 Found 312 hits with Last Name = 'song' and Initial = 'n'
Found 312 hits with Last Name = 'song' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50571444

(CHEMBL4858291)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cccc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50571445
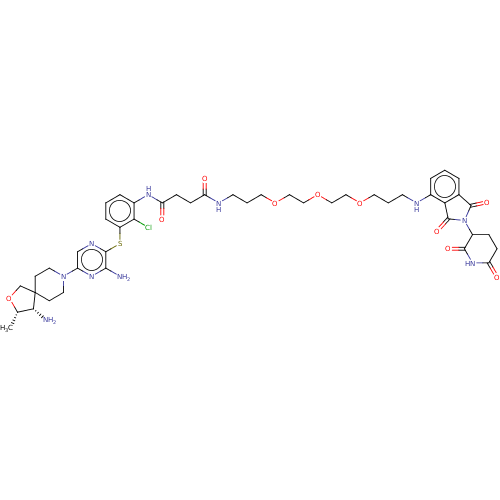
(CHEMBL4871539)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cccc(NC(=O)CCC(=O)NCCCOCCOCCOCCCNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.202 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50139171
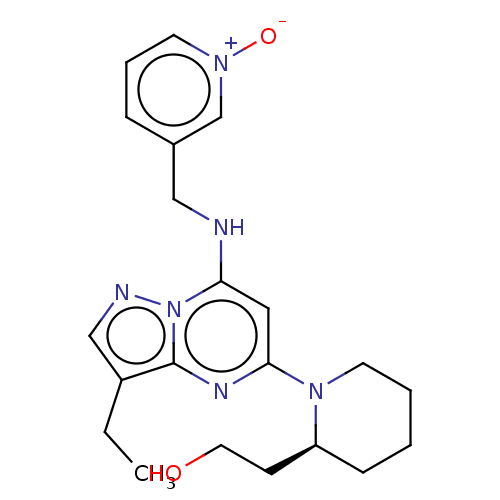
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9 (unknown origin) expressed in Sf9 insect cells using biotinylated peptide derived Histone H1 as substrate incubated for 1 hr in pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK1 (unknown origin) expressed in Sf9 insect cells using biotinylated peptide derived Histone H1 as substrate incubated for 1 hr in pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK2 (unknown origin) expressed in Sf9 insect cells using biotinylated peptide derived Histone H1 as substrate incubated for 1 hr in pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK5 (unknown origin) expressed in Sf9 insect cells using biotinylated peptide derived Histone H1 as substrate incubated for 1 hr in pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK4 (unknown origin) expressed in Sf9 insect cells incubated for 1 hr in presence of [gamma33P]ATP by liquid scintillation counter met... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057581
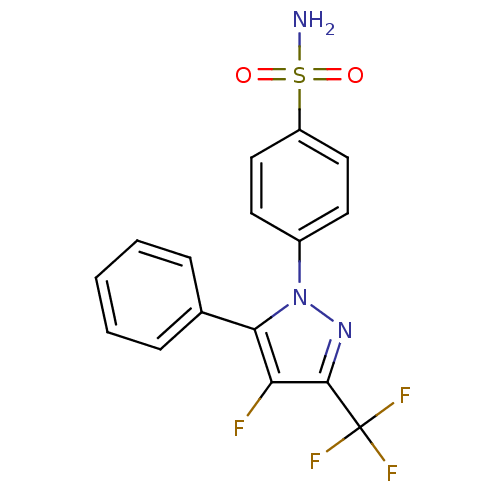
(4-(4-Fluoro-5-phenyl-3-trifluoromethyl-pyrazol-1-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c(F)c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-13-14(10-4-2-1-3-5-10)23(22-15(13)16(18,19)20)11-6-8-12(9-7-11)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057618
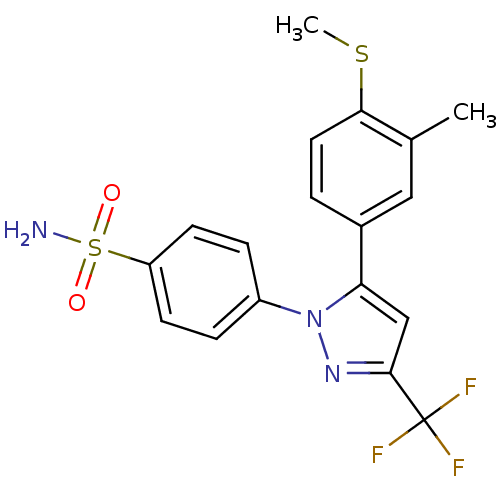
(4-[5-(3-Methyl-4-methylsulfanyl-phenyl)-3-trifluor...)Show SMILES CSc1ccc(cc1C)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O2S2/c1-11-9-12(3-8-16(11)27-2)15-10-17(18(19,20)21)23-24(15)13-4-6-14(7-5-13)28(22,25)26/h3-10H,1-2H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50546171
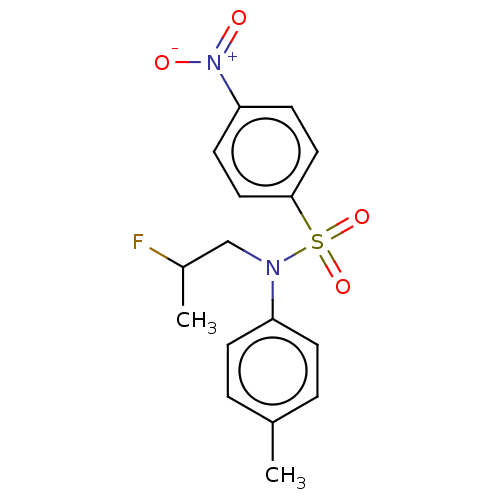
(CHEMBL4790443)Show SMILES CC(F)CN(c1ccc(C)cc1)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111731
BindingDB Entry DOI: 10.7270/Q24B34WR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057551
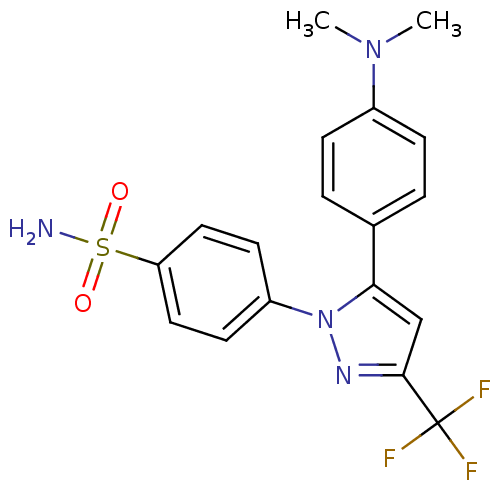
(4-[5-(4-Dimethylamino-phenyl)-3-trifluoromethyl-py...)Show SMILES CN(C)c1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H17F3N4O2S/c1-24(2)13-5-3-12(4-6-13)16-11-17(18(19,20)21)23-25(16)14-7-9-15(10-8-14)28(22,26)27/h3-11H,1-2H3,(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50553495

(CHEMBL4786559)Show SMILES COC[C@H](C)N[C@H]1CC[C@@H](CC1)Nc1cc(c(Cl)cn1)-c1cccc(NCC2(CCOCC2)C#N)n1 |r,wU:9.12,wD:6.5,3.3,(4.26,-18.37,;5.59,-17.61,;6.92,-18.38,;8.26,-17.62,;8.26,-16.08,;9.59,-18.39,;10.93,-17.63,;12.26,-18.4,;13.6,-17.63,;13.59,-16.1,;12.26,-15.32,;10.93,-16.09,;14.93,-15.33,;16.26,-16.1,;17.59,-15.33,;18.93,-16.09,;18.93,-17.64,;20.27,-18.41,;17.59,-18.42,;16.26,-17.65,;20.25,-15.32,;20.24,-13.79,;21.57,-13.01,;22.91,-13.77,;22.92,-15.32,;24.25,-16.08,;25.59,-15.31,;26.92,-16.07,;25.58,-16.83,;25.57,-18.36,;26.9,-19.14,;28.24,-18.38,;28.25,-16.84,;28.25,-15.3,;29.58,-14.52,;21.59,-16.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50057554
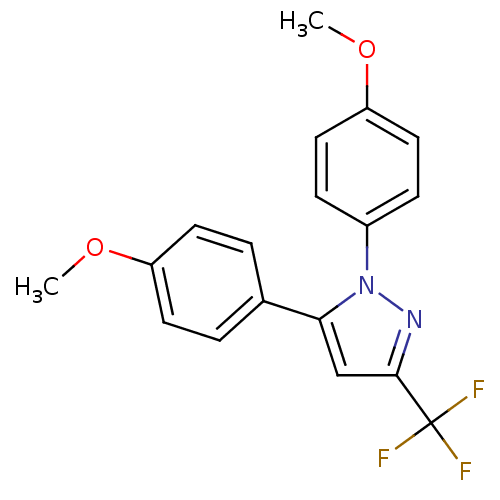
(1,5-Bis-(4-methoxy-phenyl)-3-trifluoromethyl-1H-py...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(OC)cc1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O2/c1-24-14-7-3-12(4-8-14)16-11-17(18(19,20)21)22-23(16)13-5-9-15(25-2)10-6-13/h3-11H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057606
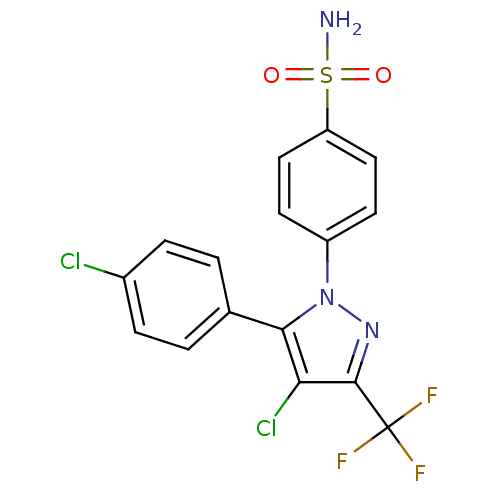
(4-[4-Chloro-5-(4-chloro-phenyl)-3-trifluoromethyl-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c(Cl)c1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H10Cl2F3N3O2S/c17-10-3-1-9(2-4-10)14-13(18)15(16(19,20)21)23-24(14)11-5-7-12(8-6-11)27(22,25)26/h1-8H,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578022
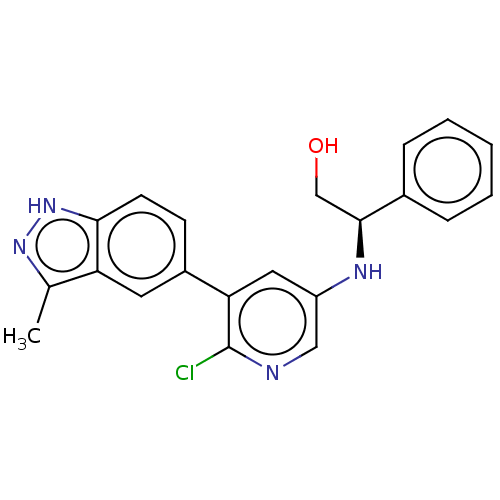
(CHEMBL4876497)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057575
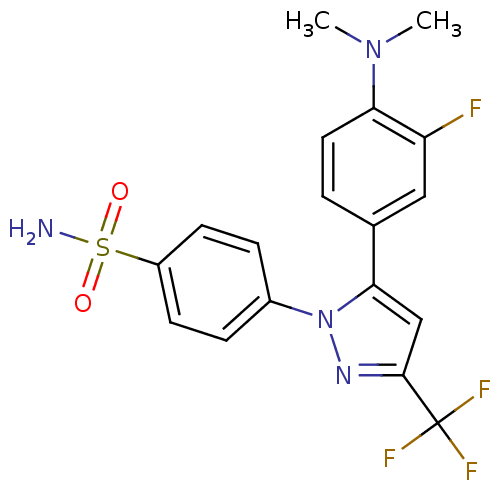
(4-[5-(4-Dimethylamino-3-fluoro-phenyl)-3-trifluoro...)Show SMILES CN(C)c1ccc(cc1F)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F4N4O2S/c1-25(2)15-8-3-11(9-14(15)19)16-10-17(18(20,21)22)24-26(16)12-4-6-13(7-5-12)29(23,27)28/h3-10H,1-2H3,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578008
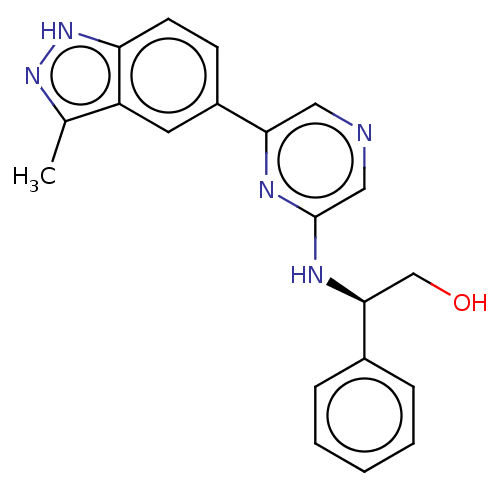
(CHEMBL4856767)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(N[C@@H](CO)c2ccccc2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578017
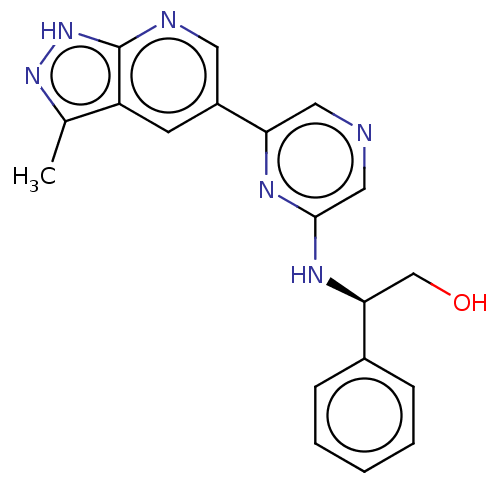
(CHEMBL4875272)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cncc(N[C@@H](CO)c2ccccc2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057609
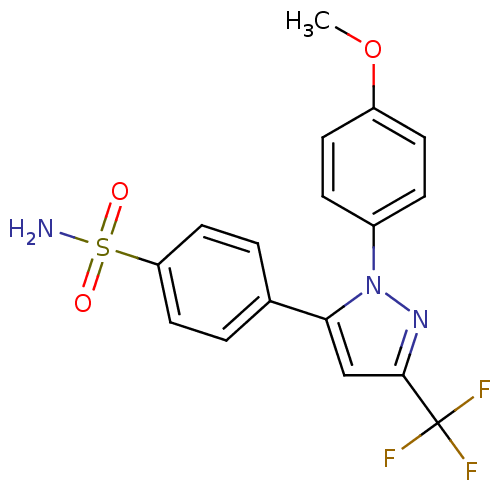
(4-[2-(4-Methoxy-phenyl)-5-trifluoromethyl-2H-pyraz...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-13-6-4-12(5-7-13)23-15(10-16(22-23)17(18,19)20)11-2-8-14(9-3-11)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13065

(5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C17H12ClF3N2O/c1-24-14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057589
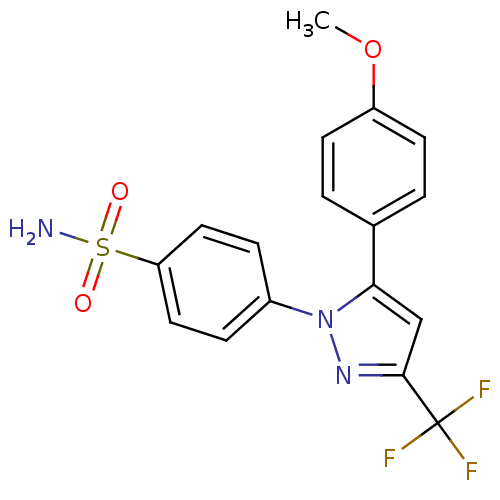
(4-[5-(4-Methoxy-phenyl)-3-trifluoromethyl-pyrazol-...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-13-6-2-11(3-7-13)15-10-16(17(18,19)20)22-23(15)12-4-8-14(9-5-12)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057591
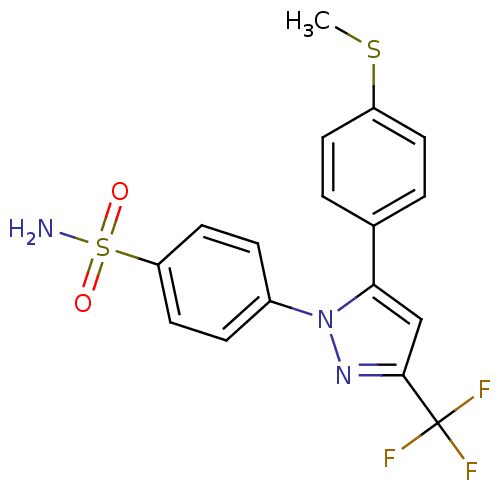
(4-[5-(4-Methylsulfanyl-phenyl)-3-trifluoromethyl-p...)Show SMILES CSc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S2/c1-26-13-6-2-11(3-7-13)15-10-16(17(18,19)20)22-23(15)12-4-8-14(9-5-12)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578014
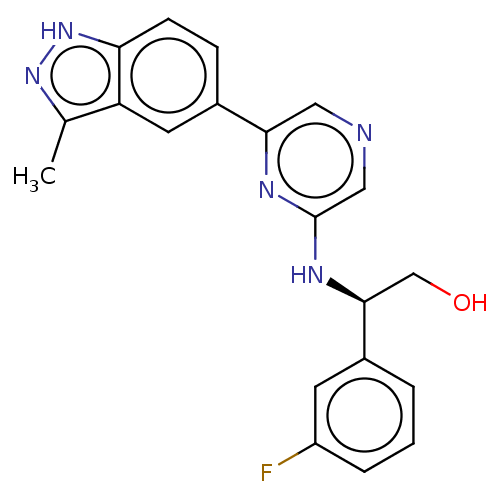
(CHEMBL4872952)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(N[C@@H](CO)c2cccc(F)c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057620
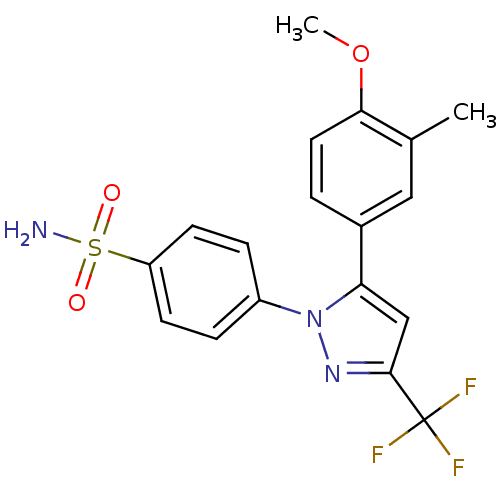
(4-[5-(4-Methoxy-3-methyl-phenyl)-3-trifluoromethyl...)Show SMILES COc1ccc(cc1C)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O3S/c1-11-9-12(3-8-16(11)27-2)15-10-17(18(19,20)21)23-24(15)13-4-6-14(7-5-13)28(22,25)26/h3-10H,1-2H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057572
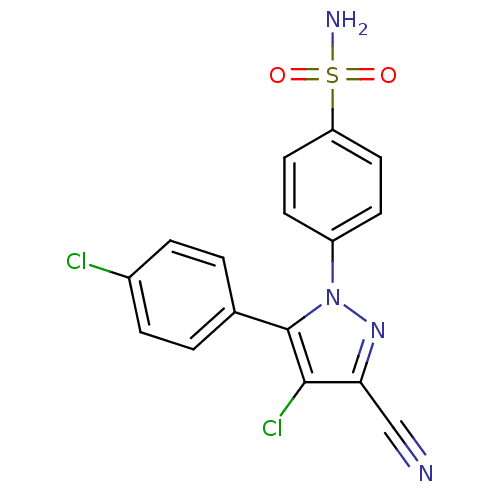
(4-[4-Chloro-5-(4-chloro-phenyl)-3-cyano-pyrazol-1-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(C#N)c(Cl)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H10Cl2N4O2S/c17-11-3-1-10(2-4-11)16-15(18)14(9-19)21-22(16)12-5-7-13(8-6-12)25(20,23)24/h1-8H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057602
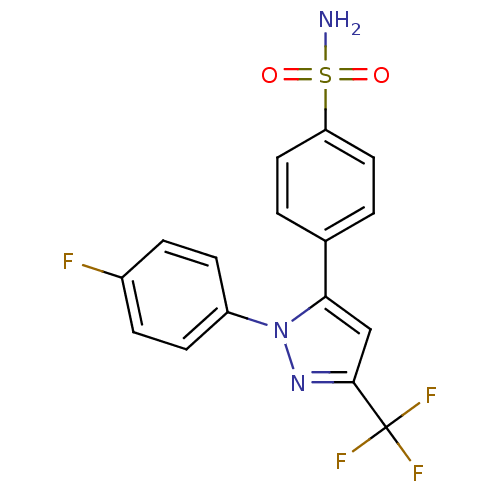
(4-[2-(4-Fluoro-phenyl)-5-trifluoromethyl-2H-pyrazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-11-3-5-12(6-4-11)23-14(9-15(22-23)16(18,19)20)10-1-7-13(8-2-10)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057610

(4-[5-(4-Chloro-phenyl)-3-difluoromethyl-pyrazol-1-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)F Show InChI InChI=1S/C16H12ClF2N3O2S/c17-11-3-1-10(2-4-11)15-9-14(16(18)19)21-22(15)12-5-7-13(8-6-12)25(20,23)24/h1-9,16H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057527
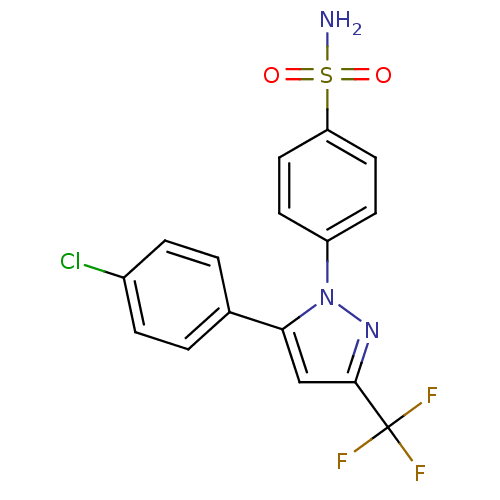
(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057552

(4-[5-(5-Bromo-thiophen-2-yl)-3-trifluoromethyl-pyr...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Br)s1)C(F)(F)F Show InChI InChI=1S/C14H9BrF3N3O2S2/c15-13-6-5-11(24-13)10-7-12(14(16,17)18)20-21(10)8-1-3-9(4-2-8)25(19,22)23/h1-7H,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057608
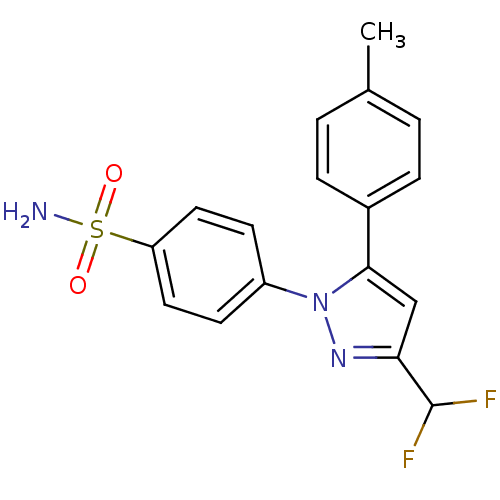
(4-(3-Difluoromethyl-5-p-tolyl-pyrazol-1-yl)-benzen...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O2S/c1-11-2-4-12(5-3-11)16-10-15(17(18)19)21-22(16)13-6-8-14(9-7-13)25(20,23)24/h2-10,17H,1H3,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578001
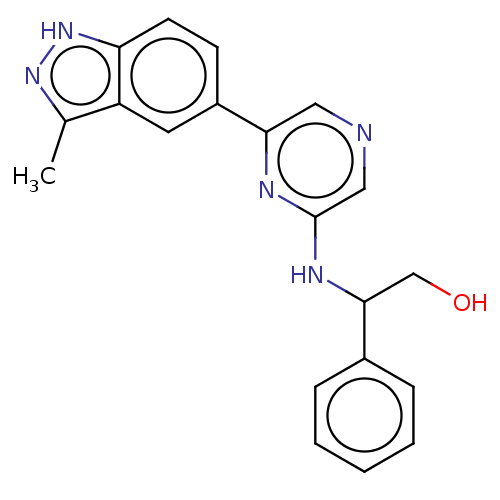
(CHEMBL4855785)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(NC(CO)c2ccccc2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057562

(4-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O3S/c1-25-13-6-2-11(3-7-13)16-10-15(17(18)19)21-22(16)12-4-8-14(9-5-12)26(20,23)24/h2-10,17H,1H3,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057560
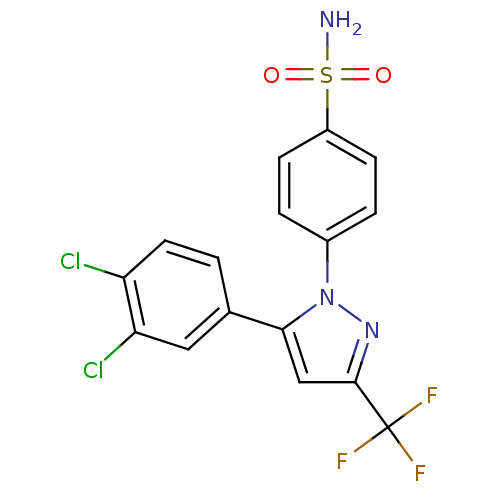
(4-[5-(3,4-Dichloro-phenyl)-3-trifluoromethyl-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C16H10Cl2F3N3O2S/c17-12-6-1-9(7-13(12)18)14-8-15(16(19,20)21)23-24(14)10-2-4-11(5-3-10)27(22,25)26/h1-8H,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057573
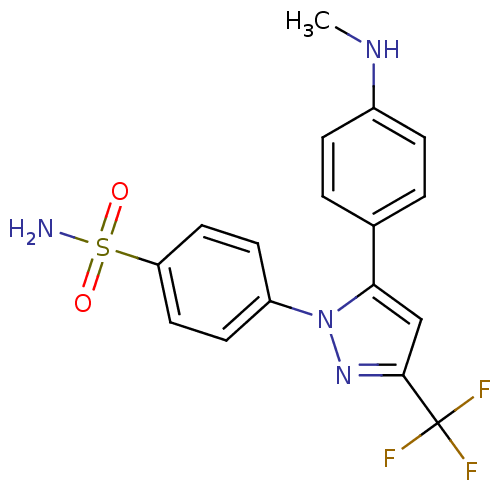
(4-[5-(4-Methylamino-phenyl)-3-trifluoromethyl-pyra...)Show SMILES CNc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c1-22-12-4-2-11(3-5-12)15-10-16(17(18,19)20)23-24(15)13-6-8-14(9-7-13)27(21,25)26/h2-10,22H,1H3,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50057532

(1-(4-Chloro-phenyl)-5-(4-methoxy-phenyl)-3-trifluo...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C17H12ClF3N2O/c1-24-14-8-2-11(3-9-14)15-10-16(17(19,20)21)22-23(15)13-6-4-12(18)5-7-13/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578010
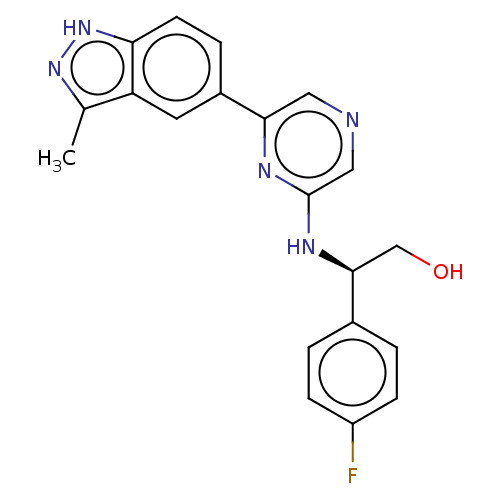
(CHEMBL4860265)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(N[C@@H](CO)c2ccc(F)cc2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057592
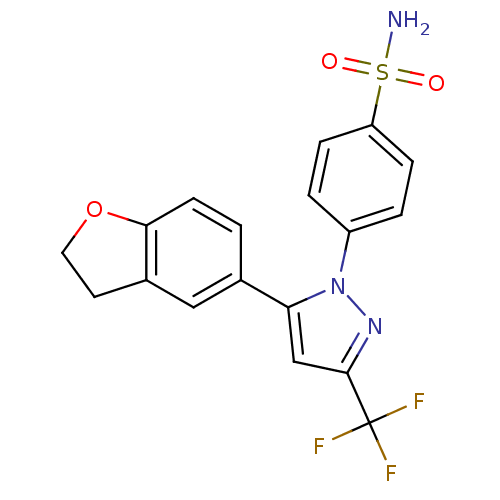
(4-[5-(2,3-Dihydro-benzofuran-5-yl)-3-trifluorometh...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc2OCCc2c1)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O3S/c19-18(20,21)17-10-15(11-1-6-16-12(9-11)7-8-27-16)24(23-17)13-2-4-14(5-3-13)28(22,25)26/h1-6,9-10H,7-8H2,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578021
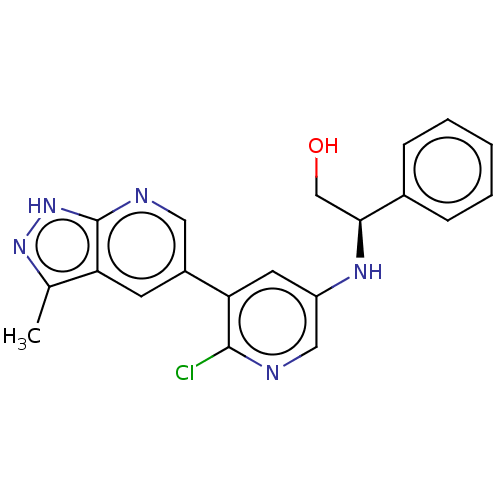
(CHEMBL4857229)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057544
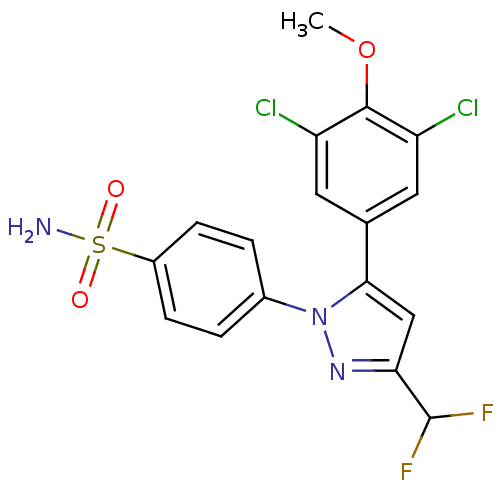
(4-[5-(3,5-Dichloro-4-methoxy-phenyl)-3-difluoromet...)Show SMILES COc1c(Cl)cc(cc1Cl)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H13Cl2F2N3O3S/c1-27-16-12(18)6-9(7-13(16)19)15-8-14(17(20)21)23-24(15)10-2-4-11(5-3-10)28(22,25)26/h2-8,17H,1H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057553
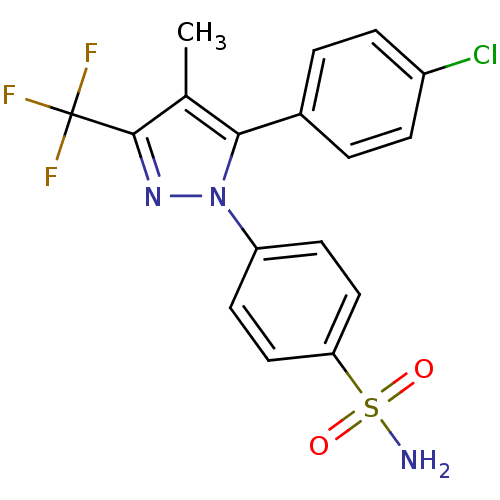
(4-[5-(4-Chloro-phenyl)-4-methyl-3-trifluoromethyl-...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H13ClF3N3O2S/c1-10-15(11-2-4-12(18)5-3-11)24(23-16(10)17(19,20)21)13-6-8-14(9-7-13)27(22,25)26/h2-9H,1H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578007
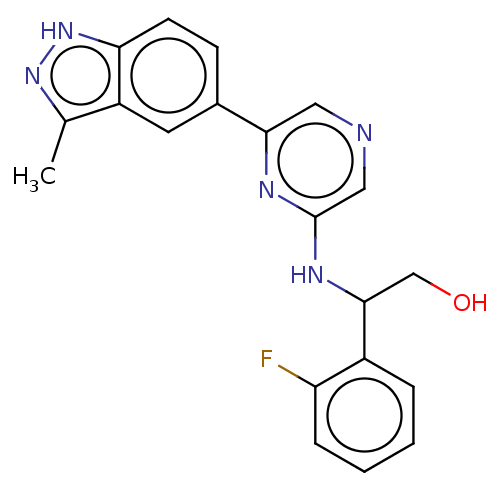
(CHEMBL4848043)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(NC(CO)c2ccccc2F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057600
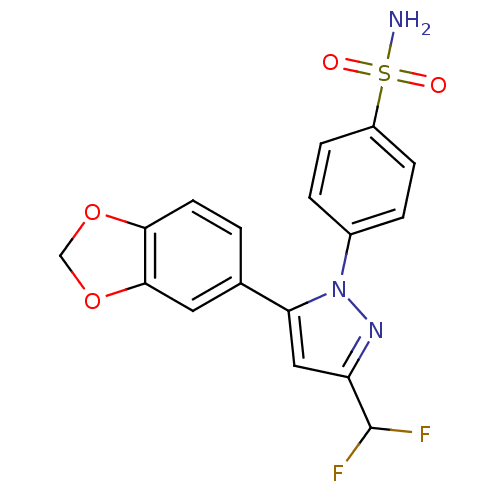
(4-(5-Benzo[1,3]dioxol-5-yl-3-difluoromethyl-pyrazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc2OCOc2c1)C(F)F Show InChI InChI=1S/C17H13F2N3O4S/c18-17(19)13-8-14(10-1-6-15-16(7-10)26-9-25-15)22(21-13)11-2-4-12(5-3-11)27(20,23)24/h1-8,17H,9H2,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578013
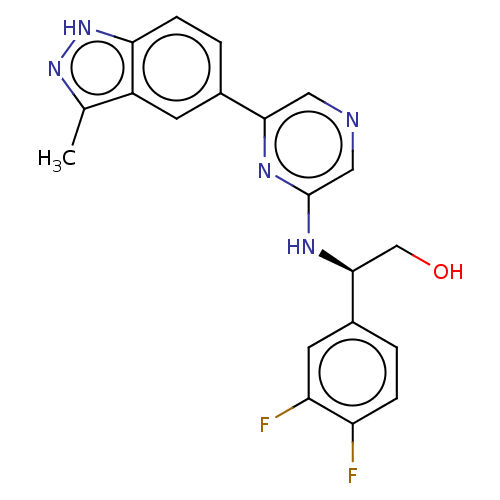
(CHEMBL4871323)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(N[C@@H](CO)c2ccc(F)c(F)c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057605
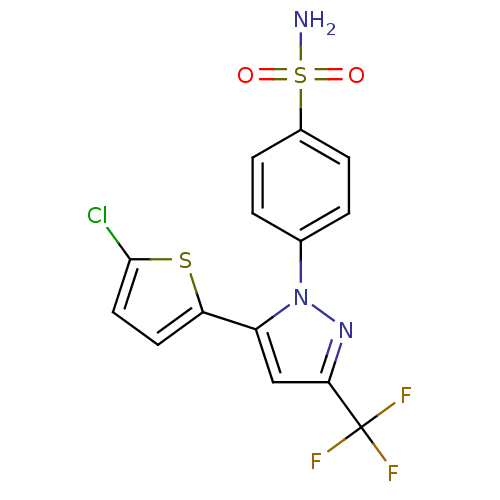
(4-[5-(5-Chloro-thiophen-2-yl)-3-trifluoromethyl-py...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)s1)C(F)(F)F Show InChI InChI=1S/C14H9ClF3N3O2S2/c15-13-6-5-11(24-13)10-7-12(14(16,17)18)20-21(10)8-1-3-9(4-2-8)25(19,22)23/h1-7H,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578023
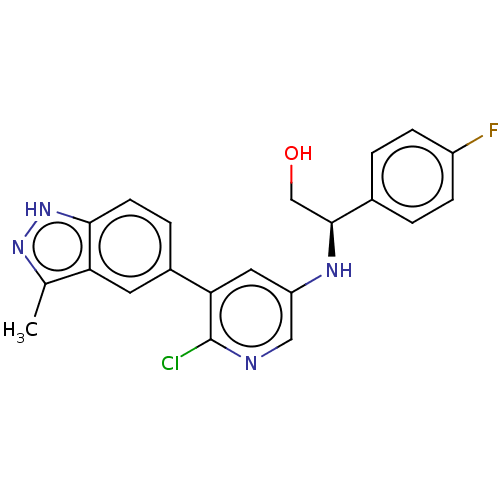
(CHEMBL4864763)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccc(F)cc2)cnc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50578003
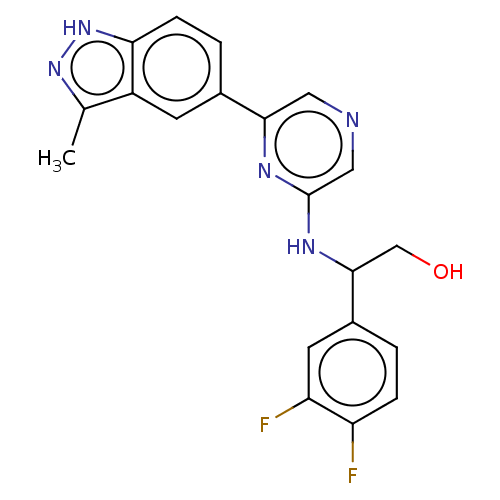
(CHEMBL4856958)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(NC(CO)c2ccc(F)c(F)c2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) incubated for 2 hrs in presence of ATP by FRET based LANCE assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01148
BindingDB Entry DOI: 10.7270/Q2154MVT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057596
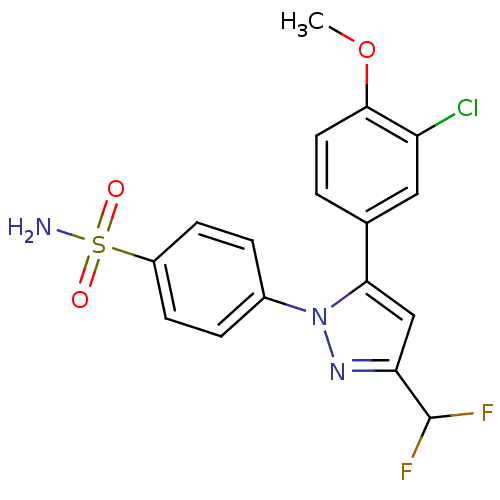
(4-[5-(3-Chloro-4-methoxy-phenyl)-3-difluoromethyl-...)Show SMILES COc1ccc(cc1Cl)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H14ClF2N3O3S/c1-26-16-7-2-10(8-13(16)18)15-9-14(17(19)20)22-23(15)11-3-5-12(6-4-11)27(21,24)25/h2-9,17H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057541
(4-[5-(3-Chloro-4-methylamino-phenyl)-3-trifluorome...)Show SMILES CNc1ccc(cc1Cl)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14ClF3N4O2S/c1-23-14-7-2-10(8-13(14)18)15-9-16(17(19,20)21)24-25(15)11-3-5-12(6-4-11)28(22,26)27/h2-9,23H,1H3,(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057528
(4-[5-(4-Chloro-phenyl)-4-ethyl-3-trifluoromethyl-p...)Show SMILES CCc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H15ClF3N3O2S/c1-2-15-16(11-3-5-12(19)6-4-11)25(24-17(15)18(20,21)22)13-7-9-14(10-8-13)28(23,26)27/h3-10H,2H2,1H3,(H2,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data