

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095997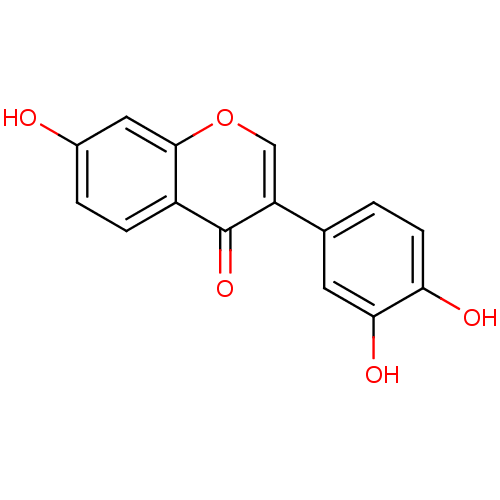 (3',4',7-trihydroxyisoflavone | CHEMBL13486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096004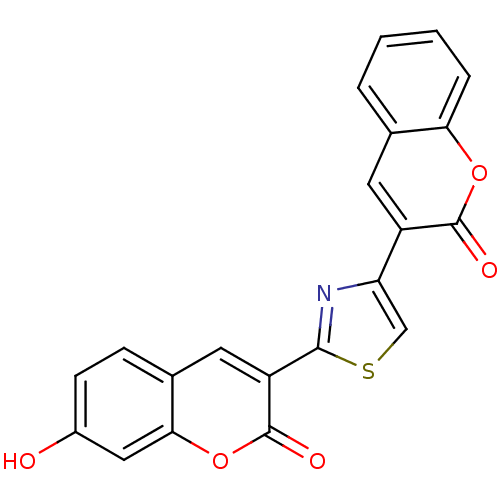 (7-Hydroxy-3-[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096003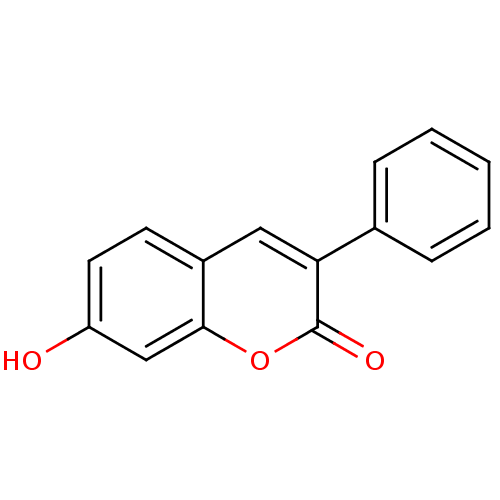 (7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096001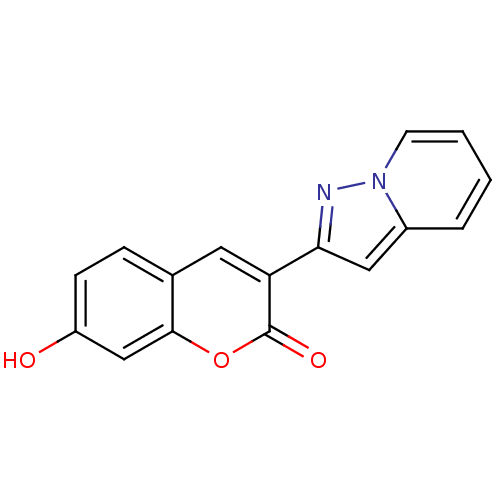 (7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095993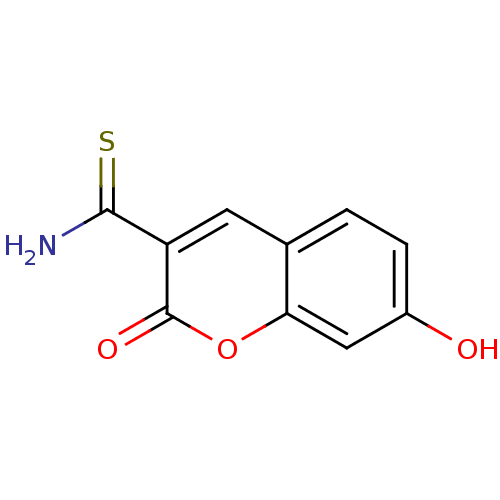 (7-Hydroxy-2-oxo-2H-chromene-3-carbothioic acid ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096002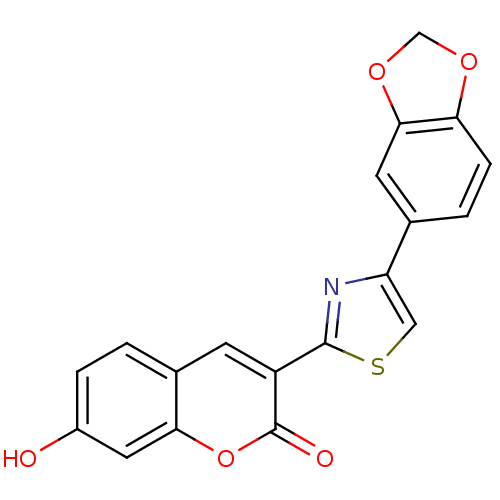 (3-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-7-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096007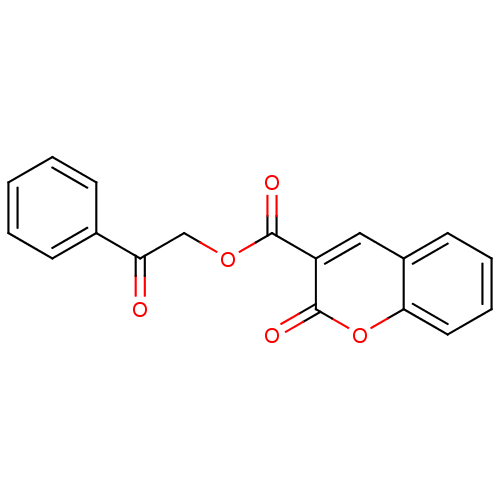 (2-Oxo-2H-chromene-3-carboxylic acid 2-oxo-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096006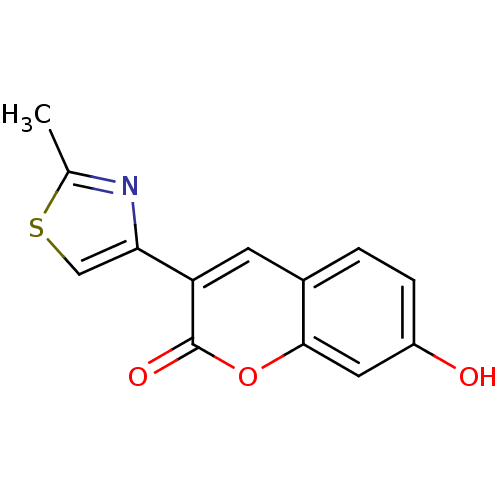 (7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096000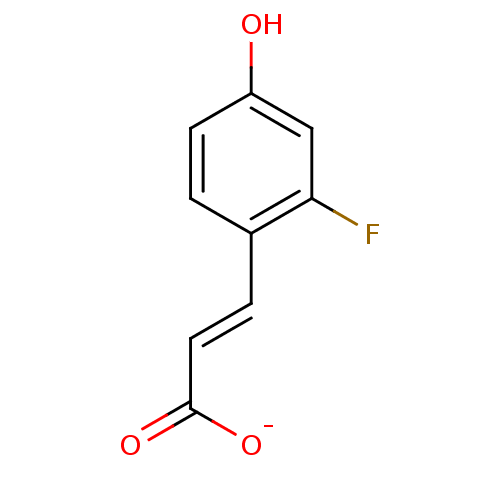 (3-(2-Fluoro-4-hydroxy-phenyl)-acrylic acid anion) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095994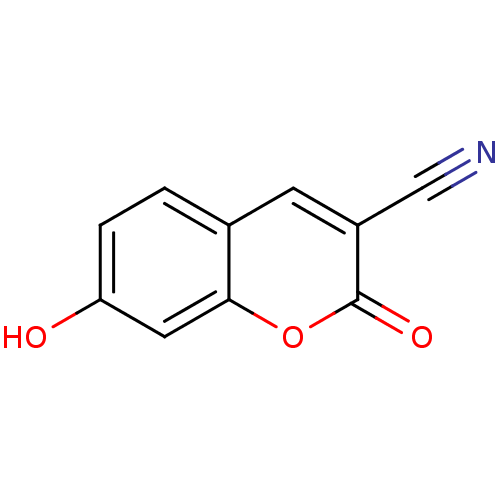 (3-Cyano-7-hydroxycoumarin (2) | 7-Hydroxy-2-oxo-2H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096005 (7-Hydroxy-3-(4-methyl-thiazol-2-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096008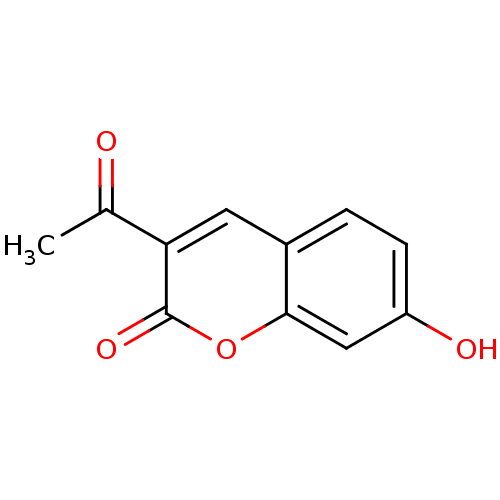 (3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095996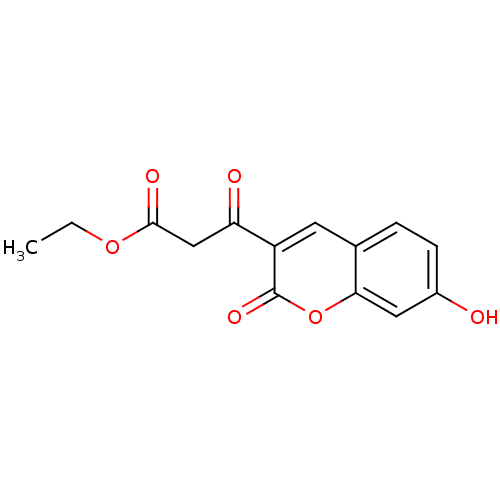 (3-(7-Hydroxy-2-oxo-2H-chromen-3-yl)-3-oxo-propioni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096009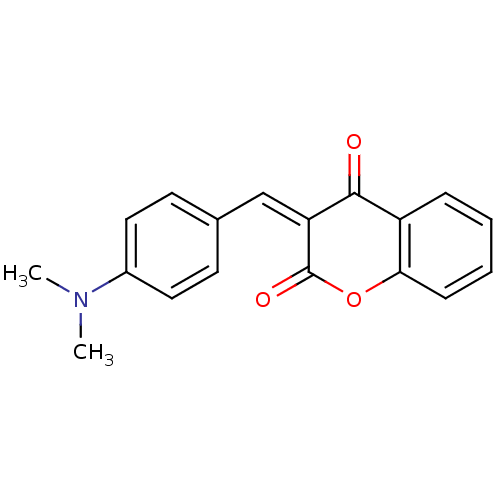 (3-(4-Dimethylamino-benzylidene)-chroman-2,4-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095995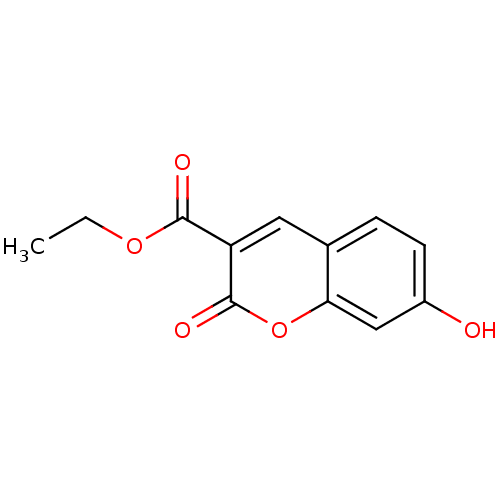 (7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273203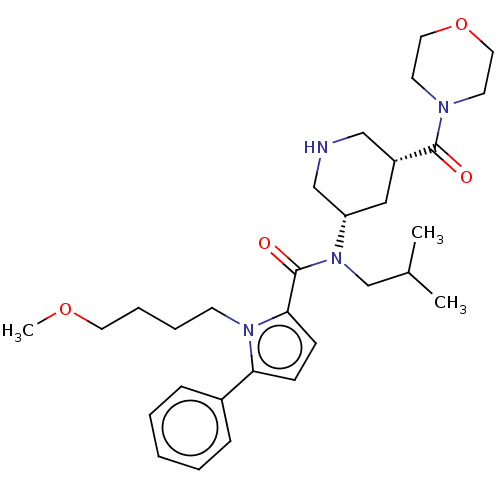 (CHEMBL4128929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451372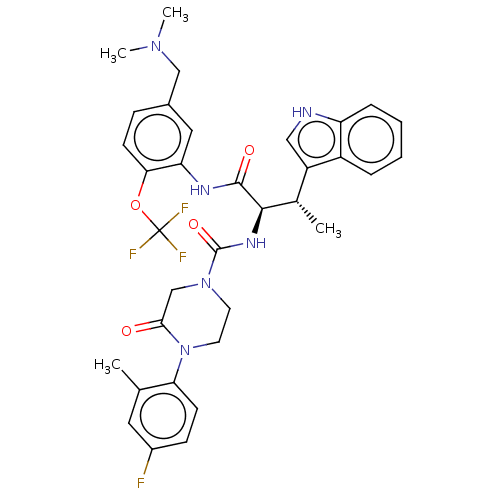 (CHEMBL4217405) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273163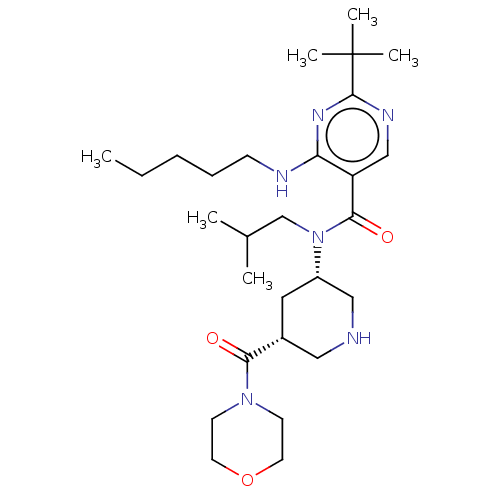 (CHEMBL4126136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451373 (CHEMBL4206924) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451391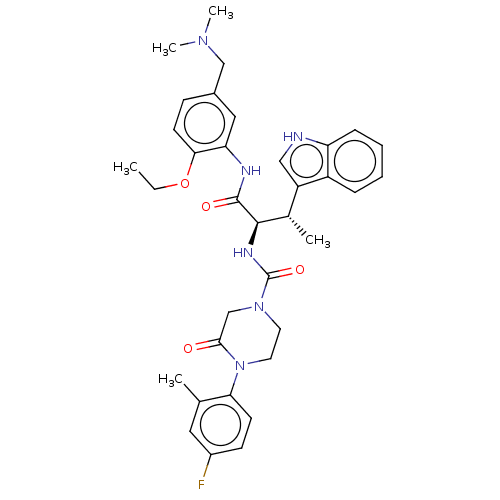 (CHEMBL4212088) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451392 (CHEMBL4214725) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451394 (CHEMBL4205606) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451384 (CHEMBL4210064) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273198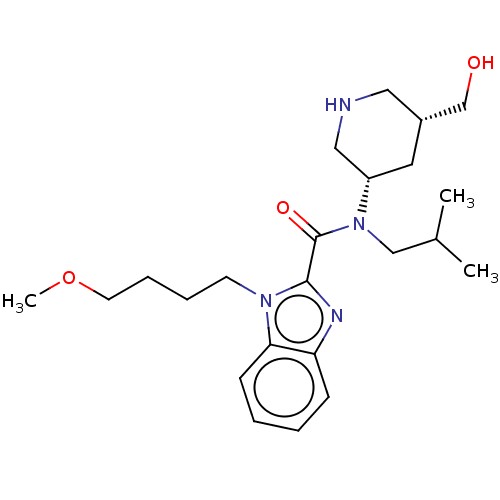 (CHEMBL4130200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451385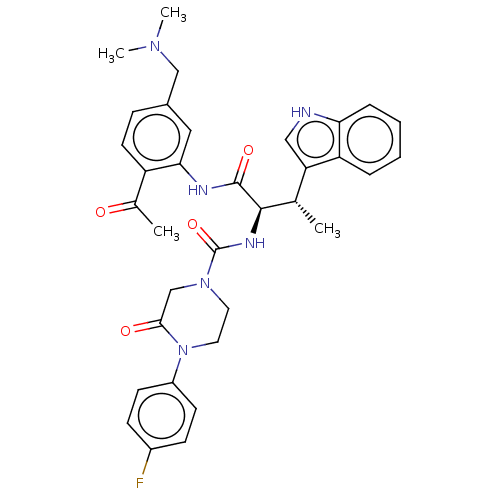 (CHEMBL4205969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273198 (CHEMBL4130200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195782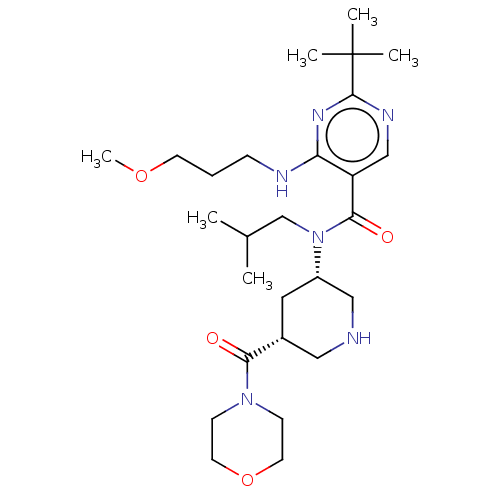 (CHEMBL3958809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273202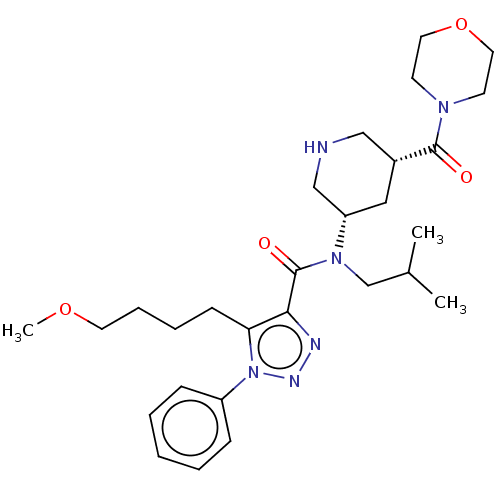 (CHEMBL4129081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273160 (IMARIKIREN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451382 (CHEMBL4205696) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451399 (CHEMBL4208528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273174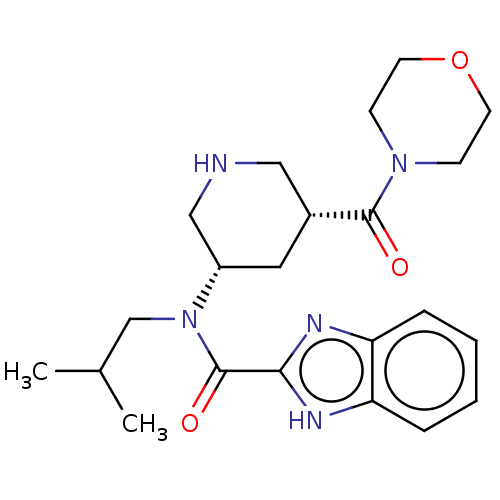 (CHEMBL4125900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451395 (CHEMBL4211422) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273161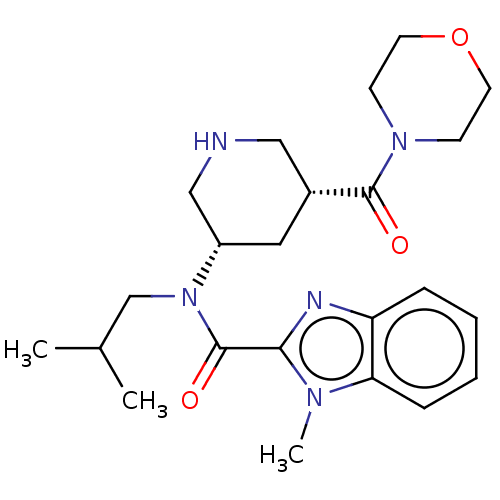 (CHEMBL4127179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273163 (CHEMBL4126136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273202 (CHEMBL4129081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273161 (CHEMBL4127179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273172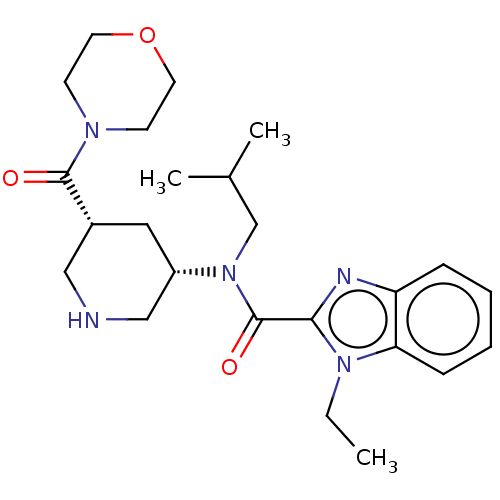 (CHEMBL4129865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451390 (CHEMBL4210627) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451388 (CHEMBL4215053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273162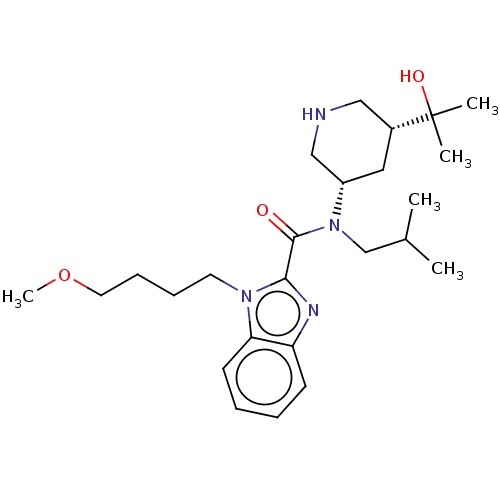 (CHEMBL4128795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273174 (CHEMBL4125900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195782 (CHEMBL3958809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273173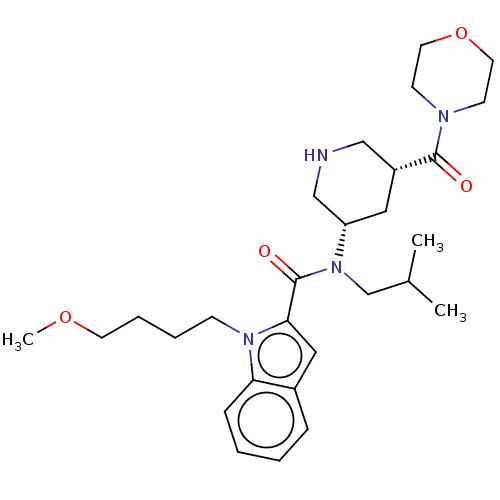 (CHEMBL4129207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273170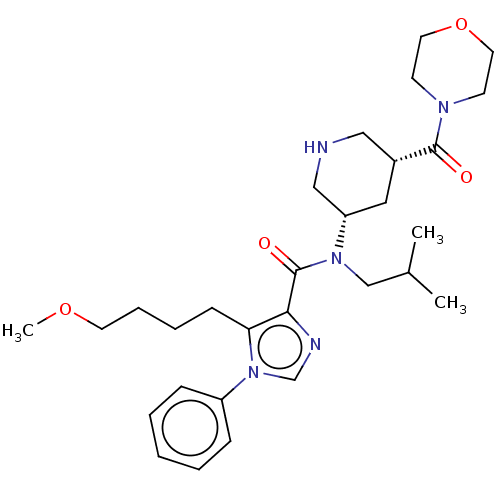 (CHEMBL4130109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273160 (IMARIKIREN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273172 (CHEMBL4129865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50451396 (CHEMBL4216387) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ... | Bioorg Med Chem 25: 5995-6006 (2017) Article DOI: 10.1016/j.bmc.2017.09.031 BindingDB Entry DOI: 10.7270/Q2PR7ZK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273199 (CHEMBL4128438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 405 total ) | Next | Last >> |