 Found 1029 hits with Last Name = 'dowling' and Initial = 'ms'
Found 1029 hits with Last Name = 'dowling' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133601
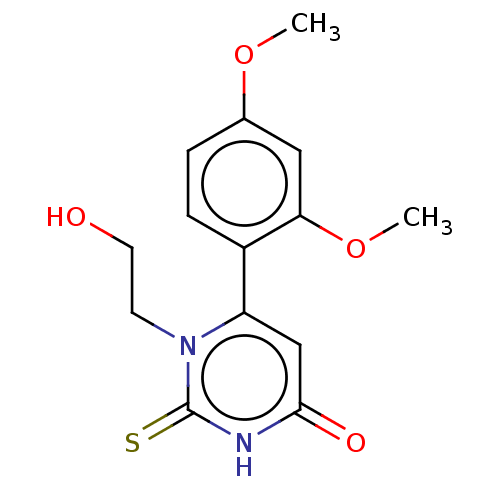
(CHEMBL3633251)Show InChI InChI=1S/C14H16N2O4S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)15-14(21)16(11)5-6-17/h3-4,7-8,17H,5-6H2,1-2H3,(H,15,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133602
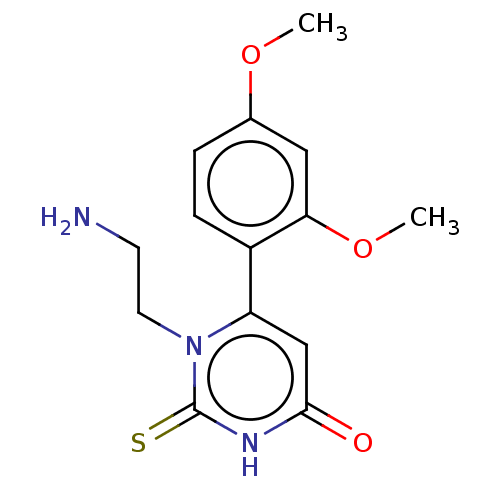
(CHEMBL3633250)Show InChI InChI=1S/C14H17N3O3S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)16-14(21)17(11)6-5-15/h3-4,7-8H,5-6,15H2,1-2H3,(H,16,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133596
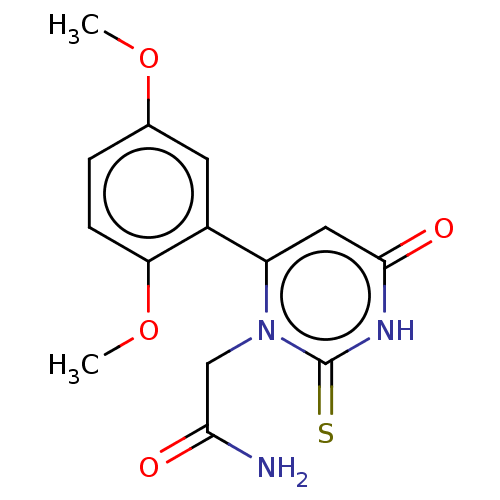
(CHEMBL3633459)Show SMILES COc1ccc(OC)c(c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-11(21-2)9(5-8)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133603
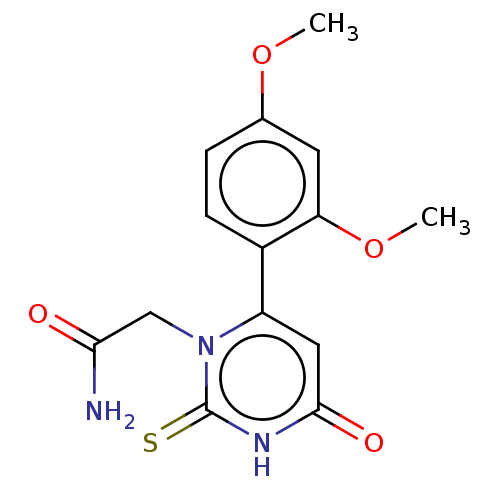
(CHEMBL3633248)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-9(11(5-8)21-2)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133600
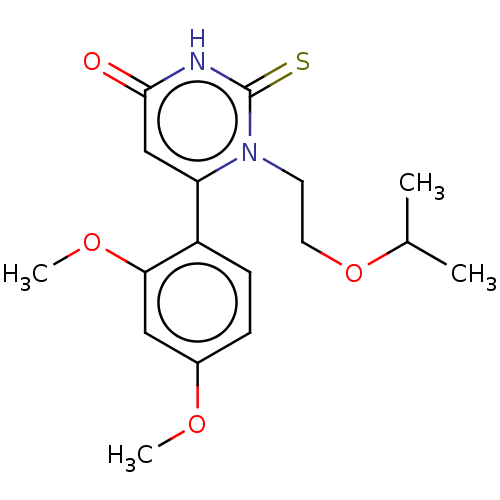
(CHEMBL3633457)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CCOC(C)C Show InChI InChI=1S/C17H22N2O4S/c1-11(2)23-8-7-19-14(10-16(20)18-17(19)24)13-6-5-12(21-3)9-15(13)22-4/h5-6,9-11H,7-8H2,1-4H3,(H,18,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287559
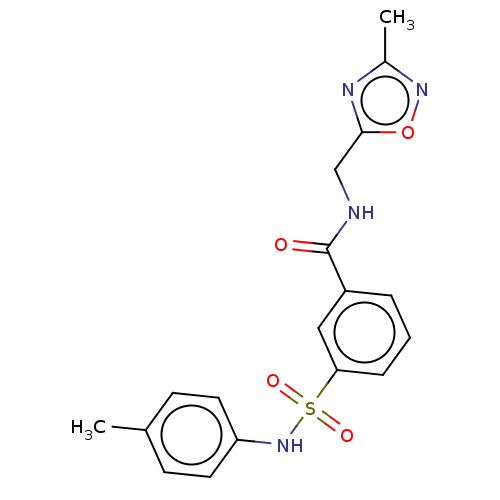
(CHEMBL4170197)Show SMILES Cc1noc(CNC(=O)c2cccc(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C18H18N4O4S/c1-12-6-8-15(9-7-12)22-27(24,25)16-5-3-4-14(10-16)18(23)19-11-17-20-13(2)21-26-17/h3-10,22H,11H2,1-2H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287558
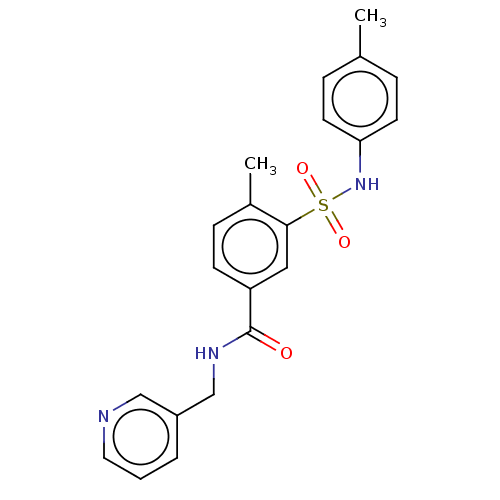
(CHEMBL1333494)Show SMILES Cc1ccc(NS(=O)(=O)c2cc(ccc2C)C(=O)NCc2cccnc2)cc1 Show InChI InChI=1S/C21H21N3O3S/c1-15-5-9-19(10-6-15)24-28(26,27)20-12-18(8-7-16(20)2)21(25)23-14-17-4-3-11-22-13-17/h3-13,24H,14H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287552
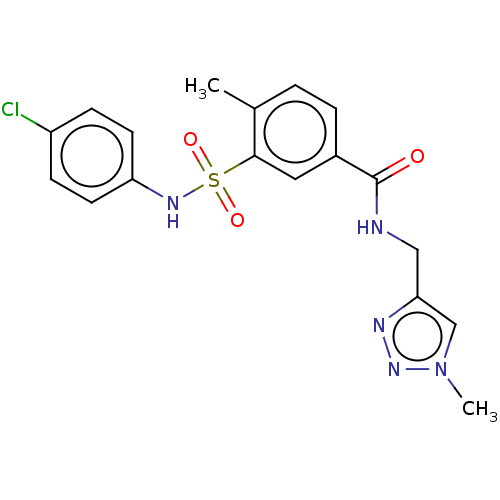
(CHEMBL4175004)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C18H18ClN5O3S/c1-12-3-4-13(18(25)20-10-16-11-24(2)23-21-16)9-17(12)28(26,27)22-15-7-5-14(19)6-8-15/h3-9,11,22H,10H2,1-2H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287556

(CHEMBL4161489)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(cc1)-c1ccno1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C21H20N6O4S/c1-14-3-4-16(21(28)22-12-18-13-27(2)26-24-18)11-20(14)32(29,30)25-17-7-5-15(6-8-17)19-9-10-23-31-19/h3-11,13,25H,12H2,1-2H3,(H,22,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287519
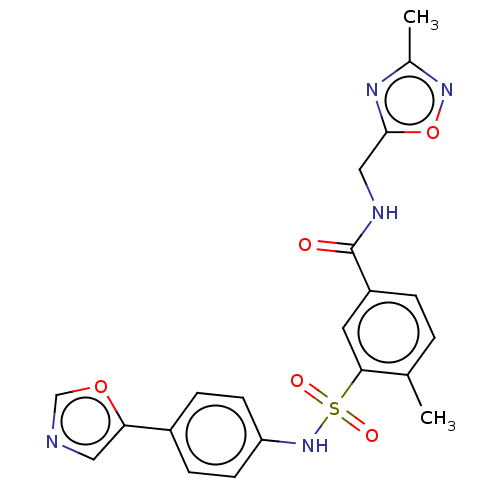
(CHEMBL4172084)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2cnco2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)23-11-20-24-14(2)25-31-20)9-19(13)32(28,29)26-17-7-5-15(6-8-17)18-10-22-12-30-18/h3-10,12,26H,11H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287553
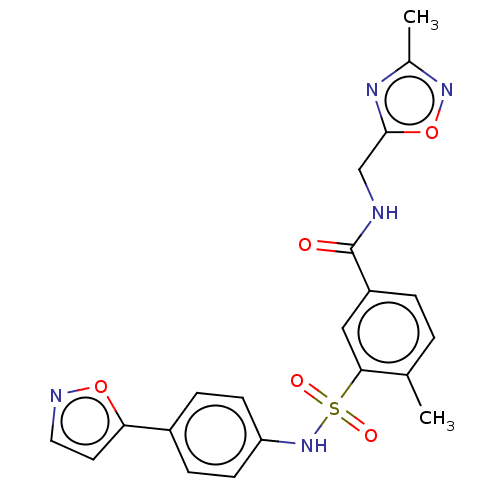
(CHEMBL4166791)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2ccno2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)22-12-20-24-14(2)25-31-20)11-19(13)32(28,29)26-17-7-5-15(6-8-17)18-9-10-23-30-18/h3-11,26H,12H2,1-2H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287554

(CHEMBL4162926)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C19H20N4O4S/c1-12-4-8-16(9-5-12)23-28(25,26)17-10-15(7-6-13(17)2)19(24)20-11-18-21-14(3)22-27-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287555
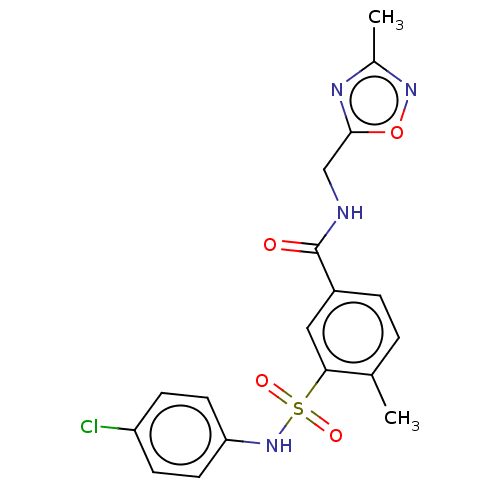
(CHEMBL4174695)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H17ClN4O4S/c1-11-3-4-13(18(24)20-10-17-21-12(2)22-27-17)9-16(11)28(25,26)23-15-7-5-14(19)6-8-15/h3-9,23H,10H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287557

(CHEMBL4159568)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(C)cc1)C(=O)NCc1nc(C)no1 Show InChI InChI=1S/C19H20N4O5S/c1-12-4-7-15(8-5-12)23-29(25,26)17-10-14(6-9-16(17)27-3)19(24)20-11-18-21-13(2)22-28-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582
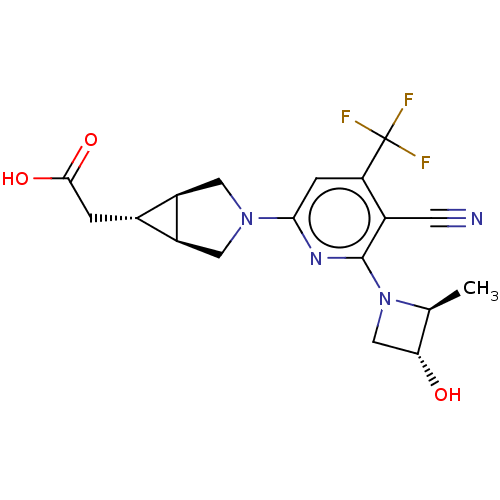
(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276763
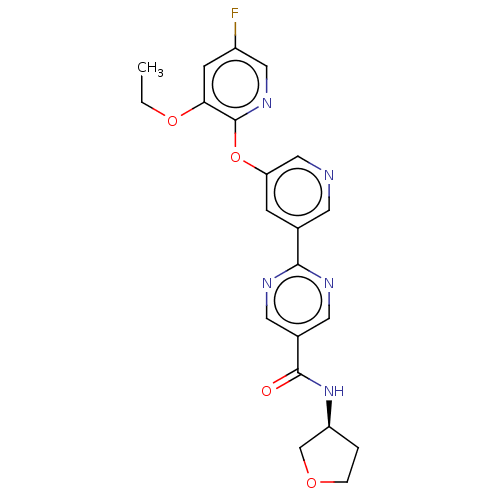
(US10071992, Example 8 | US11034678, Example 8 | US...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H20FN5O4/c1-2-30-18-6-15(22)10-26-21(18)31-17-5-13(7-23-11-17)19-24-8-14(9-25-19)20(28)27-16-3-4-29-12-16/h5-11,16H,2-4,12H2,1H3,(H,27,28)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC64BQ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276763

(US10071992, Example 8 | US11034678, Example 8 | US...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H20FN5O4/c1-2-30-18-6-15(22)10-26-21(18)31-17-5-13(7-23-11-17)19-24-8-14(9-25-19)20(28)27-16-3-4-29-12-16/h5-11,16H,2-4,12H2,1H3,(H,27,28)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
US Patent US10071992 (2018)
BindingDB Entry DOI: 10.7270/Q2RJ4MHN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771
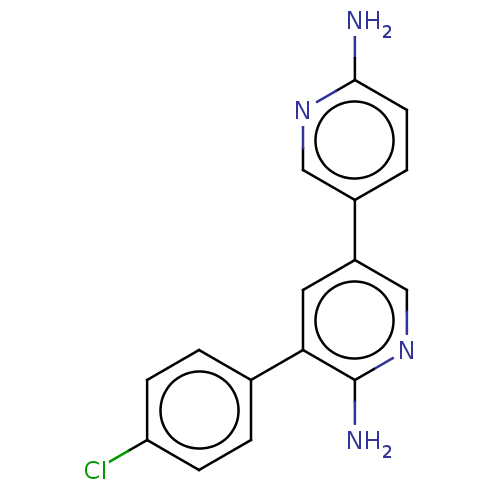
(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAP4K4 catalytic domain in presence of 10 uM ATP (Km) by FRET assay |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582

(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276748
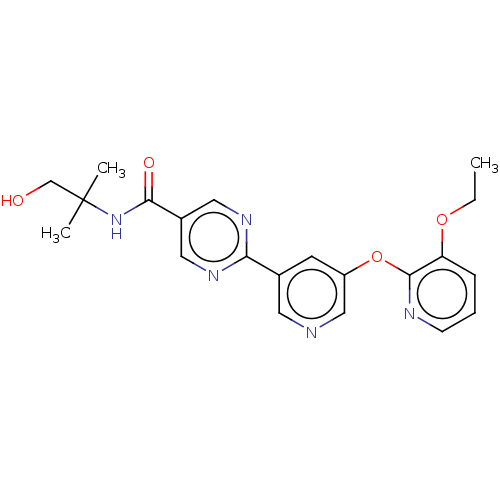
(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276759
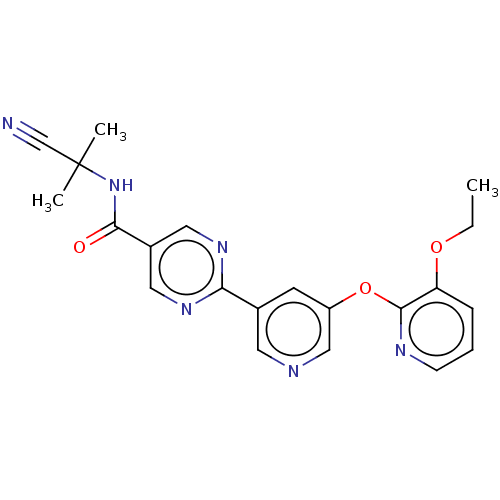
(US10071992, Example 4 | US11034678, Example 4)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)C#N Show InChI InChI=1S/C21H20N6O3/c1-4-29-17-6-5-7-24-20(17)30-16-8-14(9-23-12-16)18-25-10-15(11-26-18)19(28)27-21(2,3)13-22/h5-12H,4H2,1-3H3,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC64BQ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276759

(US10071992, Example 4 | US11034678, Example 4)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)C#N Show InChI InChI=1S/C21H20N6O3/c1-4-29-17-6-5-7-24-20(17)30-16-8-14(9-23-12-16)18-25-10-15(11-26-18)19(28)27-21(2,3)13-22/h5-12H,4H2,1-3H3,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
US Patent US10071992 (2018)
BindingDB Entry DOI: 10.7270/Q2RJ4MHN |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Rattus norvegicus (Rat)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Rattus norvegicus (Rat)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749
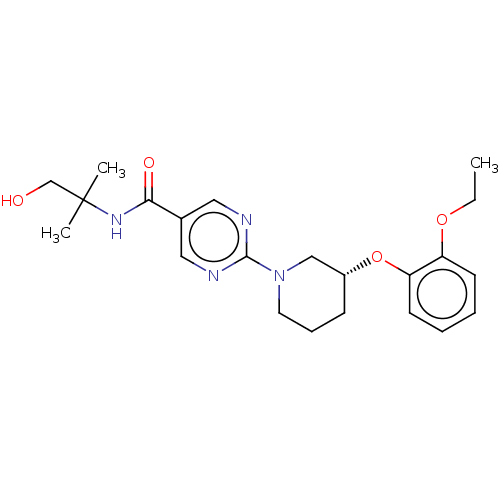
(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749

(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC64BQ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749

(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... |
US Patent US10071992 (2018)
BindingDB Entry DOI: 10.7270/Q2RJ4MHN |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MINK (unknown origin) in presence of ATP (Km) |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546611

(CHEMBL4762472)Show SMILES CN(C)c1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)CC(=O)c1nn(cc1O2)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546611

(CHEMBL4762472)Show SMILES CN(C)c1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)CC(=O)c1nn(cc1O2)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546611

(CHEMBL4762472)Show SMILES CN(C)c1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)CC(=O)c1nn(cc1O2)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546611

(CHEMBL4762472)Show SMILES CN(C)c1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)CC(=O)c1nn(cc1O2)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588577

(CHEMBL5176366)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCCOC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data