

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human thrombin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 50.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human trypsin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 57 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | 67 | -42.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 780 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human plasmin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Coagulation factor Xa (fXa) | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 4.10E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.50E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 9.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 9.20E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.60E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Activated protein C | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 4.40E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Tissue plasminogen activator (tissue plasminogen activator) | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376985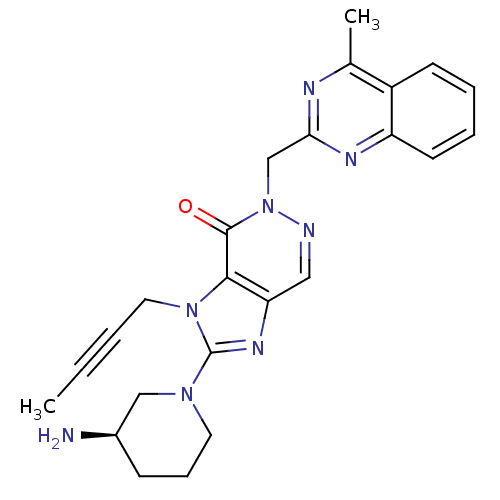 (CHEMBL403553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376986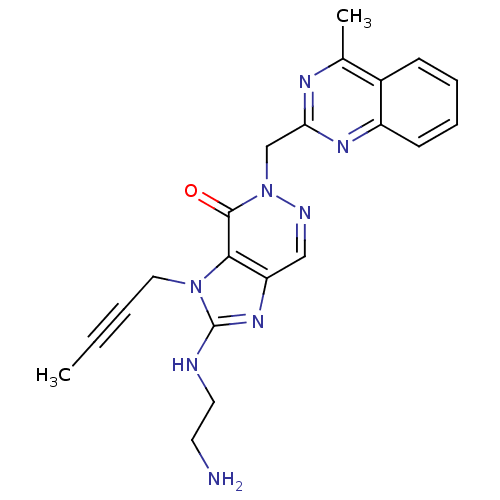 (CHEMBL257376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376984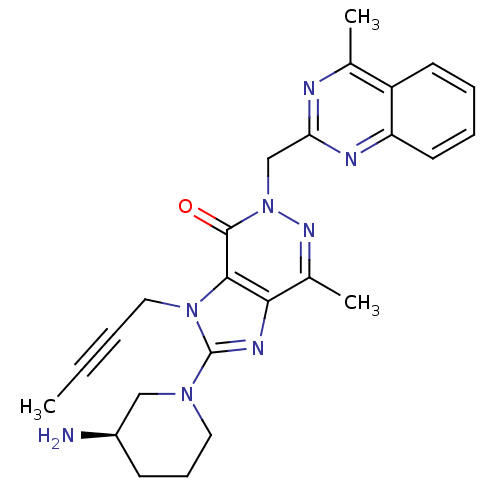 (CHEMBL256121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228406 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376982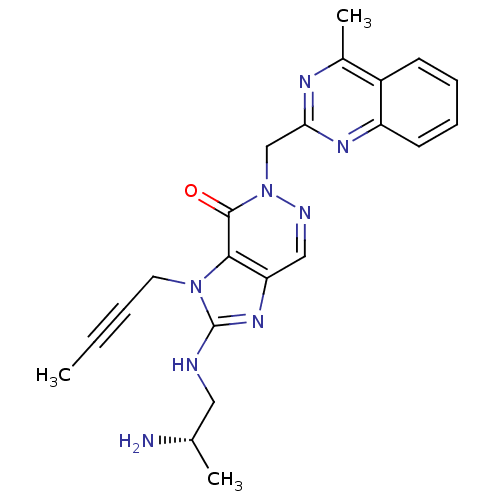 (CHEMBL403408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043244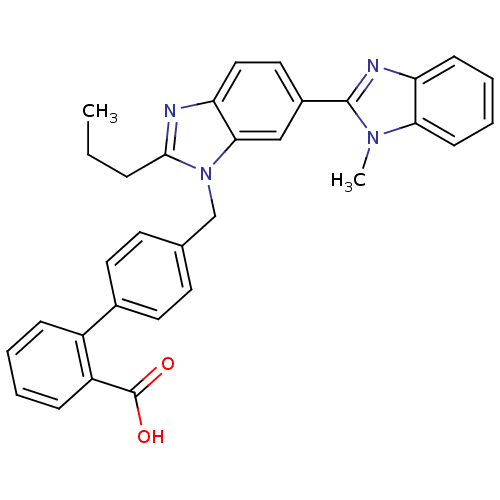 (4'-(1-Methyl-2'-propyl-1H-[2,5']bibenzoimidazolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043260 (2-Butyl-6-(1,1-dioxo-1lambda*6*-[1,2]thiazinan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50129964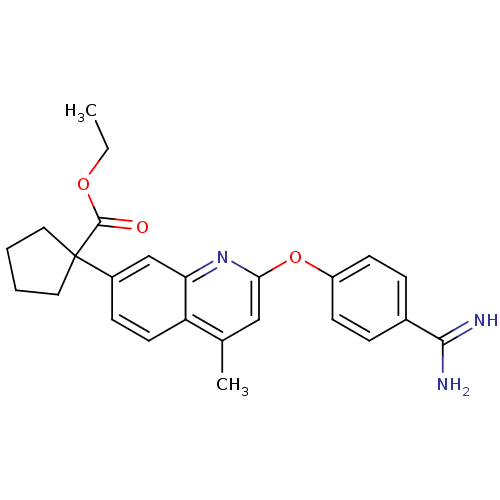 (1-[2-(4-Carbamimidoyl-phenoxy)-4-methyl-quinolin-7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description In vitro inhibitory activity against thrombin in human plasma | Bioorg Med Chem Lett 13: 2291-5 (2003) BindingDB Entry DOI: 10.7270/Q2QJ7GP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376987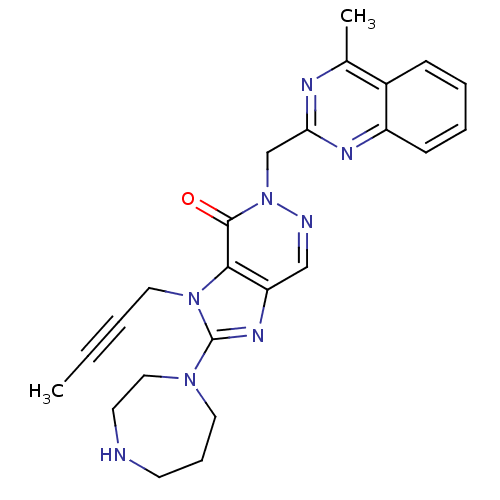 (CHEMBL257999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376991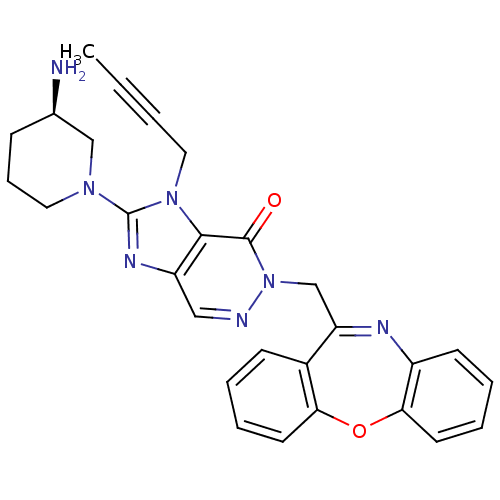 (CHEMBL401502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043257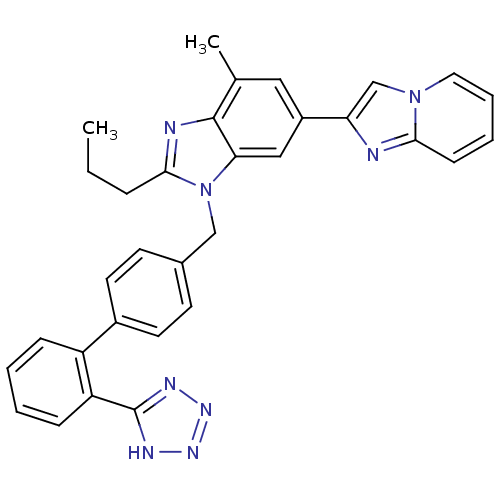 (6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl-1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043277 (1-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043246 (4'-(6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376989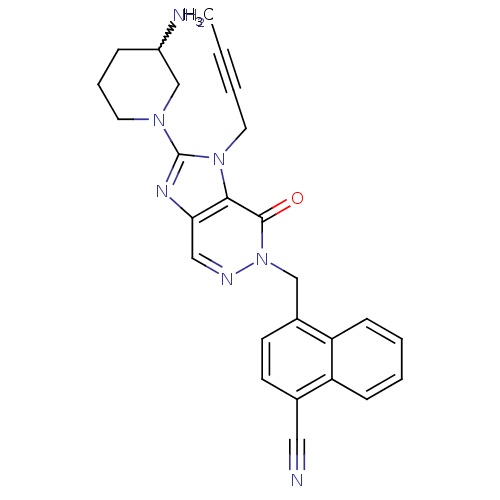 (CHEMBL404220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376988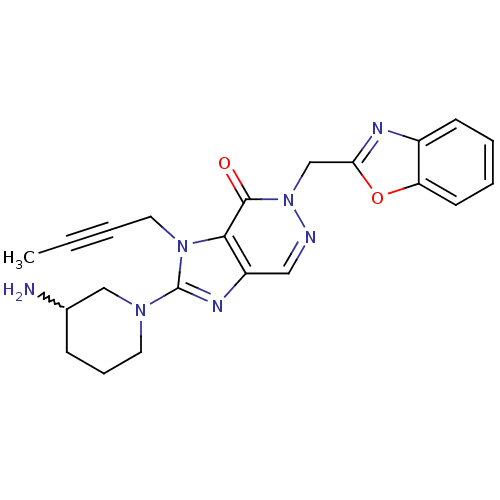 (CHEMBL256969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376983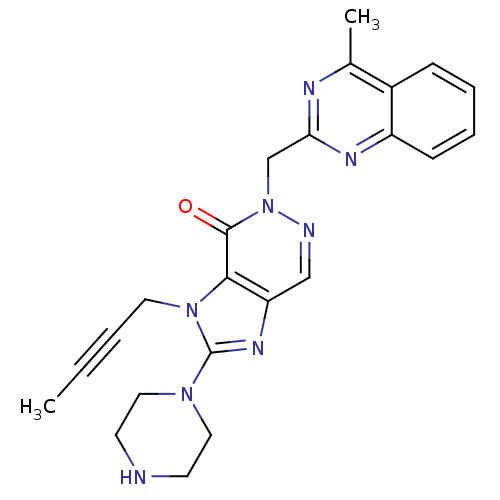 (CHEMBL401971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50377002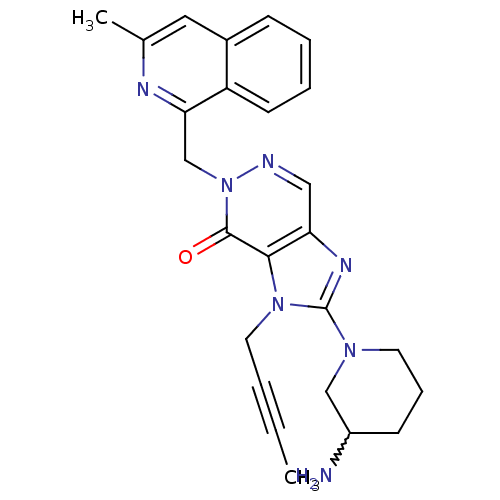 (CHEMBL256521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043279 (1-Methyl-2'-propyl-3'-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |