

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361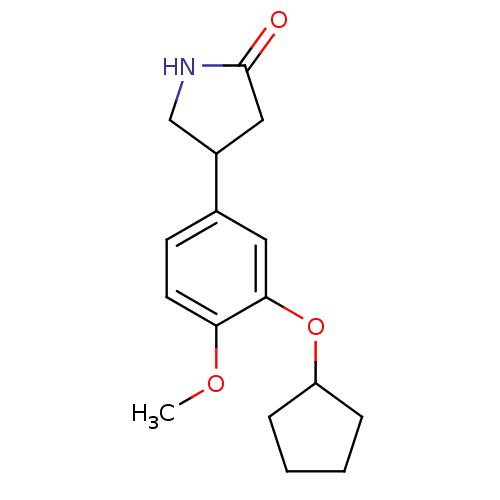 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220995 (CHEMBL77788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221005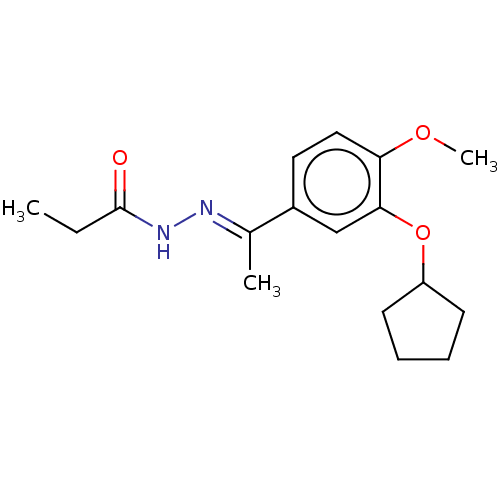 (CHEMBL75684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220998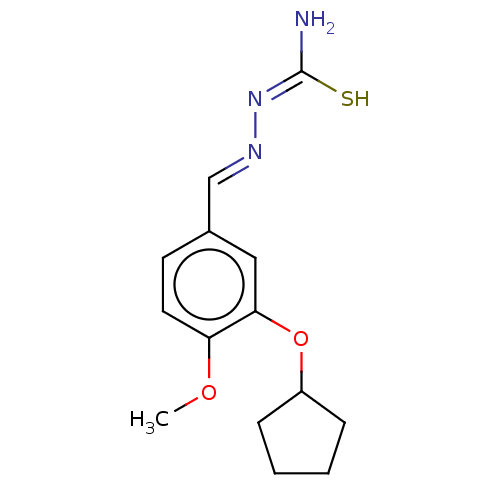 (CHEMBL76382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221003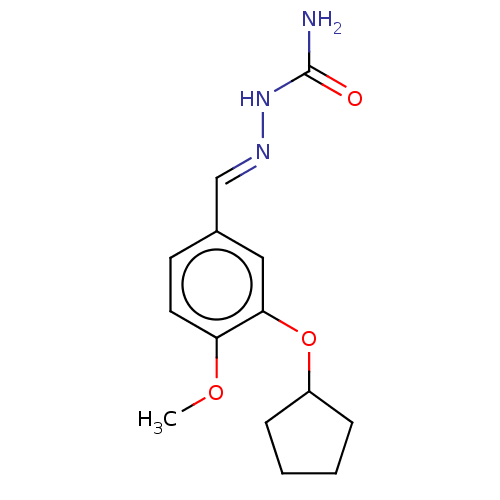 (CHEMBL432348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220997 (CHEMBL78237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221006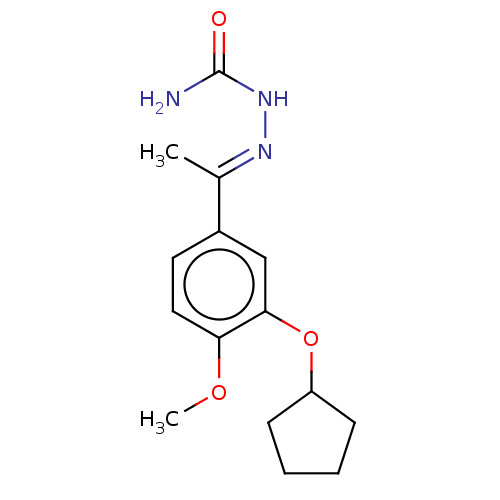 (CHEMBL77358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220999 (CHEMBL77745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474799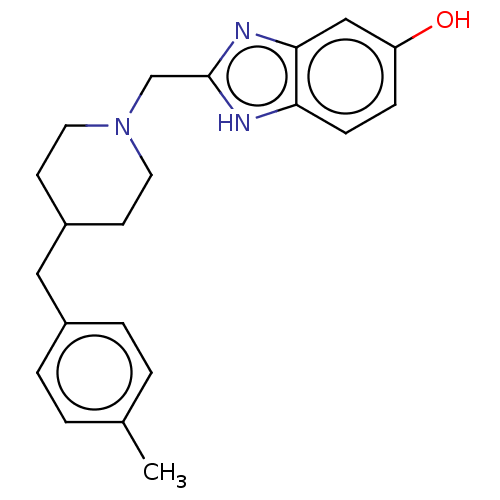 (CHEMBL416690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474791 (CHEMBL65693) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474809 (CHEMBL65454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474789 (CHEMBL68134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50204862 (CHEMBL436521 | N-(2-((4-benzylpiperidin-1-yl)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474796 (CHEMBL64941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220996 (CHEMBL76635) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50143890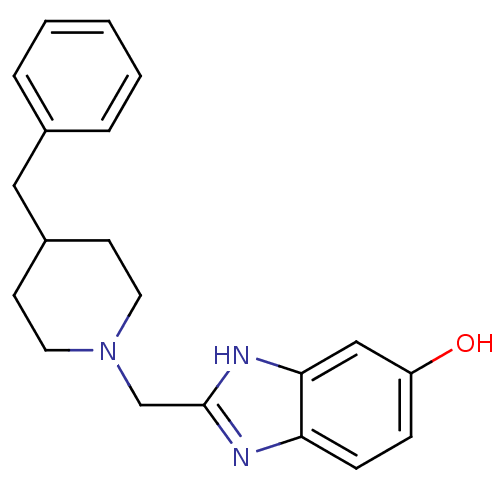 (2-(4-Benzyl-piperidin-1-ylmethyl)-3H-benzoimidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221004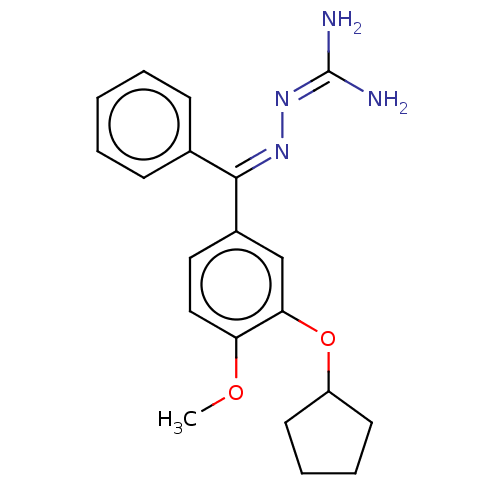 (CHEMBL77999) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221001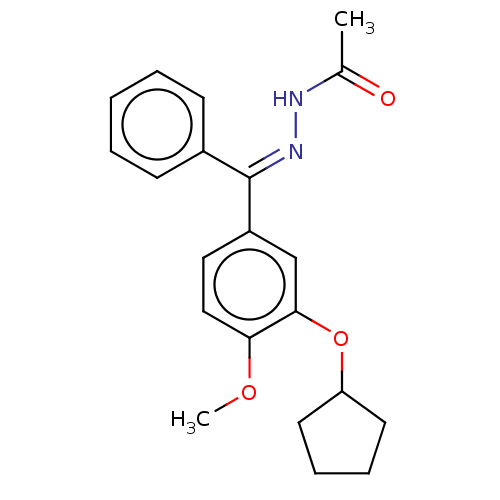 (CHEMBL76257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220993 (CHEMBL78238) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221007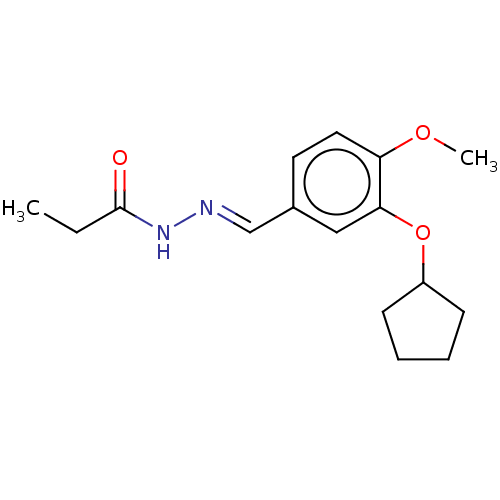 (CHEMBL80258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474808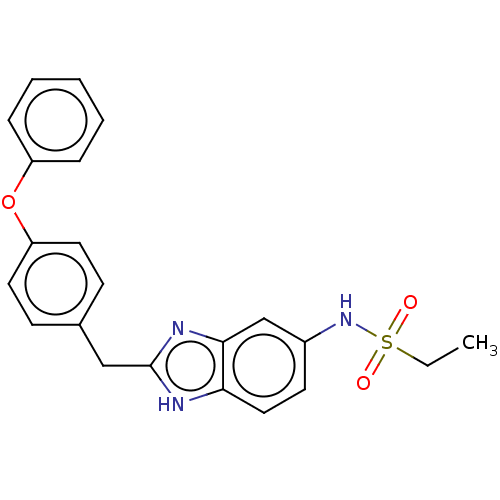 (CHEMBL65314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474792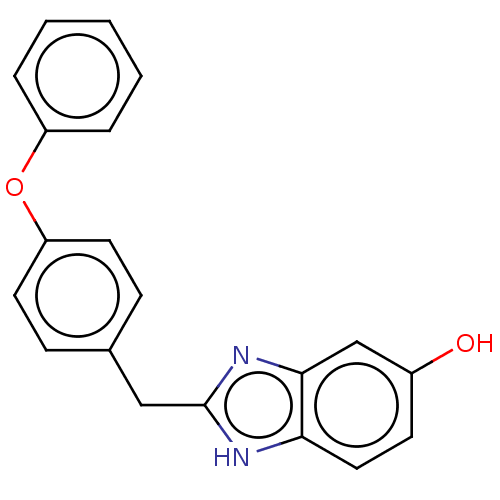 (CHEMBL63200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIP peptides (Homo sapiens (Human)) | BDBM85078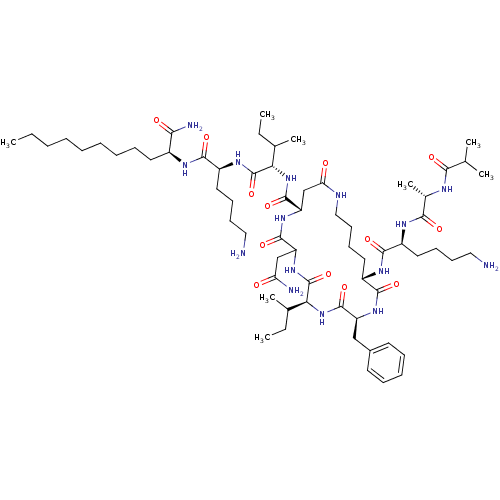 (CAS_150828-75-4 | Ro 25-1392) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, San Francisco Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 629-33 (1997) BindingDB Entry DOI: 10.7270/Q26T0K51 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221002 (CHEMBL306320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474790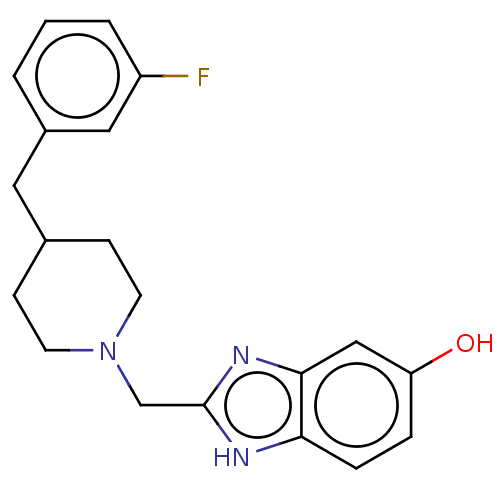 (CHEMBL303832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221000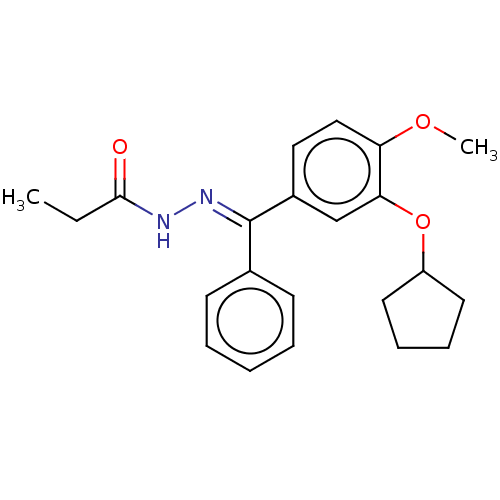 (CHEMBL431962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220994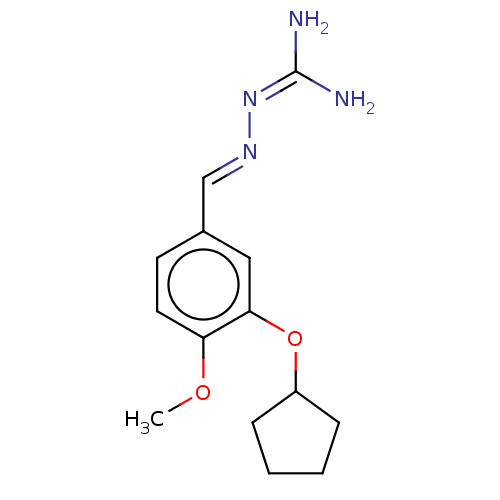 (CHEMBL77177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 2 (Homo sapiens (Human)) | BDBM85078 (CAS_150828-75-4 | Ro 25-1392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, San Francisco Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 629-33 (1997) BindingDB Entry DOI: 10.7270/Q26T0K51 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474807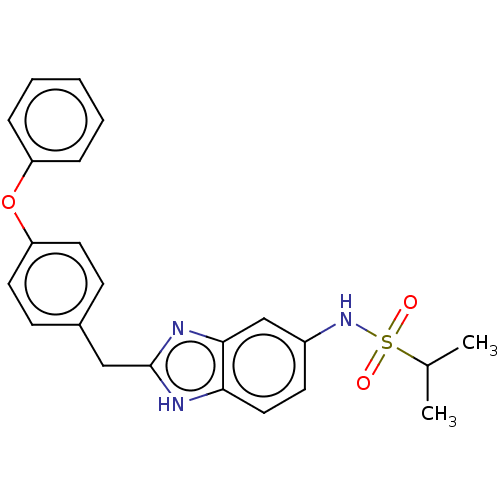 (CHEMBL304274) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 2 (Homo sapiens (Human)) | BDBM85078 (CAS_150828-75-4 | Ro 25-1392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, San Francisco Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 629-33 (1997) BindingDB Entry DOI: 10.7270/Q26T0K51 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 2 (Homo sapiens (Human)) | BDBM85078 (CAS_150828-75-4 | Ro 25-1392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, San Francisco Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 629-33 (1997) BindingDB Entry DOI: 10.7270/Q26T0K51 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474794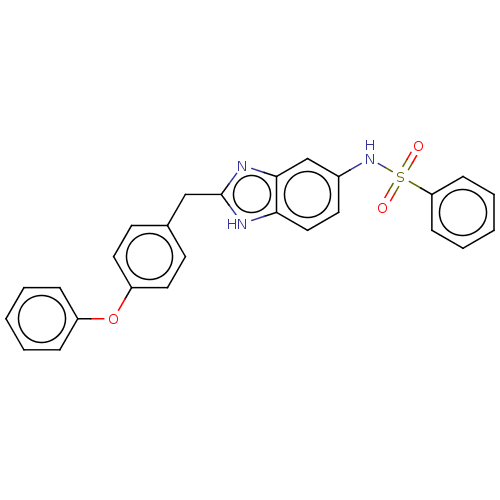 (CHEMBL66539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474793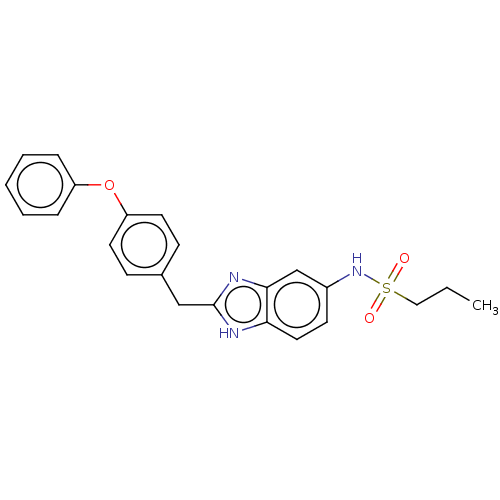 (CHEMBL63992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Rattus norvegicus) | BDBM50212873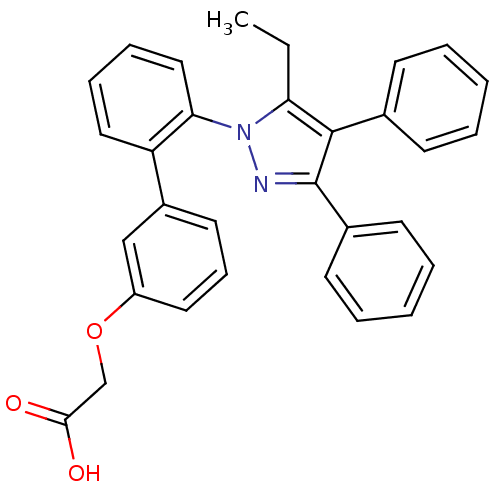 (((2'-(5-ETHYL-3,4-DIPHENYL-1H-PYRAZOL-1-YL)-3-BIPH...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay | Eur J Med Chem 52: 70-81 (2012) Article DOI: 10.1016/j.ejmech.2012.03.006 BindingDB Entry DOI: 10.7270/Q26H4JD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Rattus norvegicus) | BDBM50381857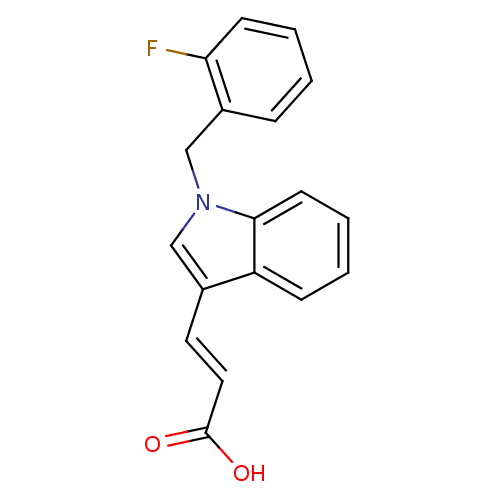 (CHEMBL2023268) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay | Eur J Med Chem 52: 70-81 (2012) Article DOI: 10.1016/j.ejmech.2012.03.006 BindingDB Entry DOI: 10.7270/Q26H4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474797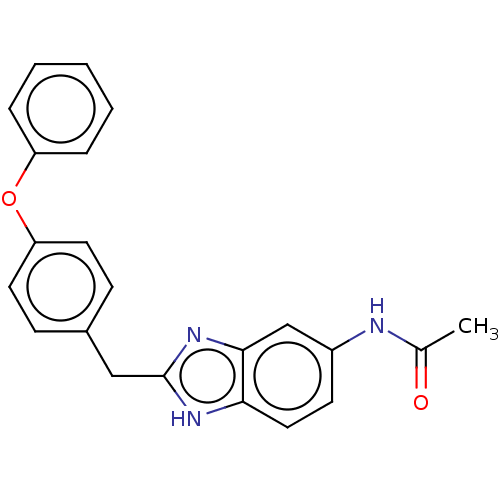 (CHEMBL65245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474801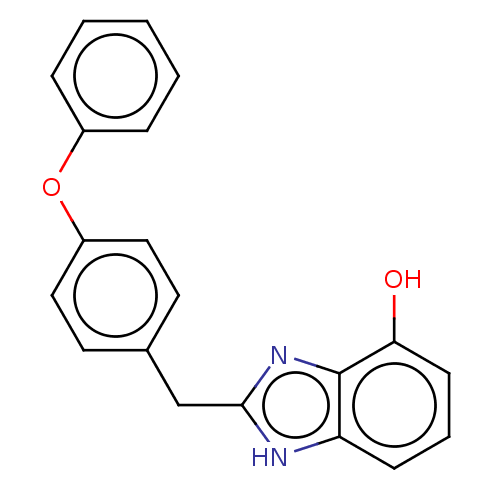 (CHEMBL63924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474802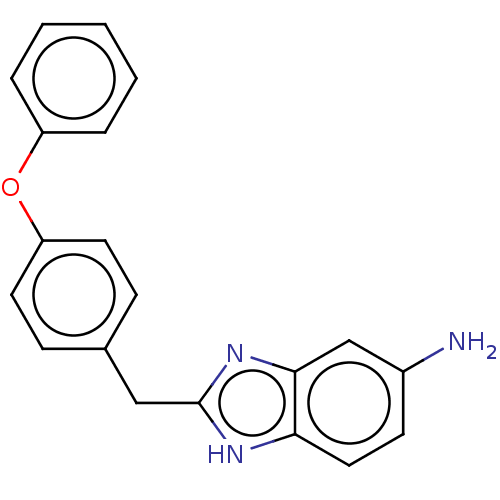 (CHEMBL65119) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474795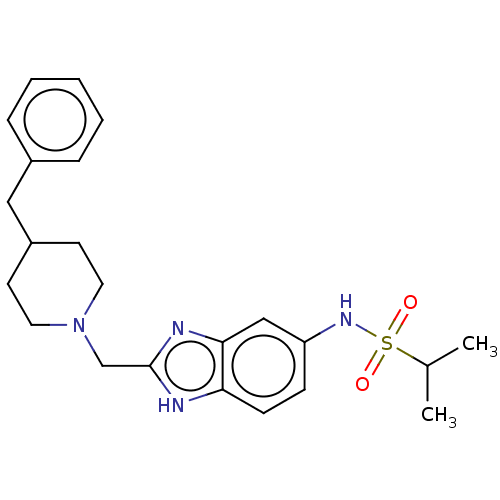 (CHEMBL65039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474804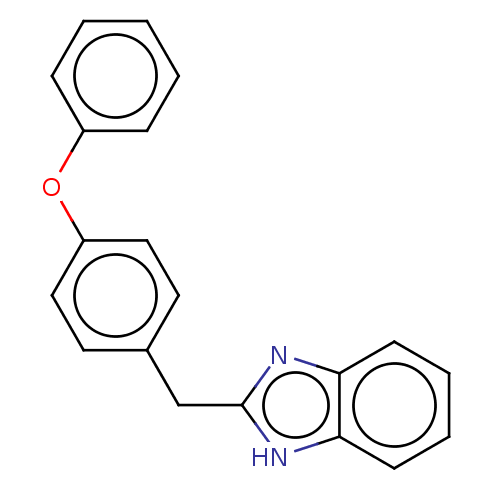 (CHEMBL67249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474800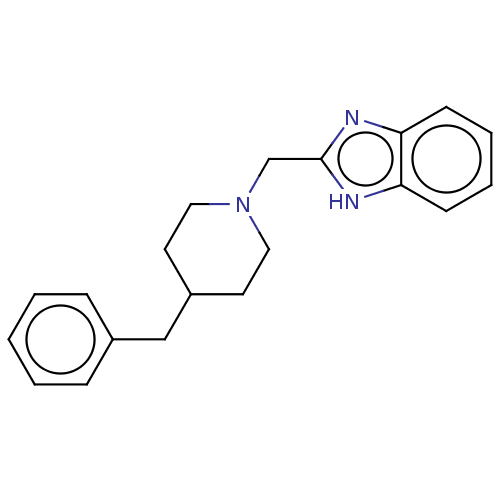 (CHEMBL304070) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474798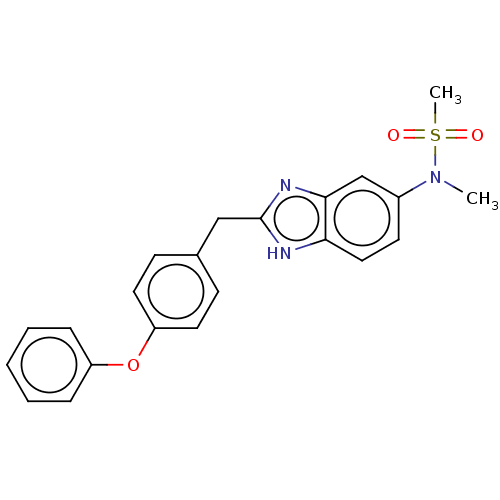 (CHEMBL61895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474806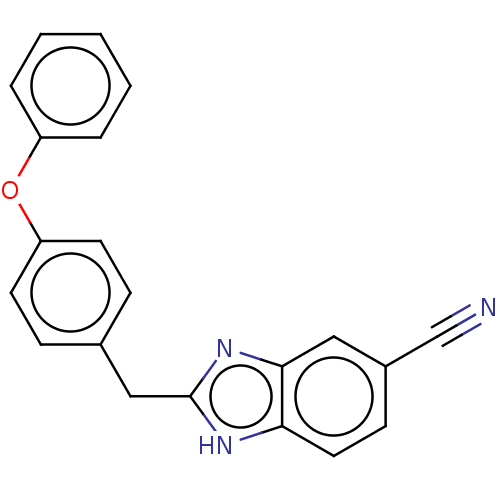 (CHEMBL63474) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474805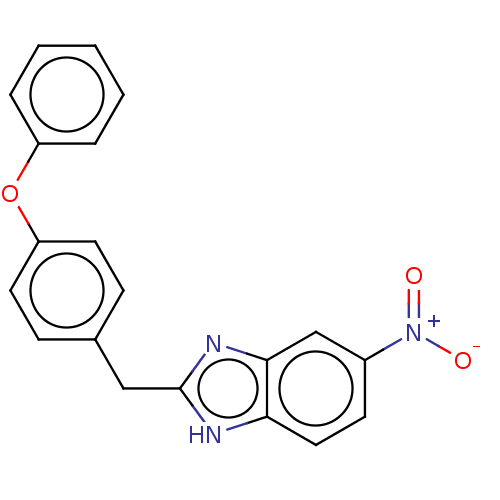 (CHEMBL63146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474803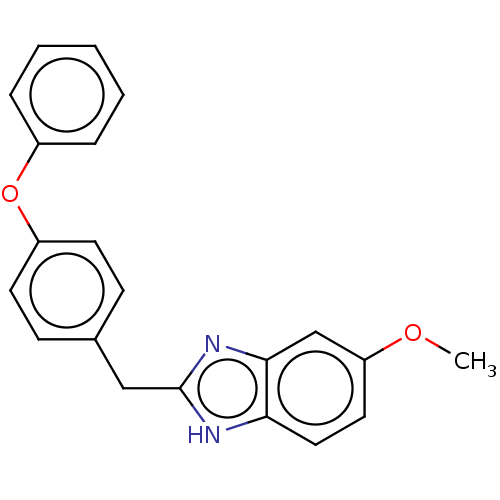 (CHEMBL67196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146577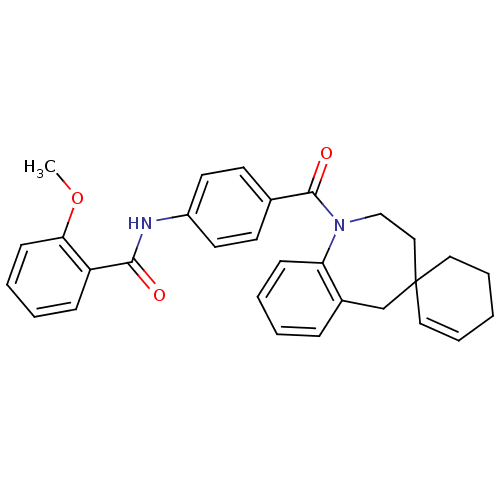 (CHEMBL102311 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50377038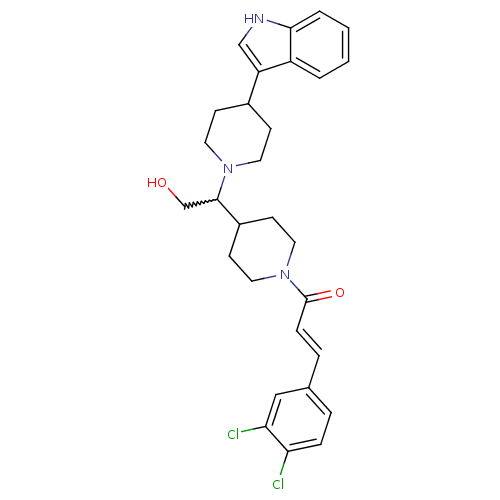 (CHEMBL258205) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at human CCR2 receptor | Bioorg Med Chem Lett 18: 3562-4 (2008) Article DOI: 10.1016/j.bmcl.2008.05.010 BindingDB Entry DOI: 10.7270/Q2Q52QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Inhibitory activity against purified rat liver phosphodiesterase 4 | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50357312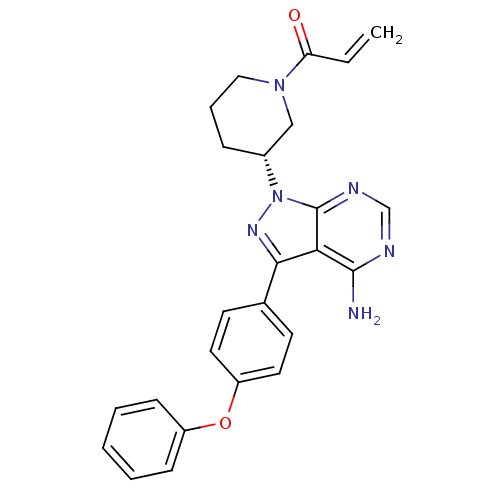 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BLK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2017.12.079 BindingDB Entry DOI: 10.7270/Q2P272TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50382932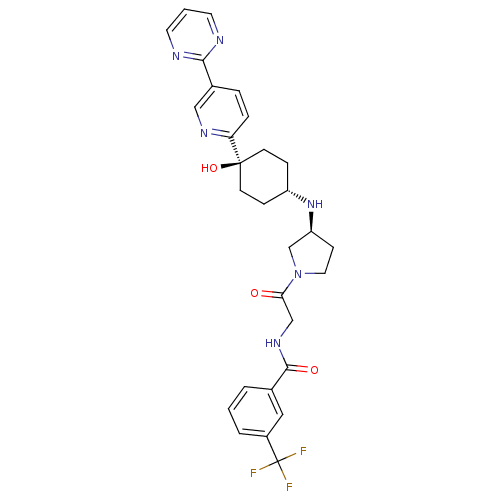 (CHEMBL2029422) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CCR2-mediated Erk phosphorylation | ACS Med Chem Lett 2: 913-918 (2011) Article DOI: 10.1021/ml200199c BindingDB Entry DOI: 10.7270/Q29024TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2169 total ) | Next | Last >> |