

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143028 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143032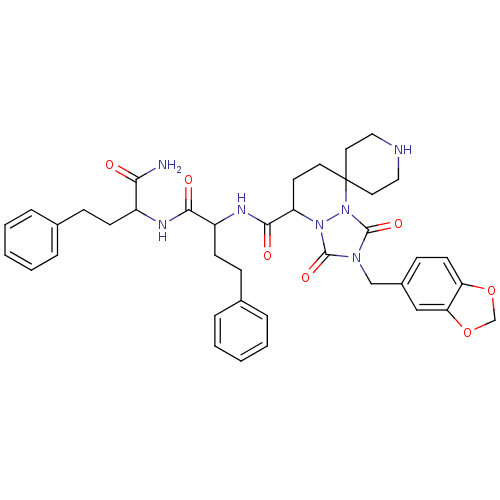 (8'N-[1-(1-carbamoyl-3-phenylpropylcarbamoyl)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition full length human recombinant HDAC2 expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143038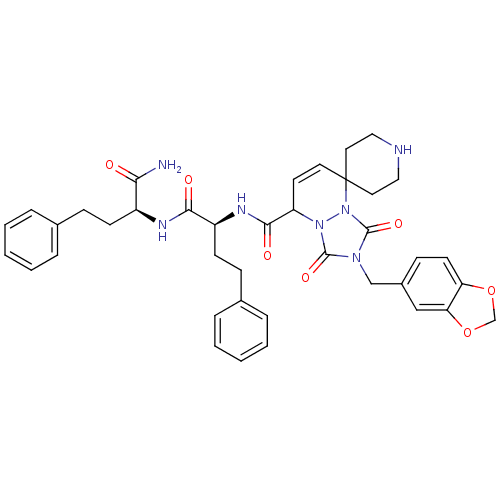 (8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143039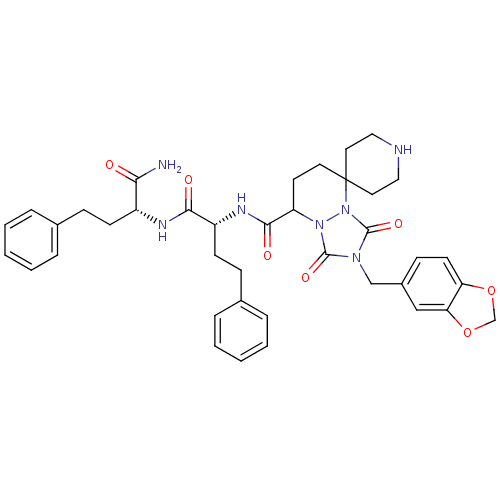 (8'N-[1-[1-carbamoyl-3-phenyl-(1R)-propylcarbamoyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150169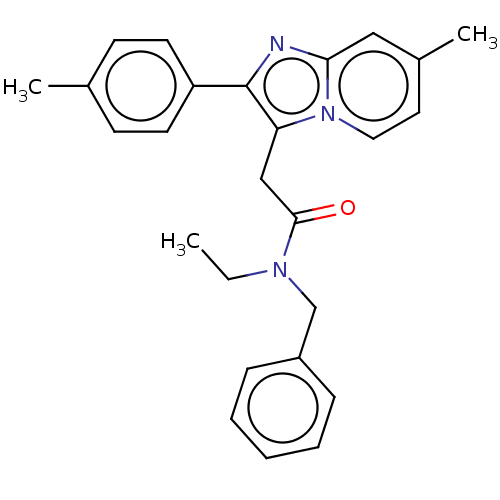 (US8980887, Compound 13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.97 | -49.5 | 6.99 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50143037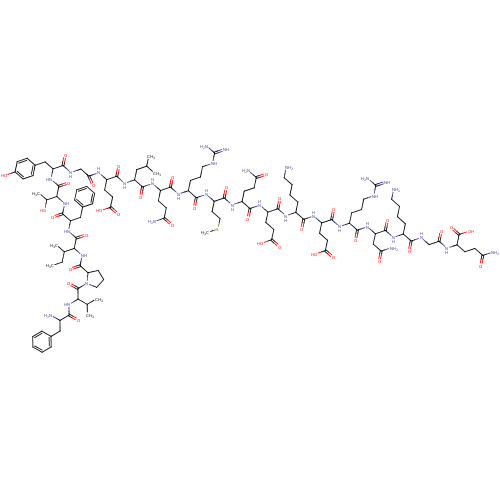 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50344952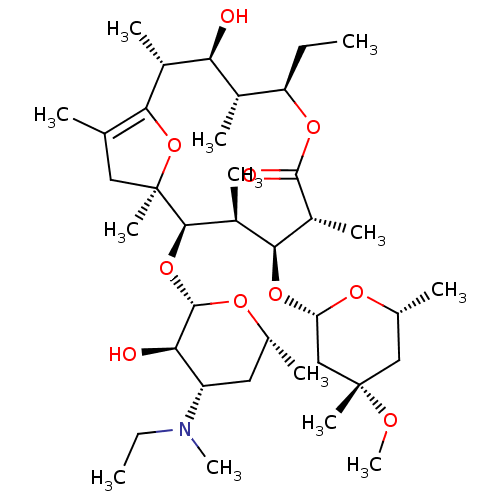 ((2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity towards human motilin receptor | J Med Chem 47: 1704-8 (2004) Article DOI: 10.1021/jm0304865 BindingDB Entry DOI: 10.7270/Q2571CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952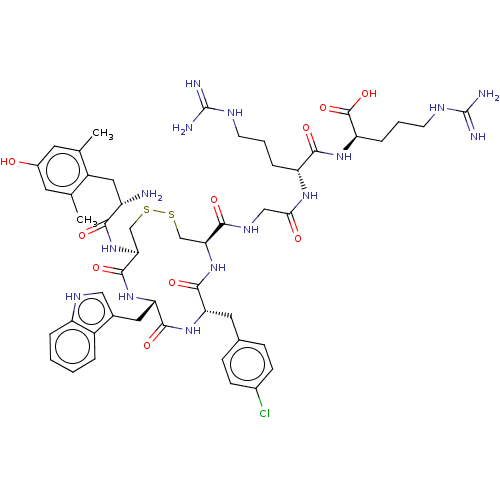 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953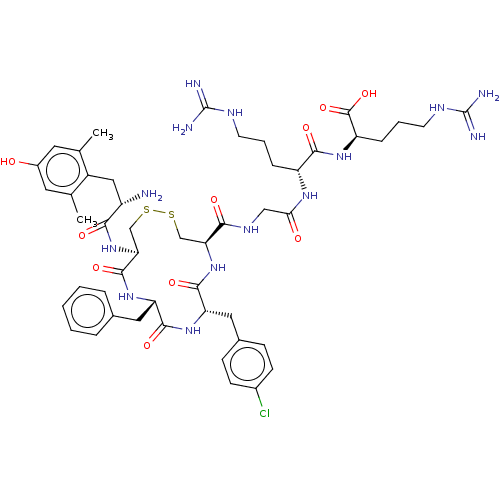 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150168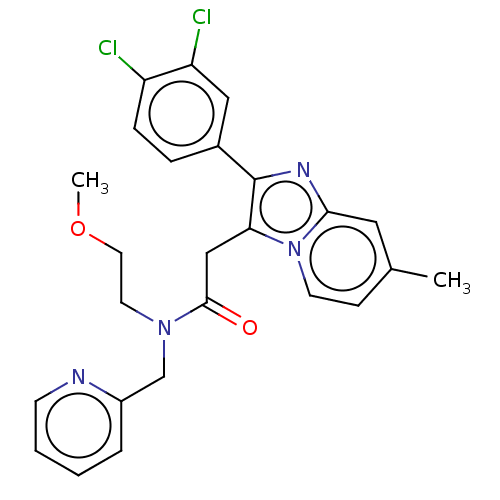 (US8980887, Compound 11) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.66 | -47.5 | 15.6 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150164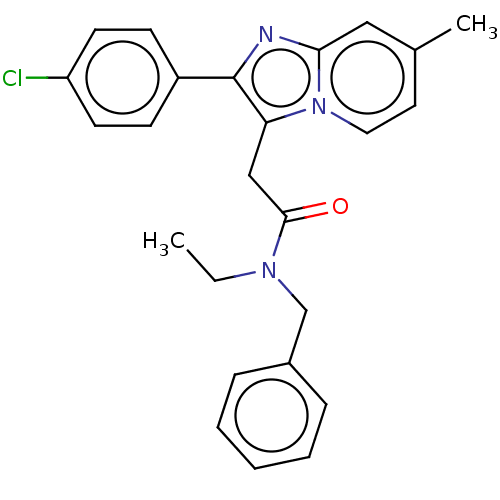 (US8980887, Compound 1) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.17 | -47.3 | 16.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150166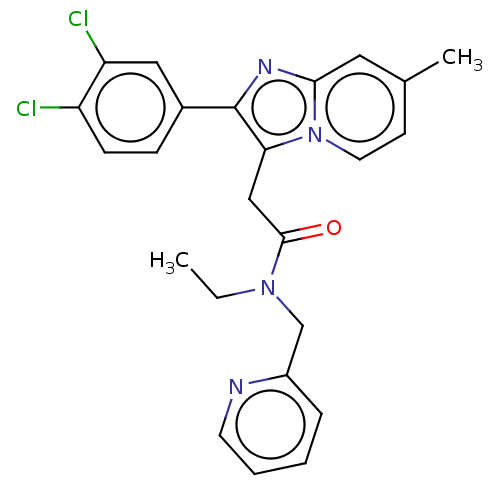 (US8980887, Compound 8) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.34 | -46.6 | 21.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150167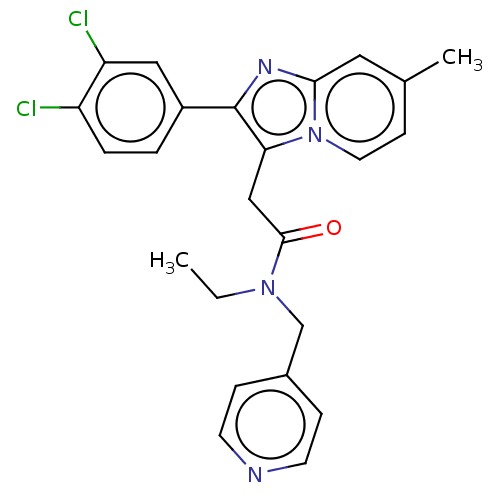 (US8980887, Compound 10) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11.1 | -46.2 | 26.1 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50198782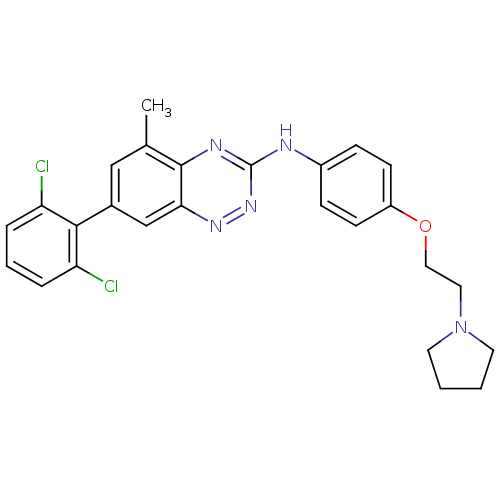 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Yes kinase | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl kinase | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lyn | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Src | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150170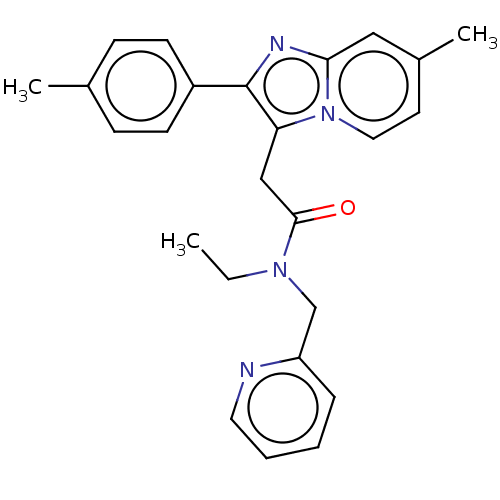 (US8980887, Compound 14) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 38.7 | -43.0 | 91.0 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579948 (CHEMBL5080666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579949 (CHEMBL5084034) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM150165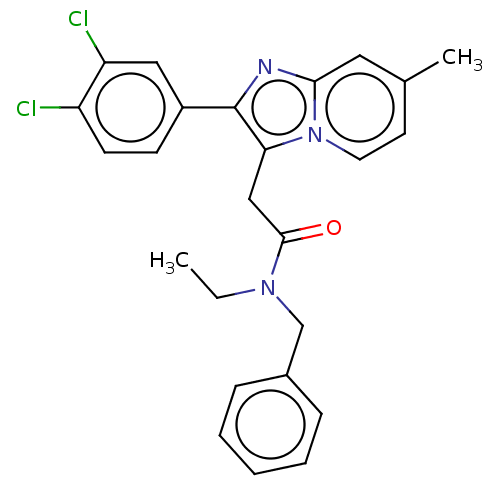 (US8980887, Compound 7) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 49.2 | -42.4 | 116 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China US Patent | Assay Description Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... | US Patent US8980887 (2015) BindingDB Entry DOI: 10.7270/Q2D50KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 4 (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of EphB4 | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579951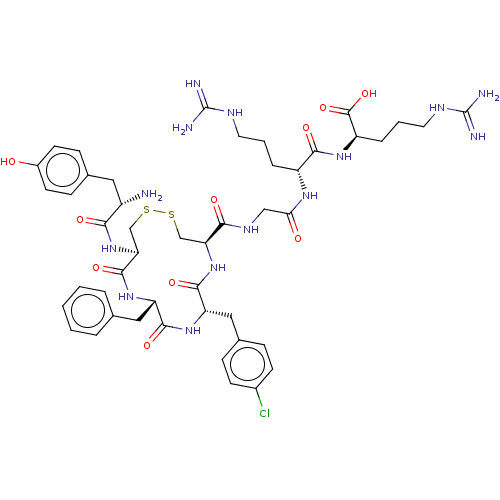 (CHEMBL5078349) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579950 (CHEMBL5080233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50189903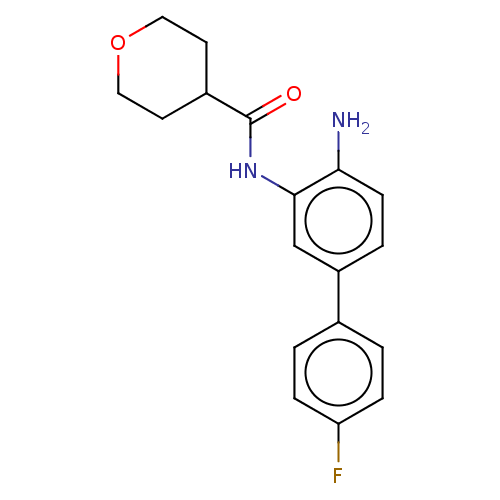 (CHEMBL3828396 | US11572368, Compound 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50189911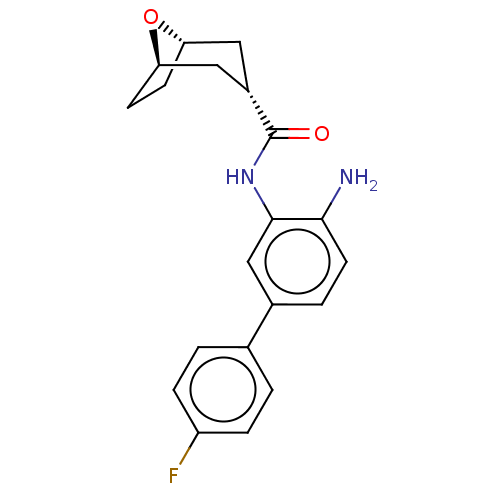 (CHEMBL3827611) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Ret | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of FGFR2 | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition full length human recombinant HDAC3 expressed in baculovirus using carboxyfluorescein (FAM)-labeled acetylated/ trifluoroacetylated peptid... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50189911 (CHEMBL3827611) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 519 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition full length human recombinant HDAC2 expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 784 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50189903 (CHEMBL3828396 | US11572368, Compound 54) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition full length human recombinant HDAC3 expressed in baculovirus using carboxyfluorescein (FAM)-labeled acetylated/ trifluoroacetylated peptid... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50198782 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579949 (CHEMBL5084034) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579948 (CHEMBL5080666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579951 (CHEMBL5078349) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579950 (CHEMBL5080233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 941 total ) | Next | Last >> |