

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512456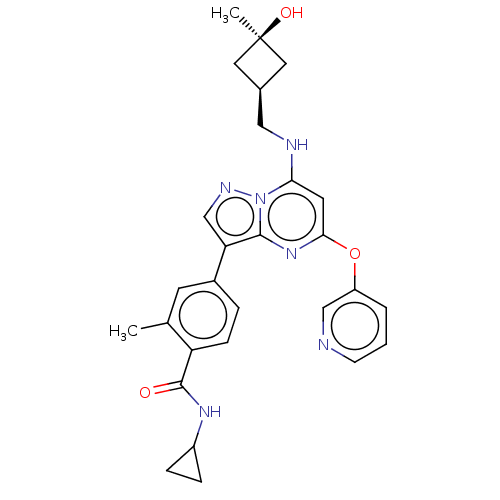 (CHEMBL4469414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512456 (CHEMBL4469414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075926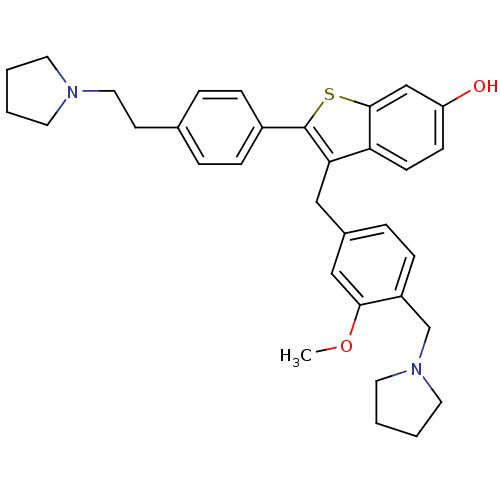 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934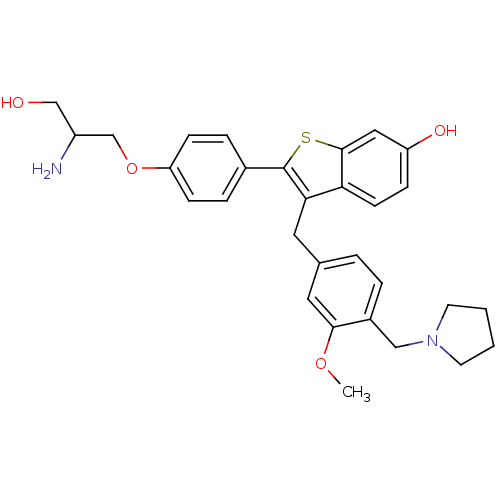 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928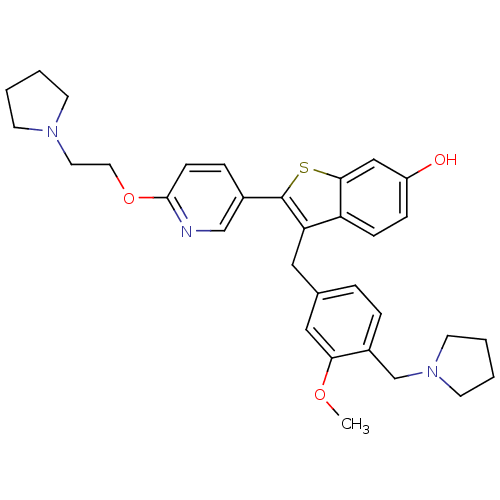 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937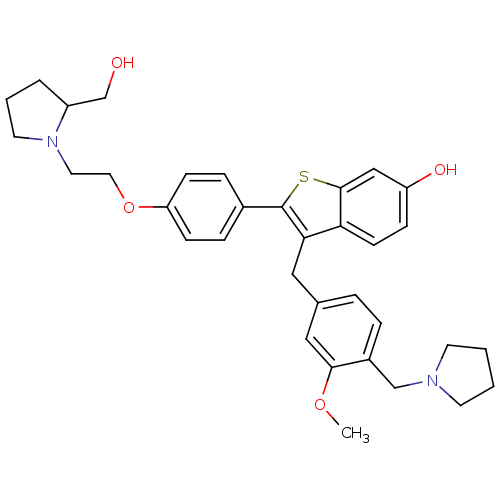 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081537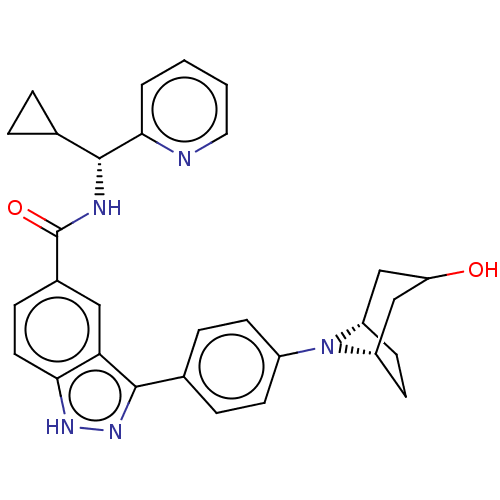 (CHEMBL3422092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Competitive inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate by Lineweaver-Burk plot analysis in prese... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938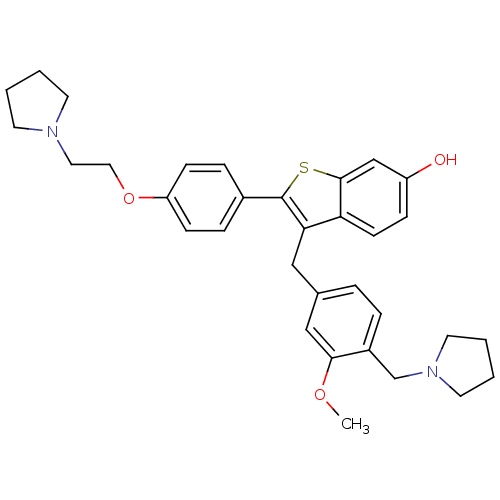 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075935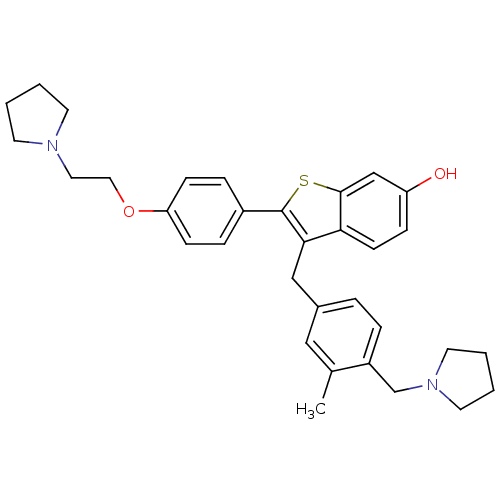 (3-(3-Methyl-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075931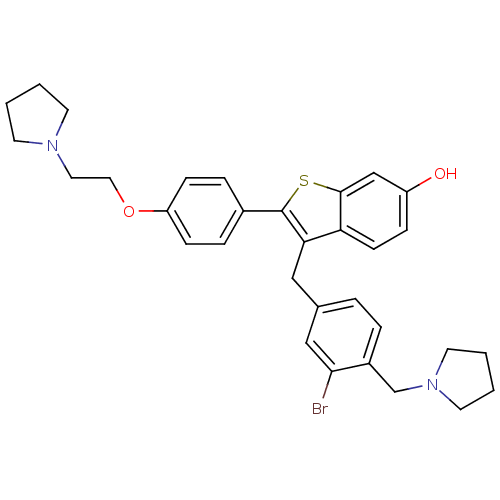 (3-(3-BROMO-4-PYRROLIDIN-1-YLMETHYL-BENZYL)-2-[4-PY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075939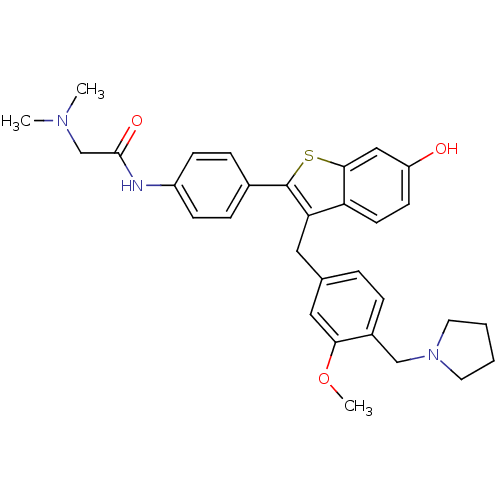 (2-Dimethylamino-N-{4-[6-hydroxy-3-(3-methoxy-4-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075927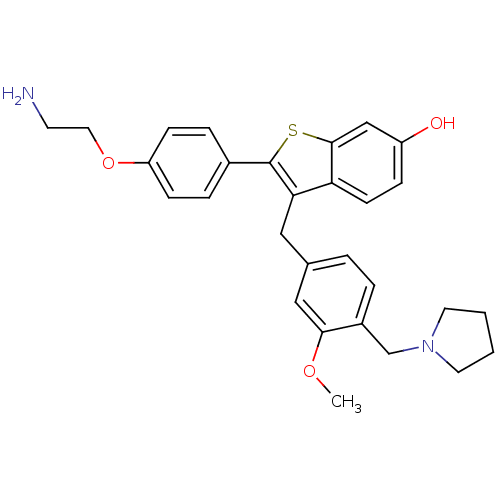 (2-[4-(2-Amino-ethoxy)-phenyl]-3-(3-methoxy-4-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075930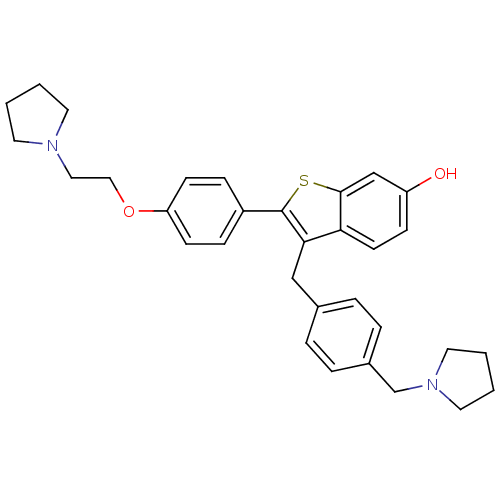 (2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-3-(4-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM25117 (AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of PLK4 (unknown origin) | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075941 (3-(3-Hydroxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075933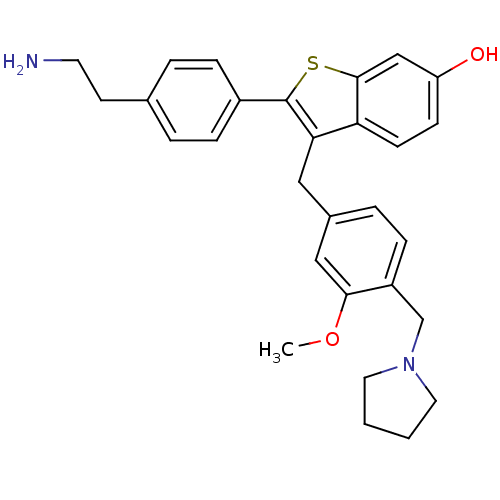 (2-[4-(2-Amino-ethyl)-phenyl]-3-(3-methoxy-4-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075929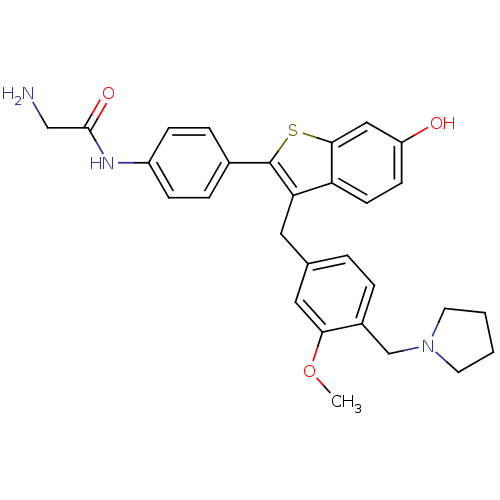 (2-Amino-N-{4-[6-hydroxy-3-(3-methoxy-4-pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075940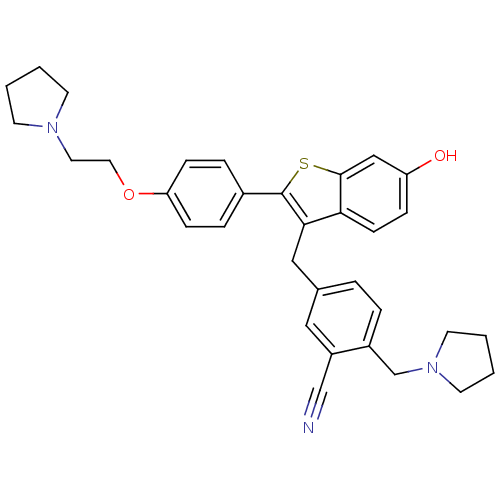 (5-{6-Hydroxy-2-[4-(2-pyrrolidin-1-yl-ethoxy)-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075936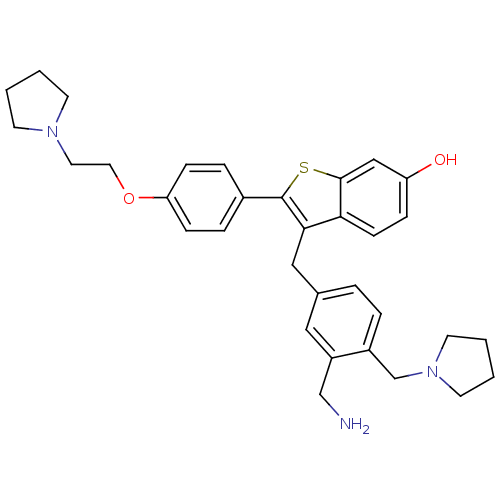 (3-(3-Aminomethyl-4-pyrrolidin-1-ylmethyl-benzyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of PLK4 (unknown origin) | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061308 ((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061305 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044653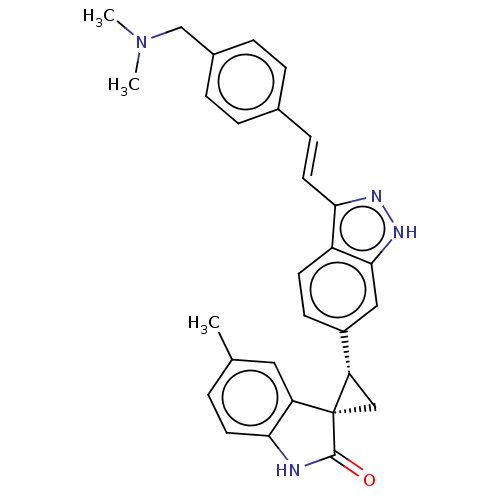 (CHEMBL3353351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044652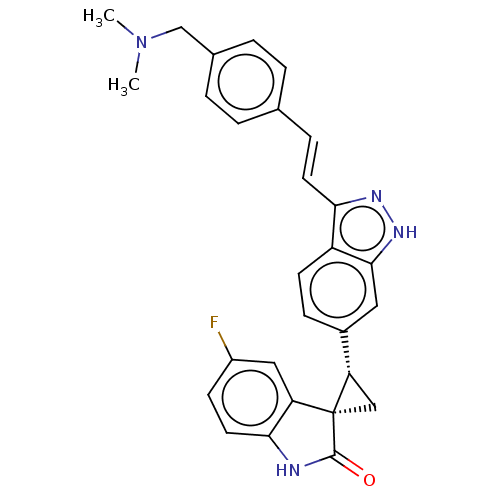 (CHEMBL3353352 | US10358436, Example A208 | US99078...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081539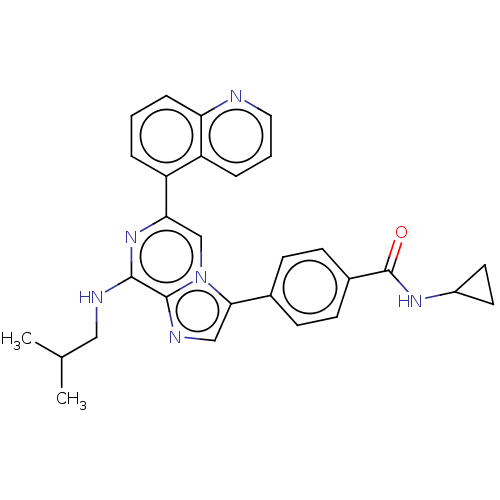 (CHEMBL3422104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human TTK | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044656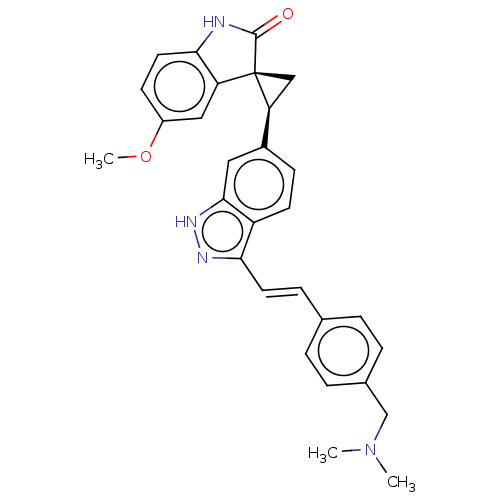 (CHEMBL3353348 | US10358436, Example A35 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50533129 (CHEMBL4526755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081597 (CHEMBL3422095) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081607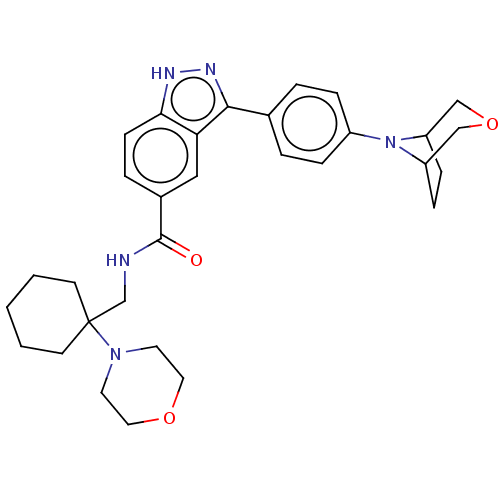 (CHEMBL3422080) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50533129 (CHEMBL4526755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044650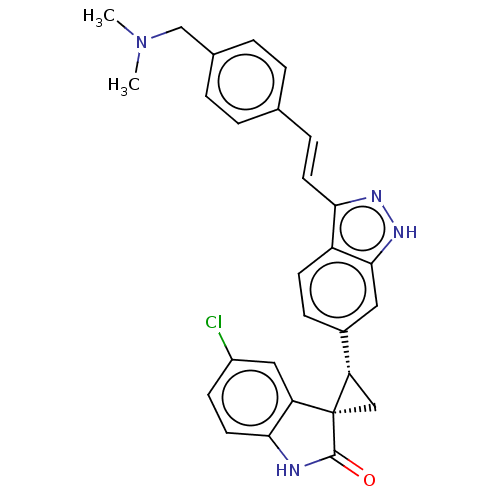 (CHEMBL3353350) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081574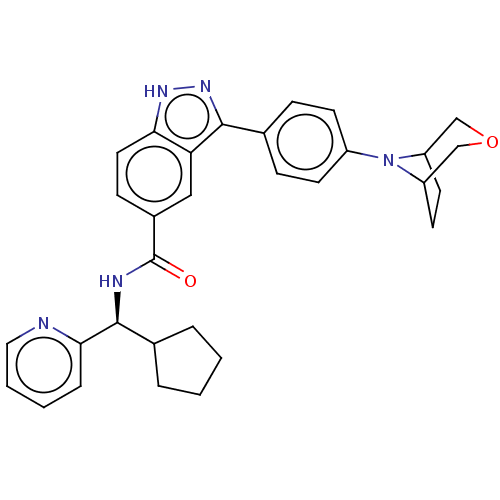 (CHEMBL3422086) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081578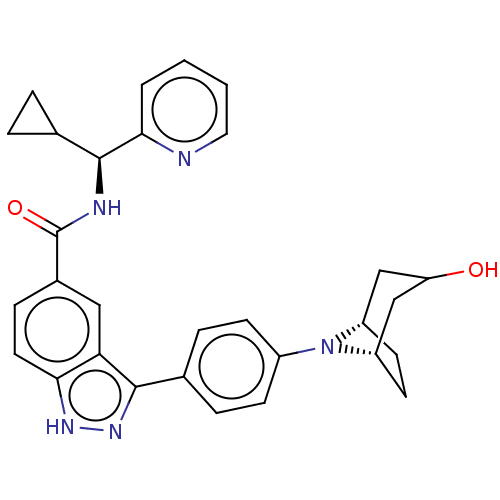 (CHEMBL3422091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081578 (CHEMBL3422091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044662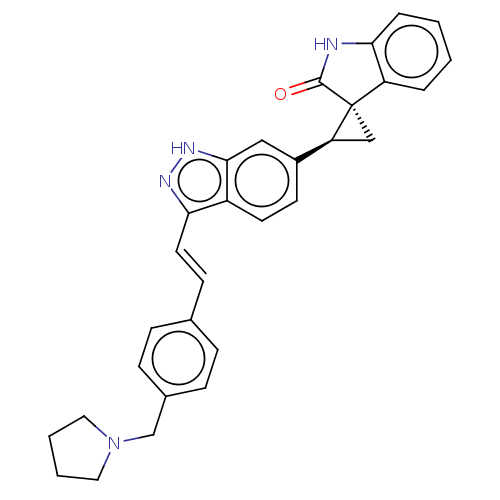 (CHEMBL3353361 | US10358436, Example A61 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50183019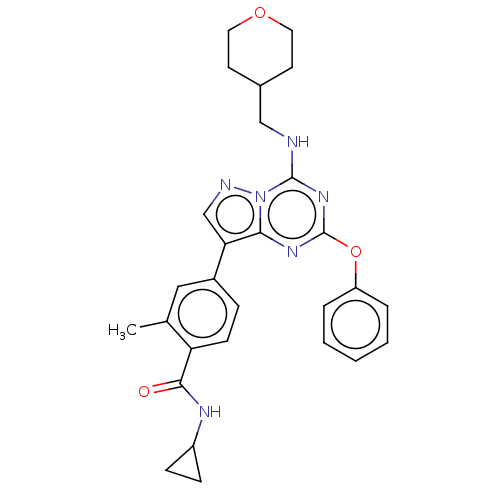 (CHEMBL3819210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081609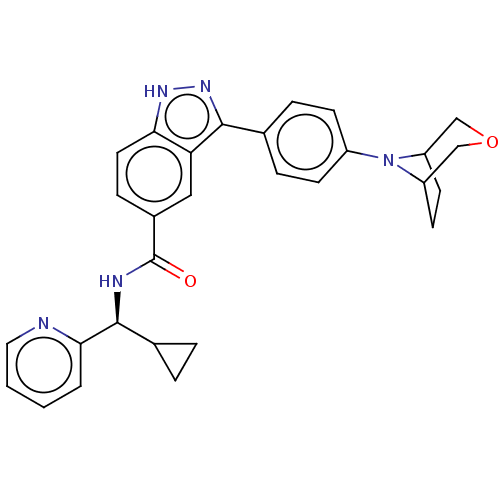 (CHEMBL3422084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044654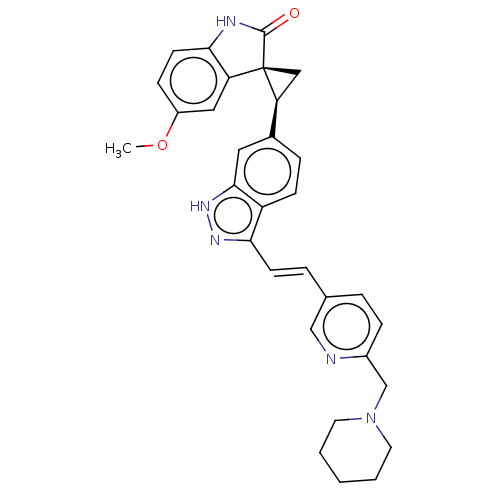 (CHEMBL3353346 | US9907800, Example A62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044657 (CHEMBL3353347 | US10358436, Example A24 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50183019 (CHEMBL3819210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081600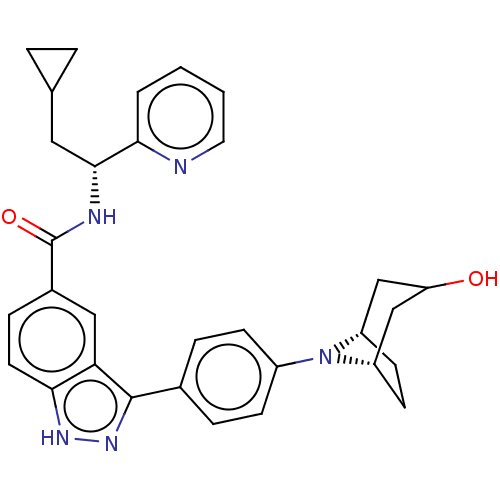 (CHEMBL3422099) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50044660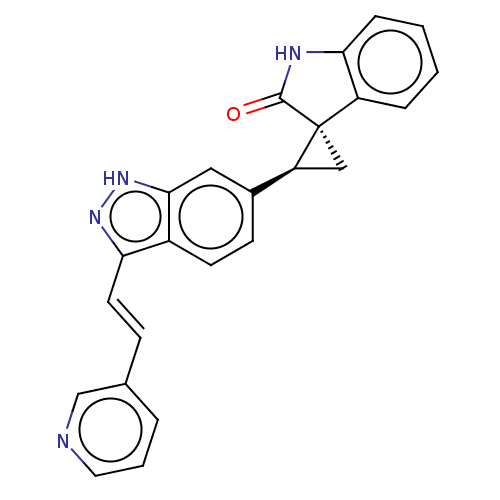 (CHEMBL3353342 | US10358436, Example A29 | US990780...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged PLK4 (1 to 391 residues) expressed in Escherichia coli incubated for 30 mins by ELISA method | J Med Chem 58: 130-46 (2015) Article DOI: 10.1021/jm500537u BindingDB Entry DOI: 10.7270/Q2125V9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512456 (CHEMBL4469414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512456 (CHEMBL4469414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50081598 (CHEMBL3422097) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate preincubated for 15 mins prior to ATP addition by ind... | J Med Chem 58: 3366-92 (2015) Article DOI: 10.1021/jm501740a BindingDB Entry DOI: 10.7270/Q2Q52RCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50533132 (CHEMBL4579057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... | ACS Med Chem Lett 7: 671-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00485 BindingDB Entry DOI: 10.7270/Q2K35Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 724 total ) | Next | Last >> |