

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317007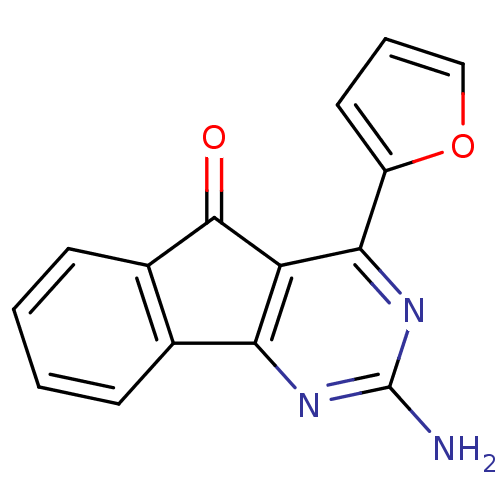 (2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adenosine receptor A2a by cAMP assay | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077798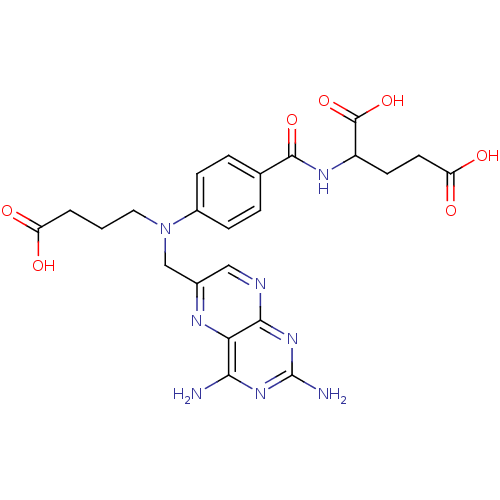 (2-{4-[(3-Carboxy-propyl)-(2,4-diamino-pteridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077796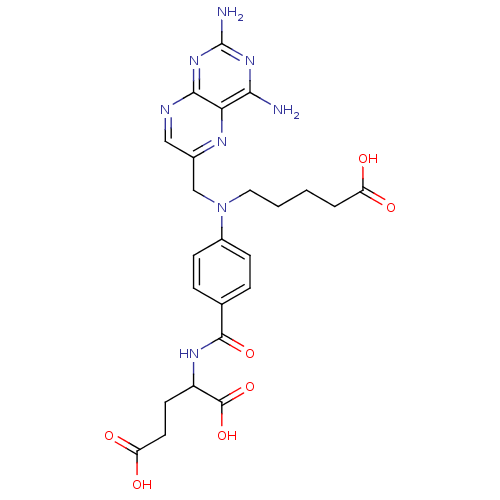 (2-{4-[(4-Carboxy-butyl)-(2,4-diamino-pteridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase in humans | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077799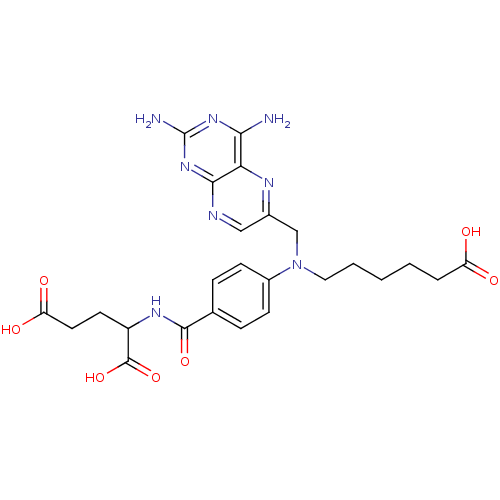 (2-{4-[(5-Carboxy-pentyl)-(2,4-diamino-pteridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773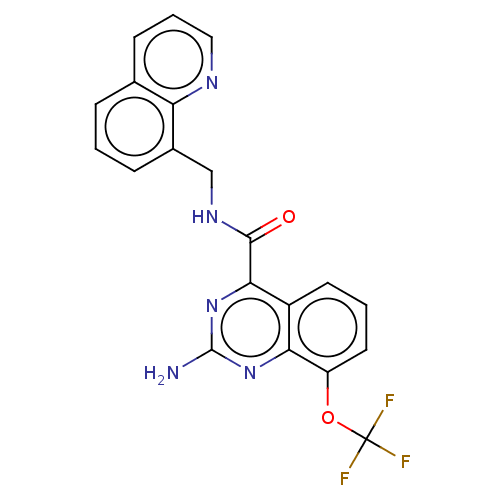 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201006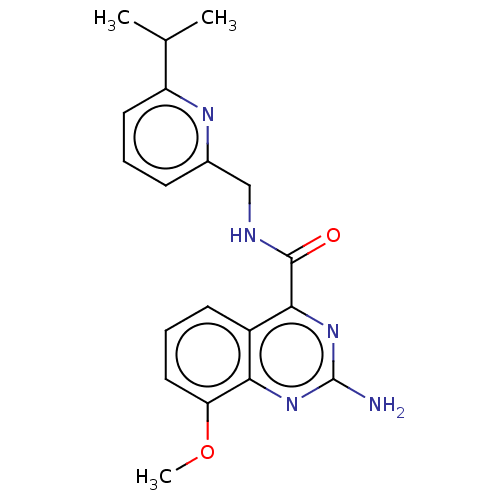 (CHEMBL3923709 | US10138212, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200981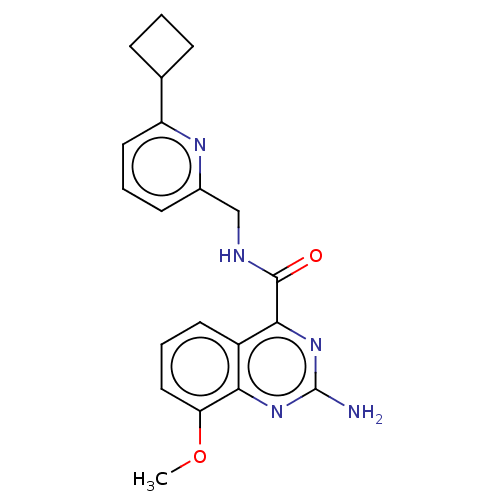 (CHEMBL3960148 | US10138212, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303246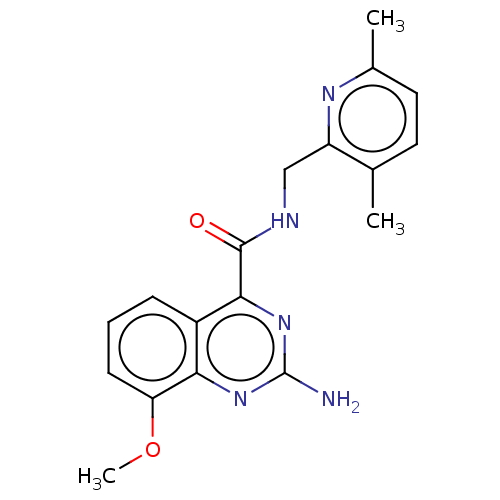 (2-amino-N-[(3,6- dimethyl-2- pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771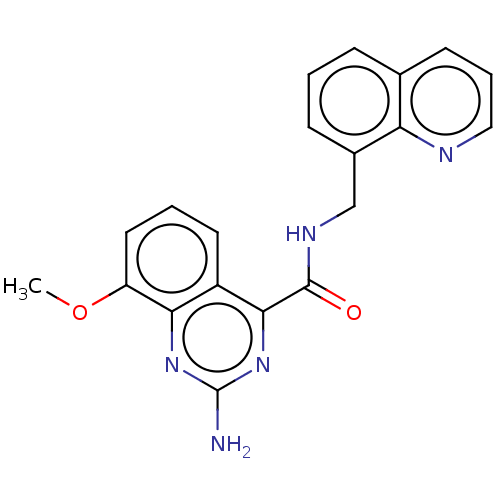 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201019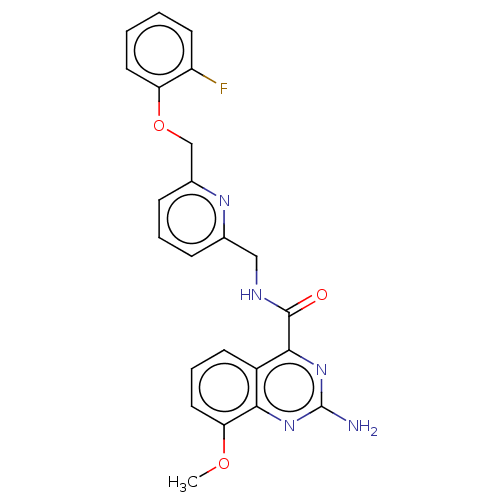 (CHEMBL3973920 | US10138212, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303248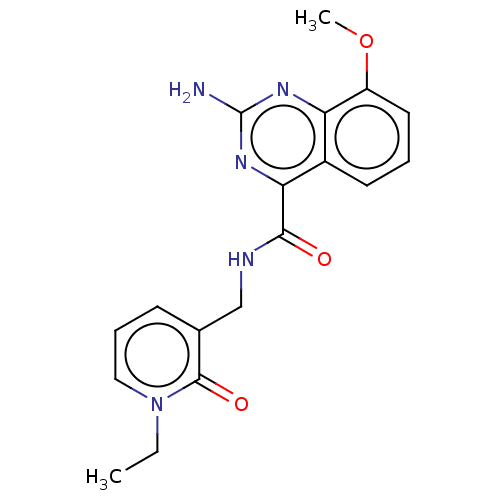 (2-amino-N-[(1-ethyl-2- oxo-3-pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from KOR in guinea pig brain membranes incubated for 30 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139748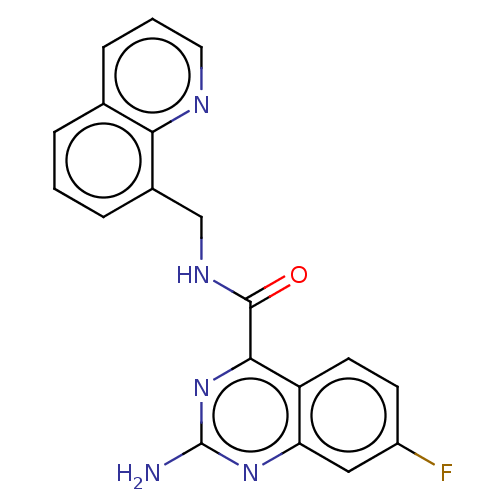 (CHEMBL3763717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303280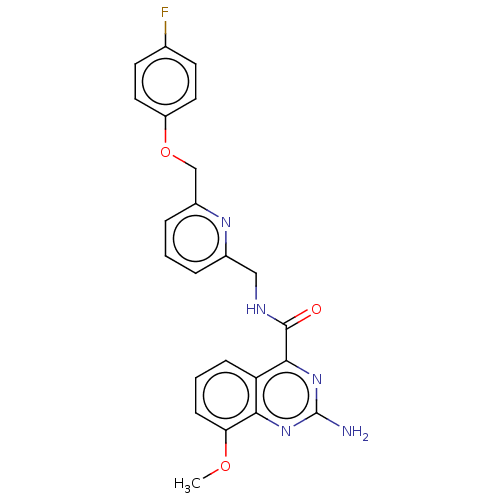 (2-amino-N-[[6-[(4- fluorophenoxy)methyl]- 2-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303251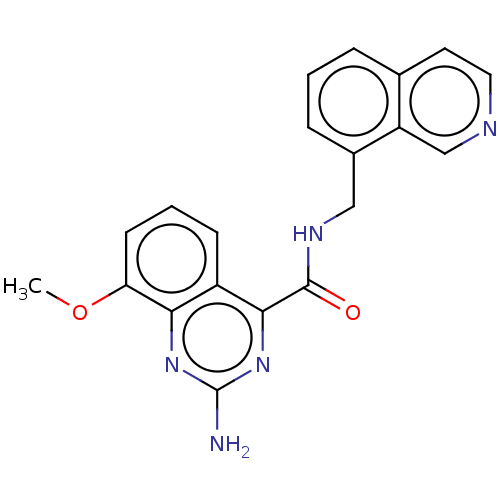 (2-amino-N-(8- isoquinolylmethyl)-8- methoxy-quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303181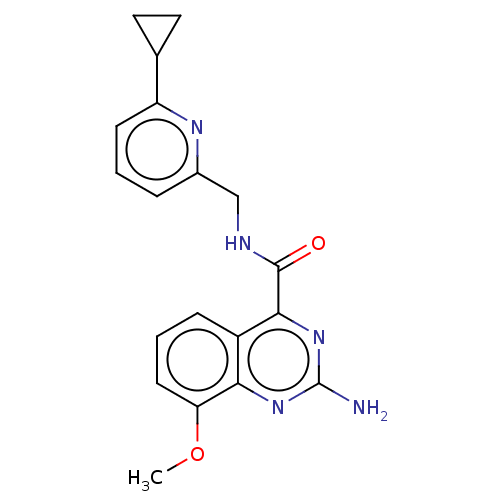 (2-amino-N-[(6- cyclopropyl-2- pyridyl)methyl]-8- m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303252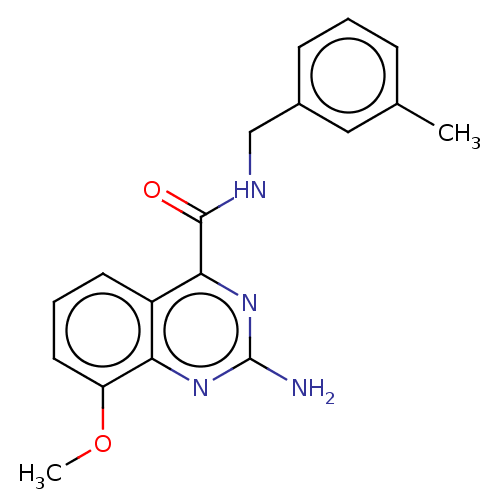 (2-amino-8-methoxy-N- (m- tolylmethyl)quinazoline- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200984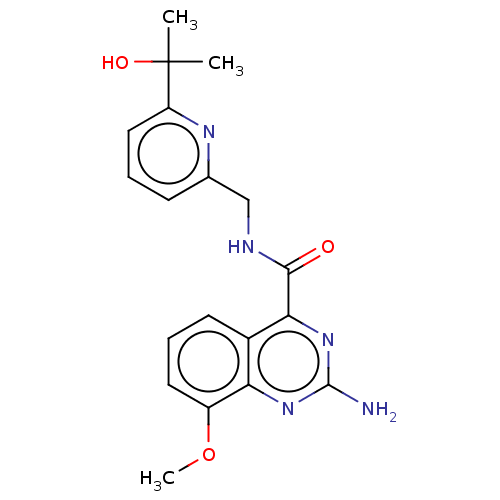 (CHEMBL3932655 | US10138212, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200989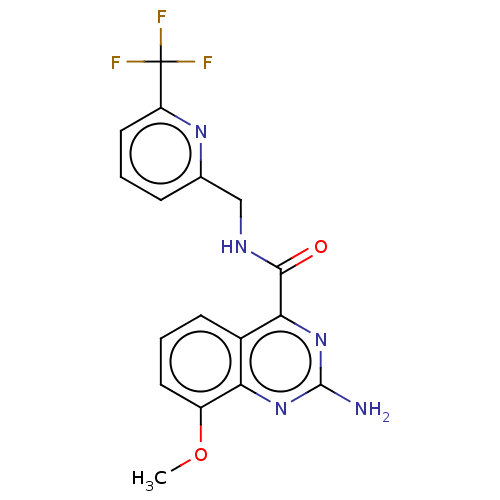 (CHEMBL3906827 | US10138212, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237064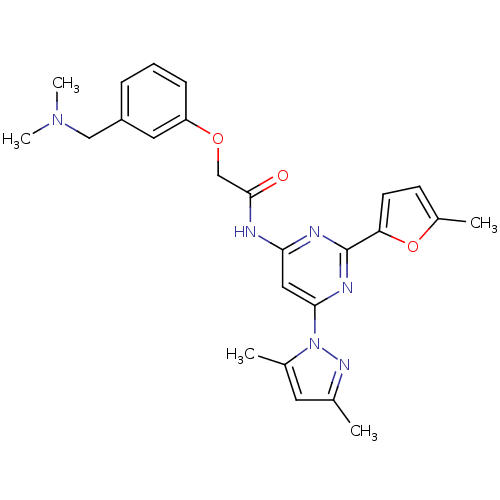 (CHEMBL401895 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26256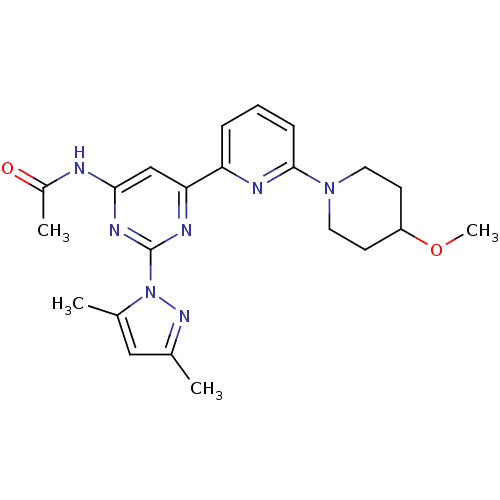 (N-[2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-[6-(4-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50317007 (2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adenosine A1 receptor by cAMP assay | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139655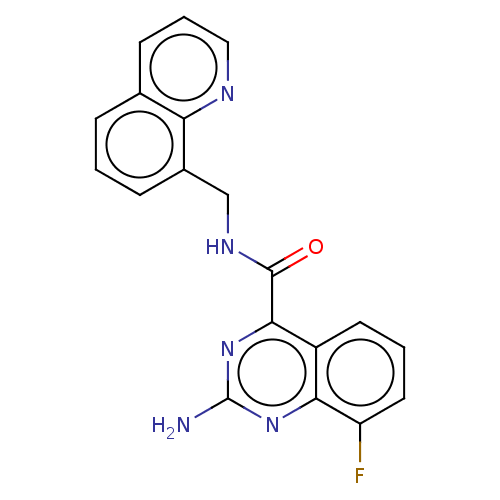 (CHEMBL3764083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201017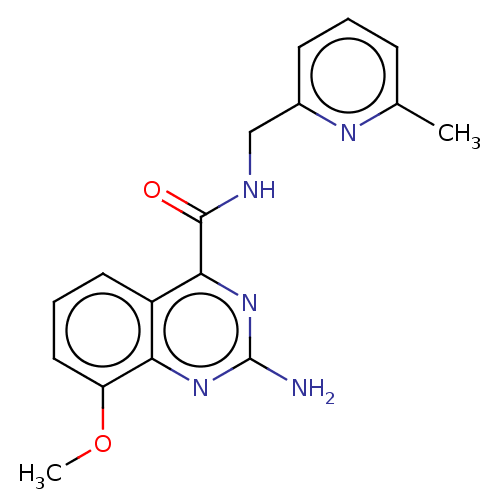 (CHEMBL3941632 | US10138212, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200986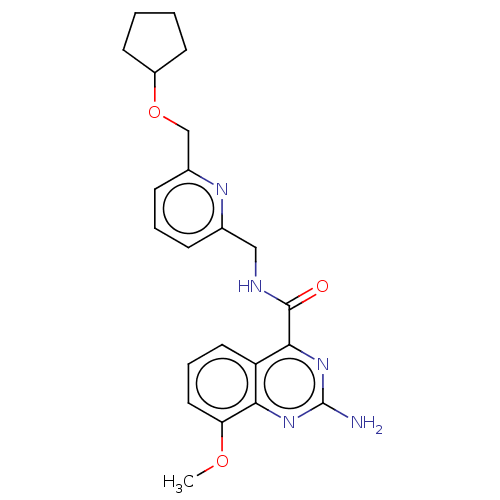 (CHEMBL3902955 | US10138212, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303255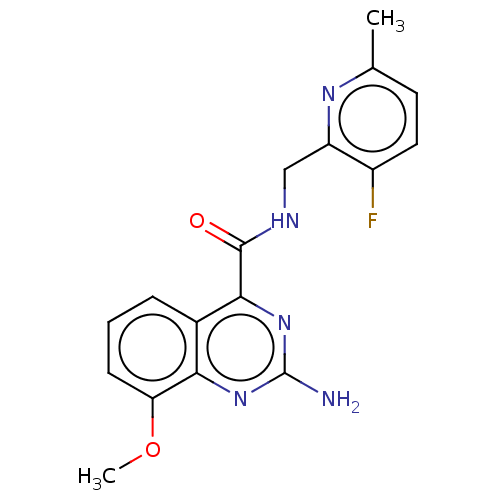 (2-amino-N-[(3-fluoro-6- methyl-2- pyridyl)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50364063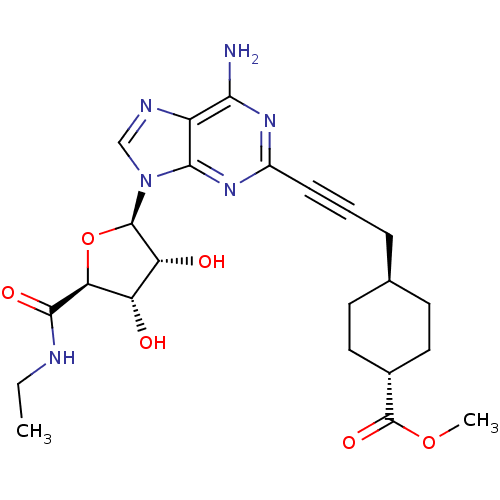 (CHEMBL1950649) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303313 (2-amino-8-fluoro-N-[(3- fluoro-6-methyl-2- pyridyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303284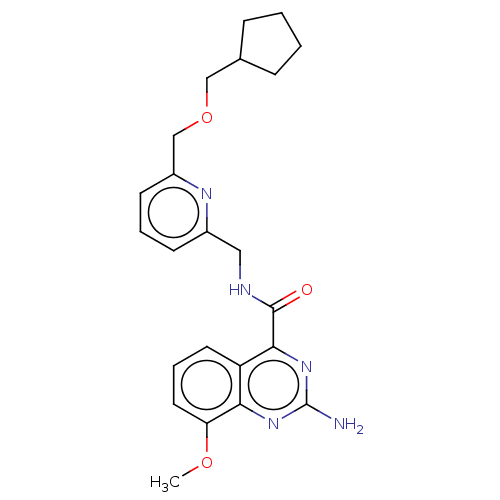 (2-amino-N-[[6- (cyclopentylmethoxy- methyl)-2-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303298 (2-amino-8-fluoro-N-[(2- pyrazol-1- ylphenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303145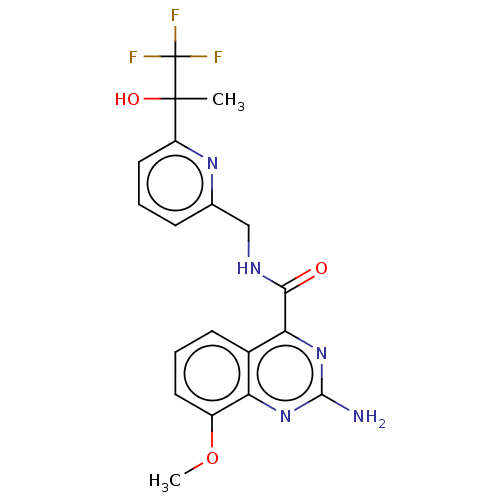 (2-amino-8-methoxy-N- [[6-(2,2,2-trifluoro-1- hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303150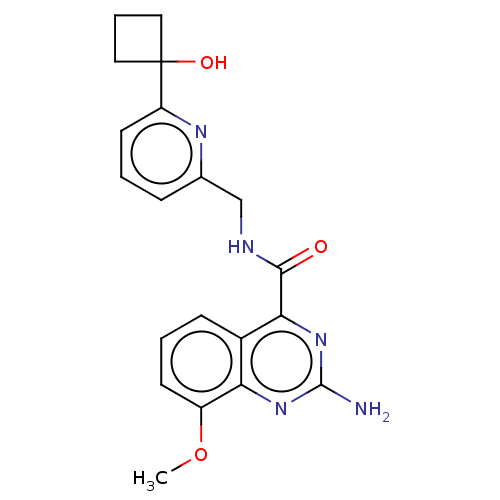 (2-amino-N-[[6-(1- hydroxycyclobutyl)-2- pyridyl]me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201020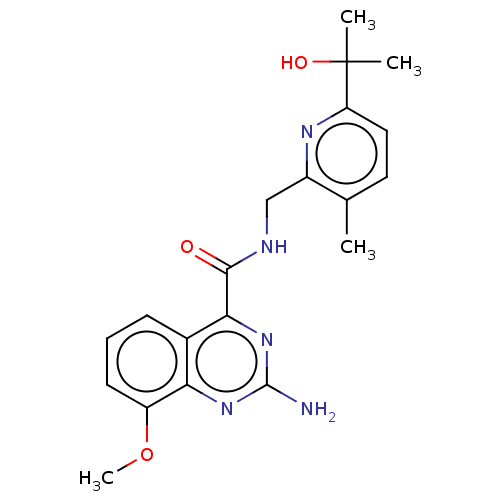 (CHEMBL3951425 | US10138212, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50232154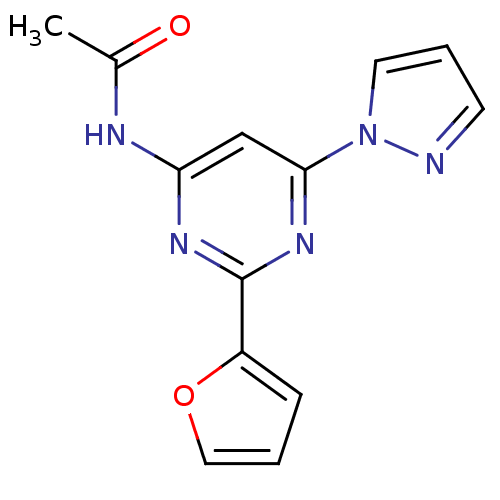 (CHEMBL401321 | N-(2-(furan-2-yl)-6-(1H-pyrazol-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202986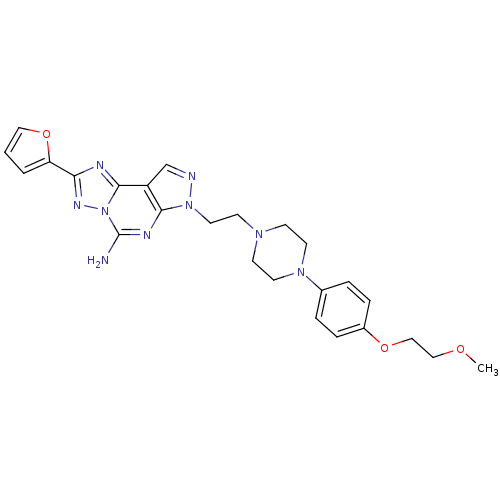 (2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202986 (2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303319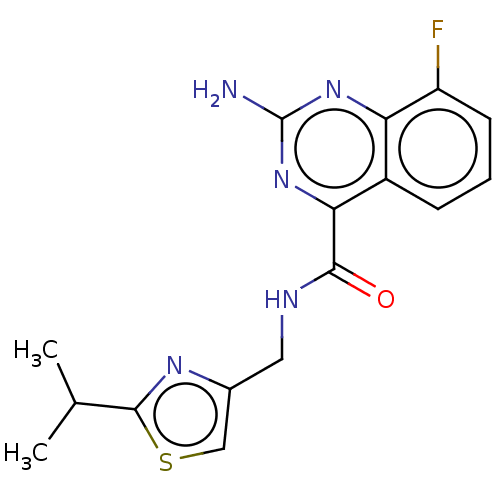 (2-amino-8-fluoro-N-[(2- isopropylthiazol-4- yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765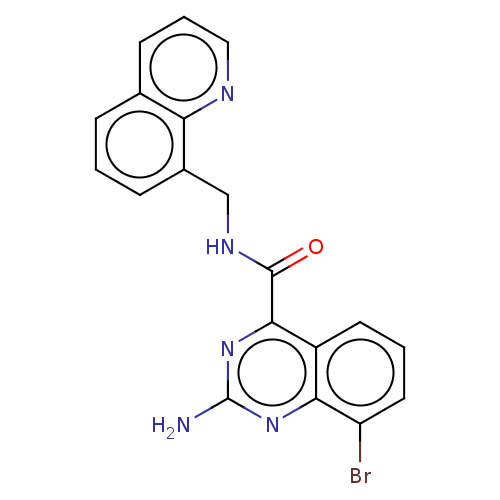 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508614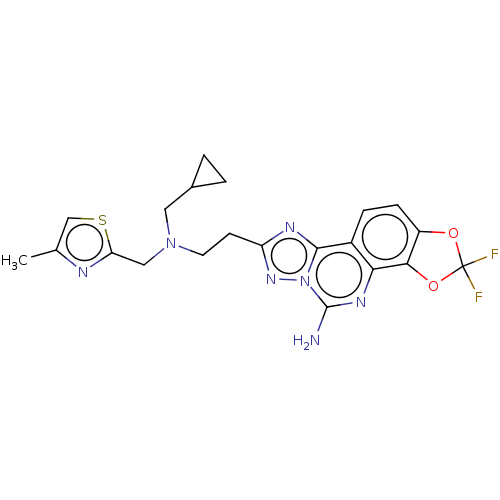 (8-(2-{(cyclopropylmethyl)[(4- methyl-1,3-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508706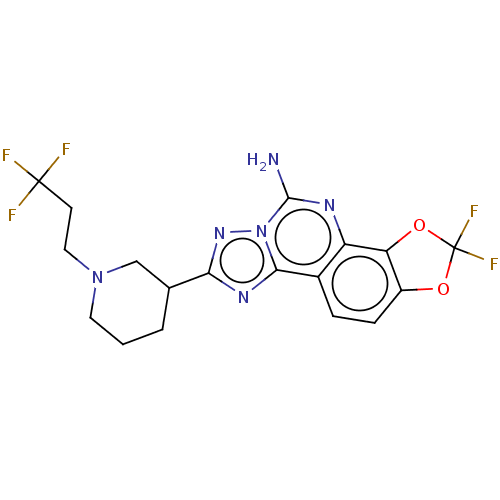 (US11046714, Example 140 | US11046714, Example 141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303299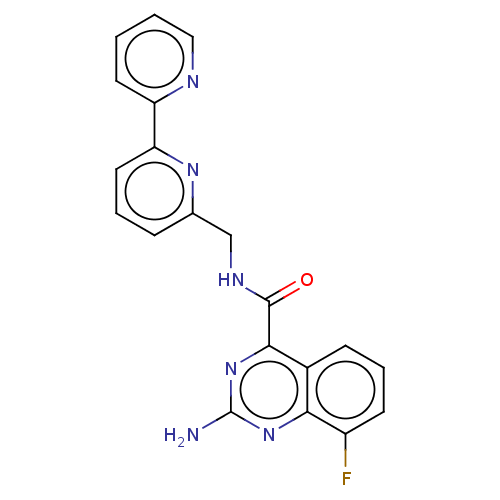 (2-amino-8-fluoro-N-[[6- (2-pyridyl)-2- pyridyl]met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508593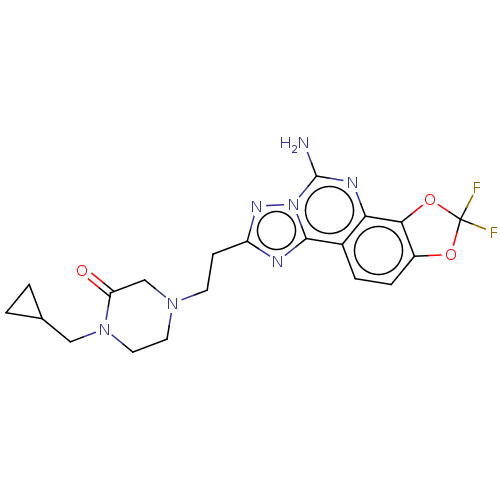 (4-[2-(5-amino-2,2-difluoro[1,3] dioxolo[4,5-h][1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303314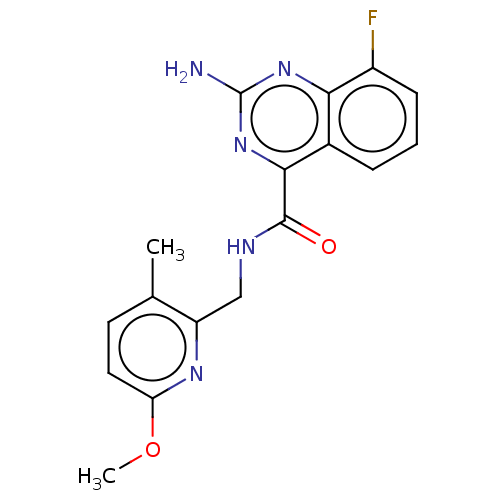 (2-amino-8-fluoro-N-[(6- methoxy-3-methyl-2- pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1867 total ) | Next | Last >> |