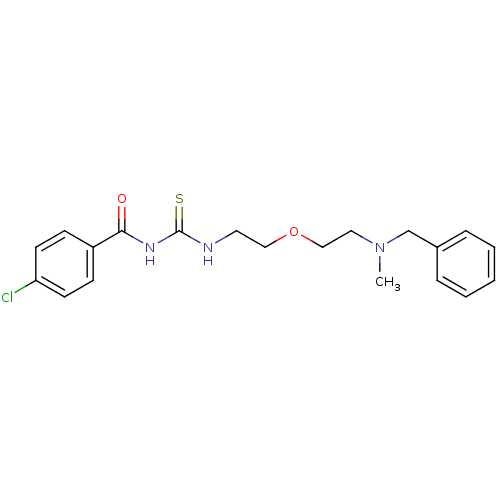 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586357 (CHEMBL5080660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040629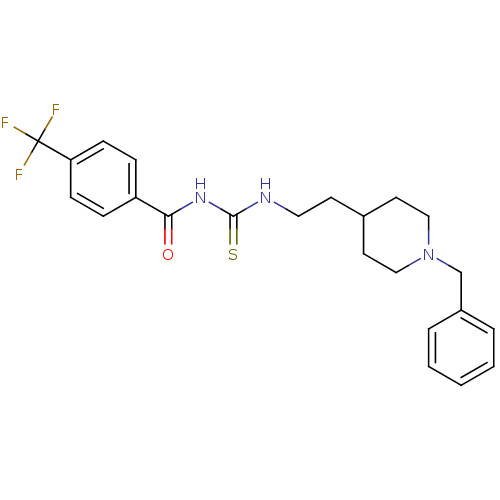 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(4-trifluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inactive phospholipase C-like protein 1 (Rattus norvegicus) | BDBM50075183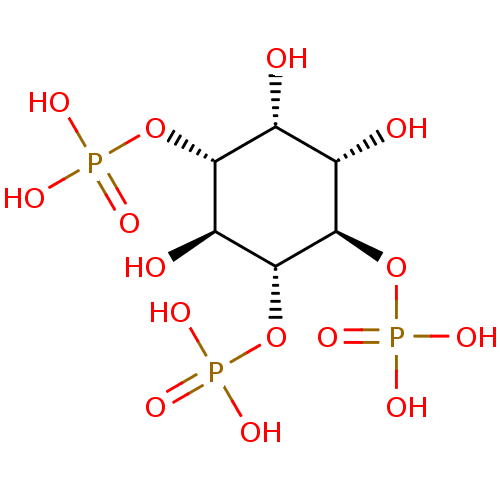 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [3H]Ins(1,4,5)P3 binding to Inositol 1,4,5-trisphosphate receptor in rat cerebellar membranes. | Bioorg Med Chem Lett 6: 2197-2200 (1996) Article DOI: 10.1016/0960-894X(96)00399-X BindingDB Entry DOI: 10.7270/Q25B030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040656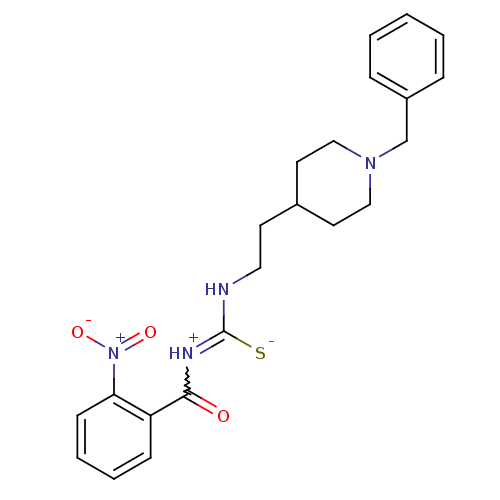 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(2-nitro-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inactive phospholipase C-like protein 1 (Rattus norvegicus) | BDBM50075183 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of specific [3H]Ins(1,4,5)P3 binding to Inositol 1,4,5-trisphosphate receptor in rat cerebellar membranes. | Bioorg Med Chem Lett 6: 2197-2200 (1996) Article DOI: 10.1016/0960-894X(96)00399-X BindingDB Entry DOI: 10.7270/Q25B030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032382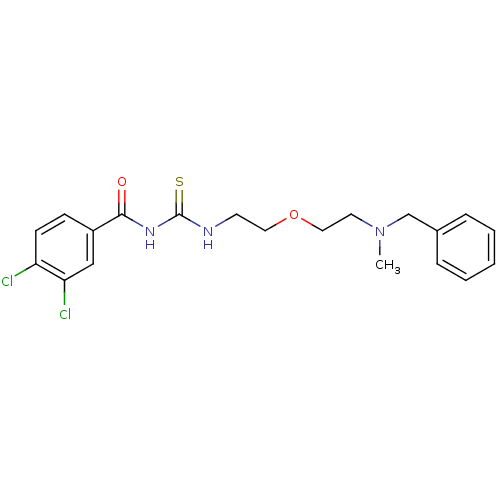 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA damage-binding protein 1/Protein cereblon (Homo sapiens (Human)) | BDBM65497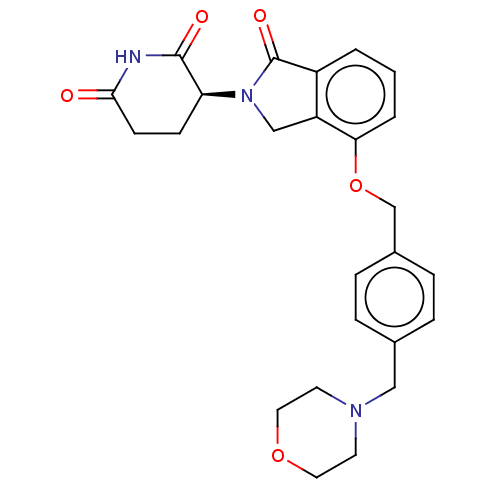 (CC-220 (Compound 6) | US9694015, 6.4S) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Celgene Corporation | Assay Description The 6XHis-tagged full length human CRBN bound to full length human DDB1 used in the assay was purified as described elsewhere with the exception that... | J Med Chem 61: 535-542 (2017) Article DOI: 10.1021/acs.jmedchem.6b01921 BindingDB Entry DOI: 10.7270/Q2PC30HG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586356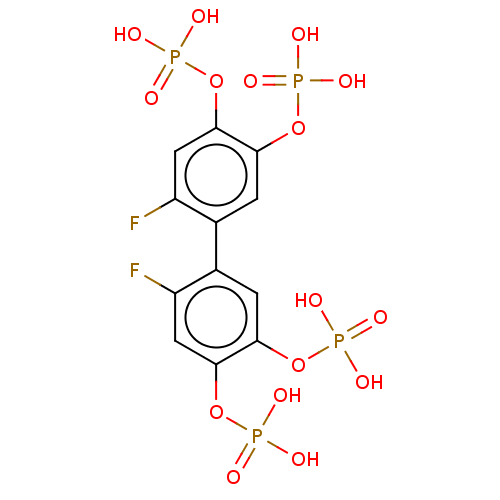 (CHEMBL5081948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032386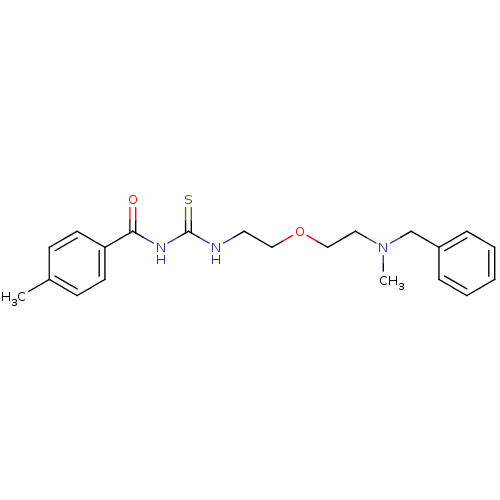 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040642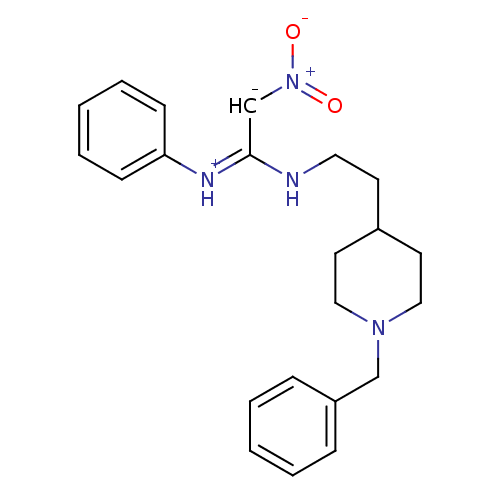 (CHEMBL161779 | N-benzyl-N''-[2-(1phenyl-4-piperidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholine esterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040636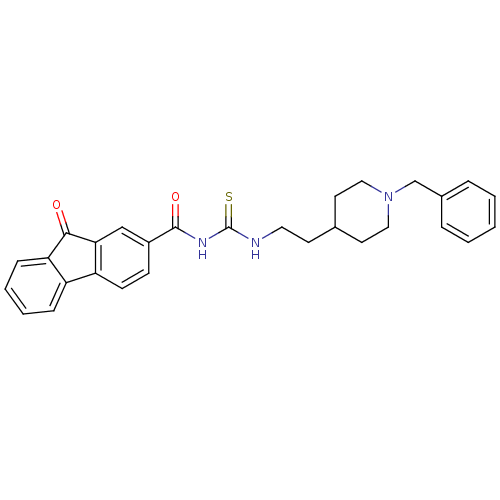 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(9-oxo-9H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040624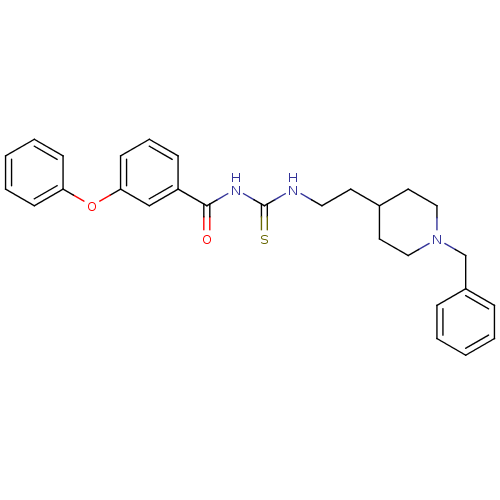 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032397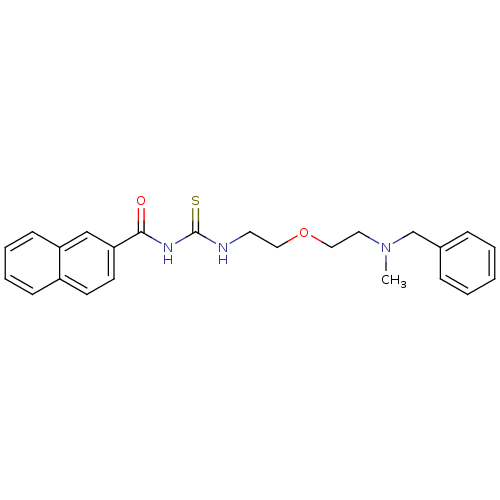 (1-{2-[2-(Benzyl-methyl-amino)-ethoxy]-ethyl}-3-(na...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50032381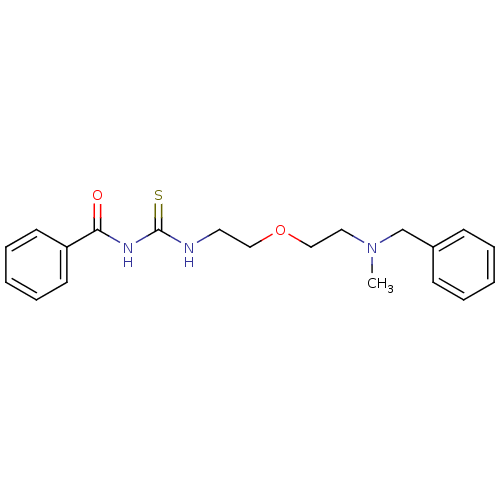 (1-Benzoyl-3-{2-[2-(benzyl-methyl-amino)-ethoxy]-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040650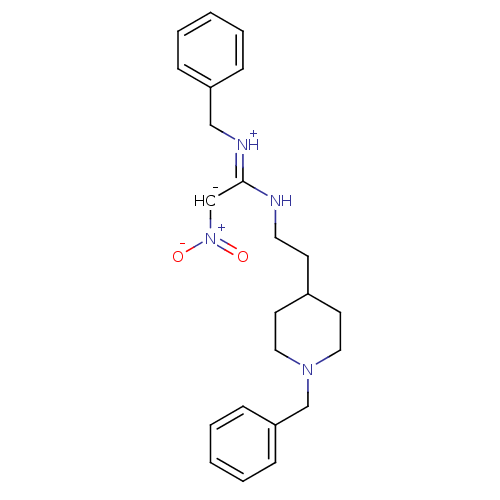 (CHEMBL162750 | N-benzyl-N''-[2-(1-benzyl-4-piperid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholine esterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040641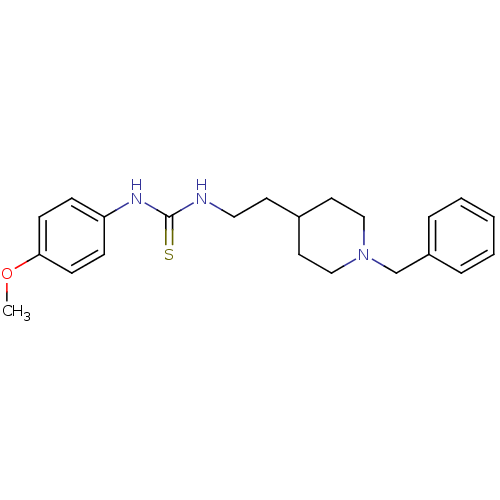 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(4-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholine esterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040627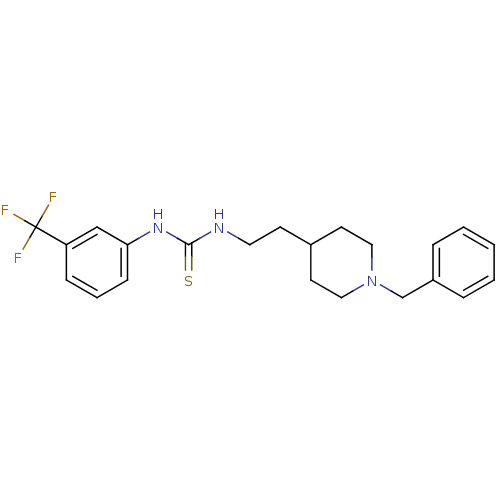 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3-trifluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 213 total ) | Next | Last >> |