

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893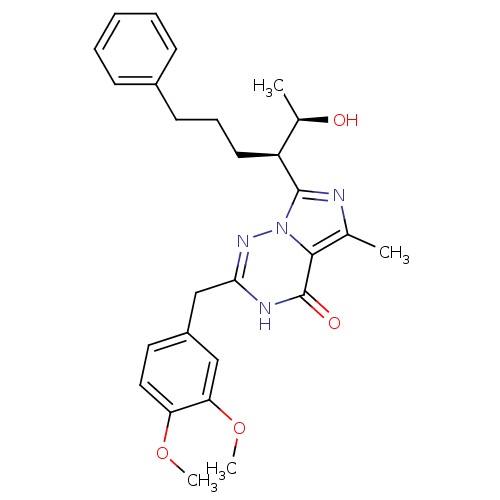 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM107767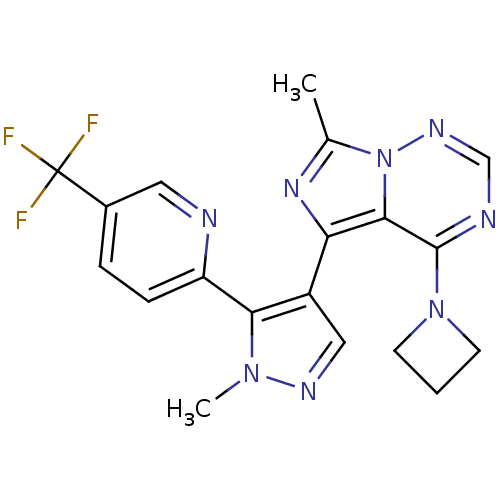 (US11419874, PF-05180999 | US8598155, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459874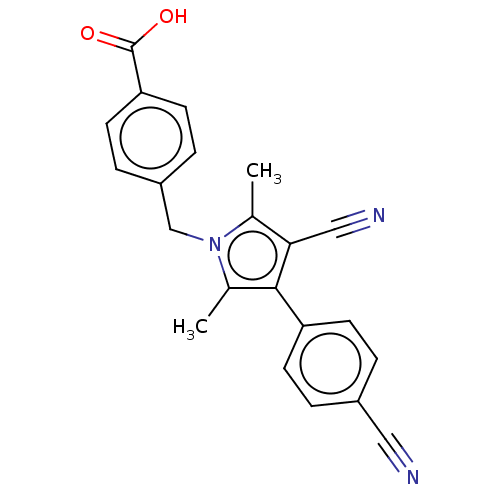 (CHEMBL4226259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Binding affinity to androgen receptor (unknown origin) | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459869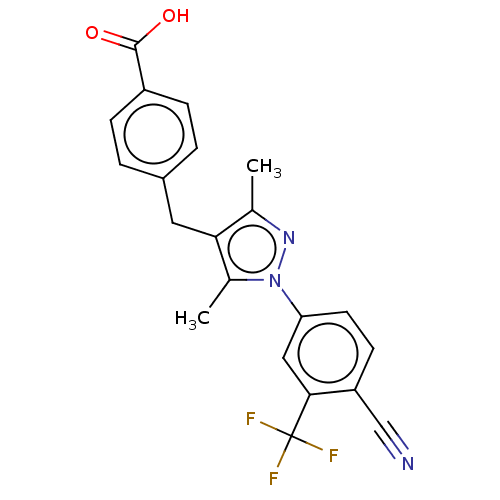 (CHEMBL4227715) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Binding affinity to androgen receptor (unknown origin) | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279272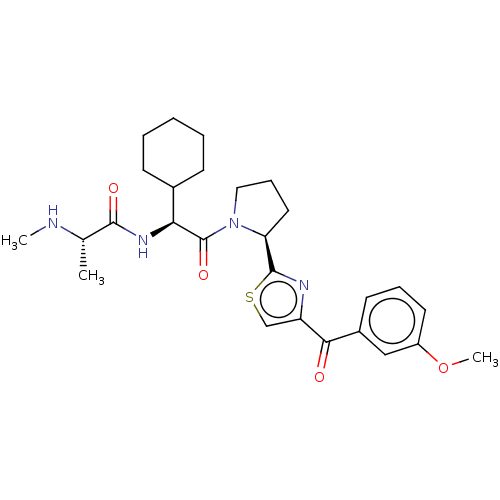 (CHEMBL4164385) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279272 (CHEMBL4164385) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50026821 (CHEMBL3331521 | US11419874, Example 10 | US9669035...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50361337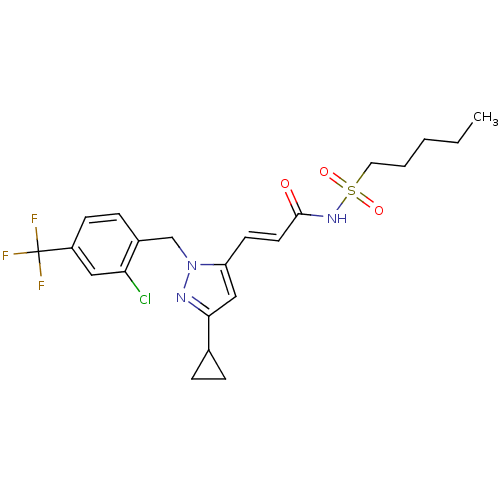 (CHEMBL1933845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma | Bioorg Med Chem 20: 714-33 (2012) Article DOI: 10.1016/j.bmc.2011.12.008 BindingDB Entry DOI: 10.7270/Q2PK0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50361338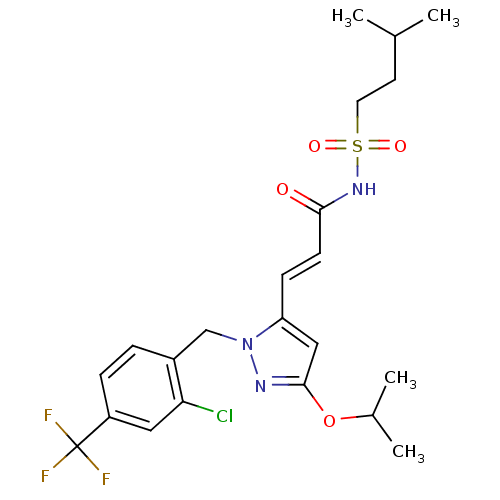 (CHEMBL1933842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma | Bioorg Med Chem 20: 714-33 (2012) Article DOI: 10.1016/j.bmc.2011.12.008 BindingDB Entry DOI: 10.7270/Q2PK0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50361340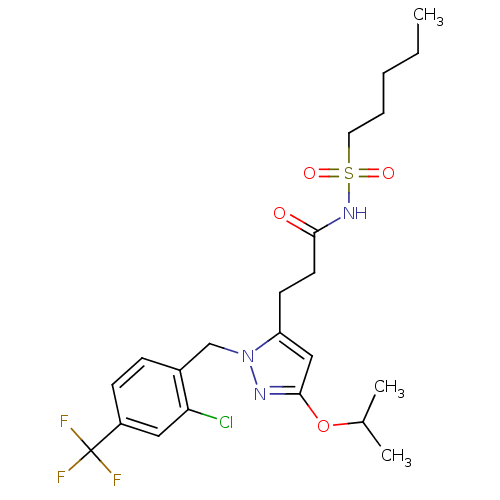 (CHEMBL1933822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma | Bioorg Med Chem 20: 714-33 (2012) Article DOI: 10.1016/j.bmc.2011.12.008 BindingDB Entry DOI: 10.7270/Q2PK0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50361339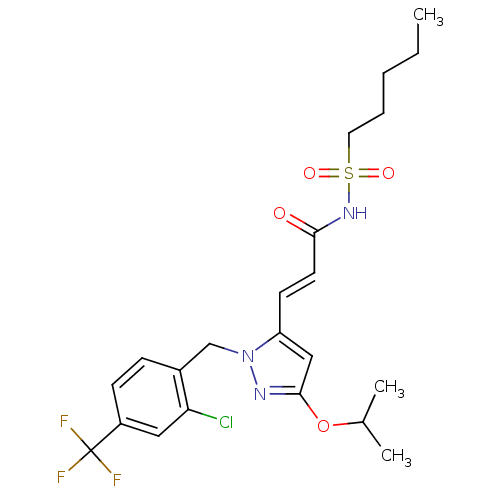 (CHEMBL1933841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma | Bioorg Med Chem 20: 714-33 (2012) Article DOI: 10.1016/j.bmc.2011.12.008 BindingDB Entry DOI: 10.7270/Q2PK0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258268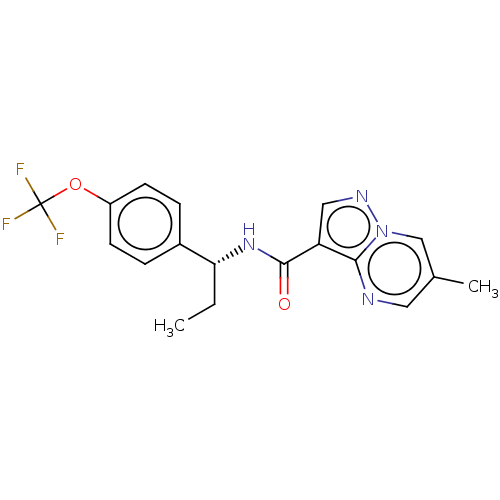 (CHEMBL4059831 | US11419874, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459873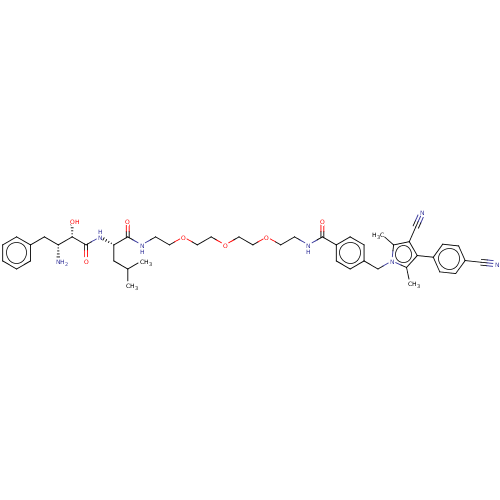 (CHEMBL4225186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50279265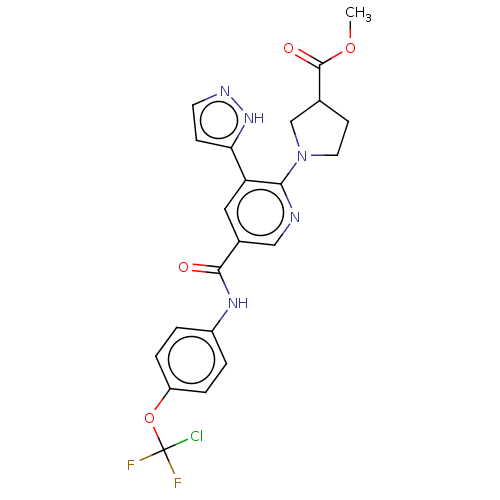 (CHEMBL4162041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258266 (CHEMBL4088107) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459875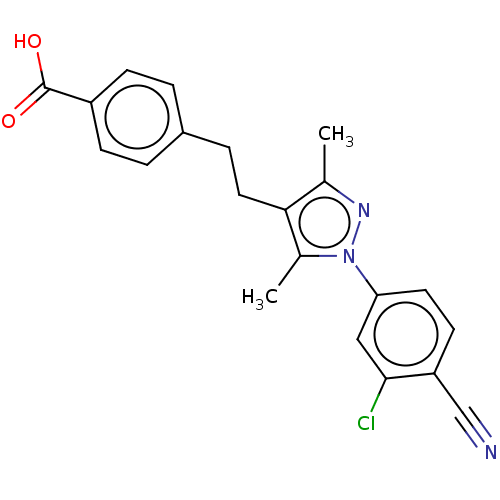 (CHEMBL4226844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Binding affinity to androgen receptor (unknown origin) | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459868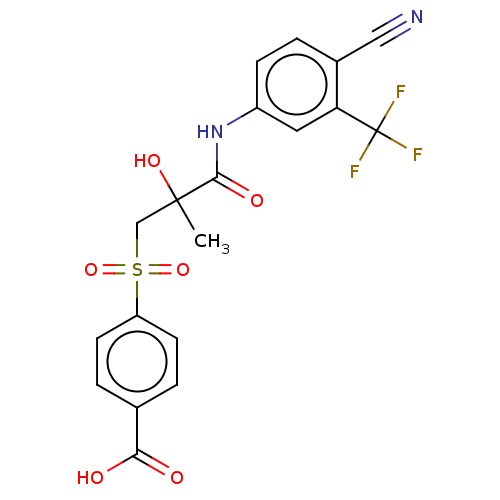 (CHEMBL4225339) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Binding affinity to androgen receptor (unknown origin) | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279272 (CHEMBL4164385) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279273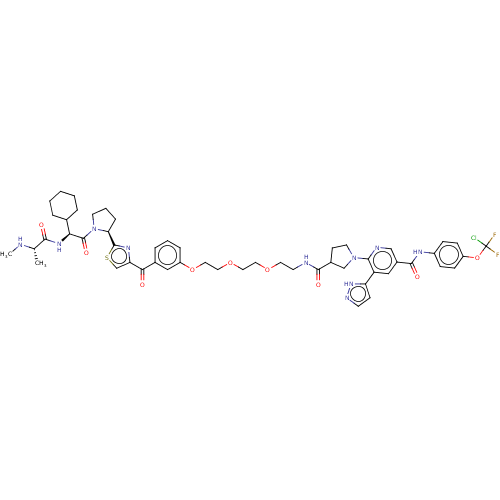 (CHEMBL4160980) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279274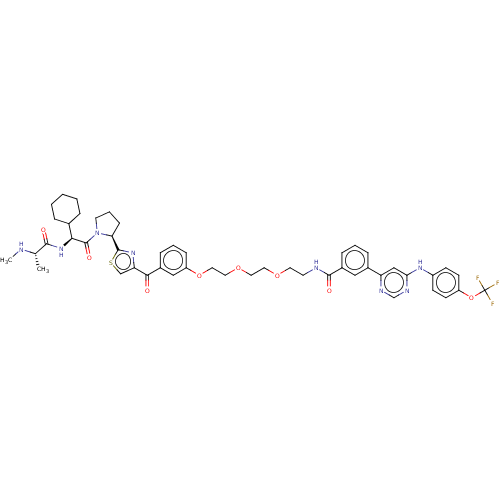 (CHEMBL4172268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279273 (CHEMBL4160980) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279273 (CHEMBL4160980) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged cIAP2 (Gln238 to Ser349 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459871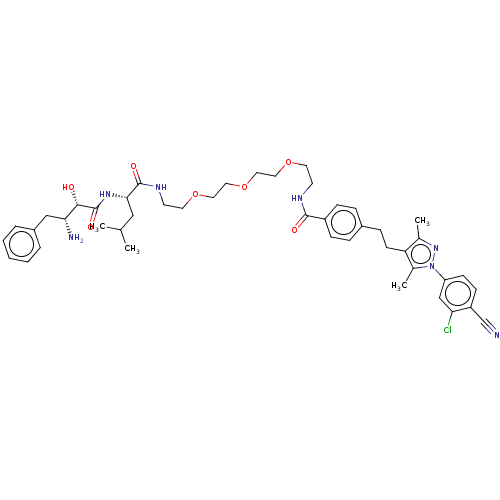 (CHEMBL4226314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459866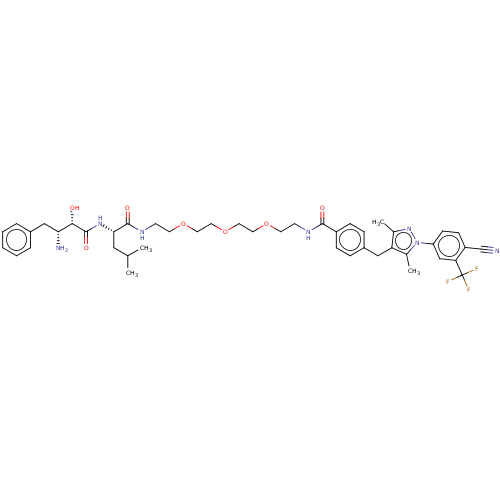 (CHEMBL4226899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279274 (CHEMBL4172268) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279274 (CHEMBL4172268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged cIAP2 (Gln238 to Ser349 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50279271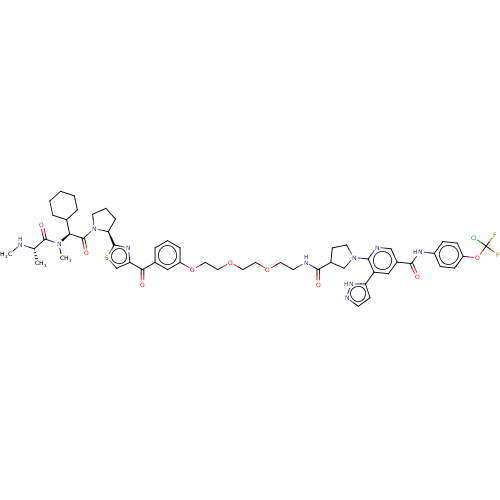 (CHEMBL4168907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50279273 (CHEMBL4160980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258301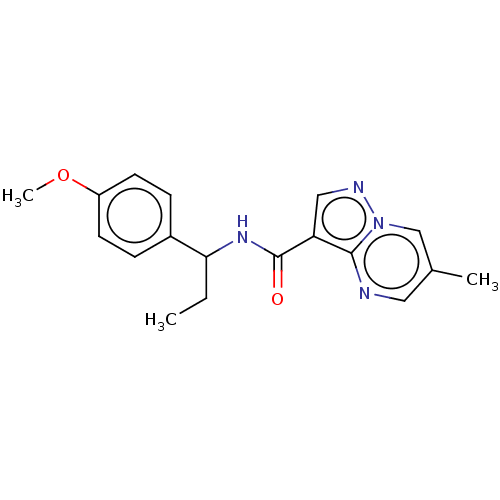 (CHEMBL4104510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279271 (CHEMBL4168907) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged cIAP2 (Gln238 to Ser349 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279271 (CHEMBL4168907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258303 (CHEMBL4064136) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279271 (CHEMBL4168907) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459870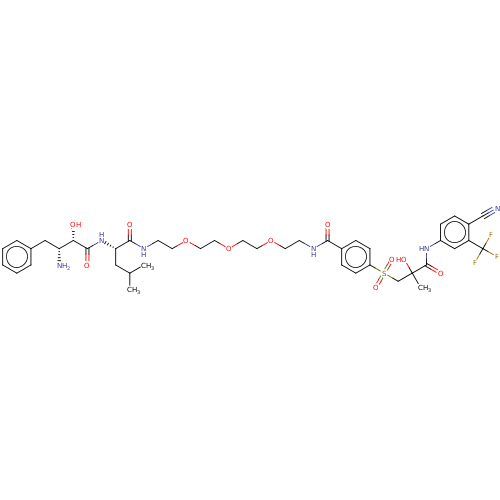 (CHEMBL4227287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258307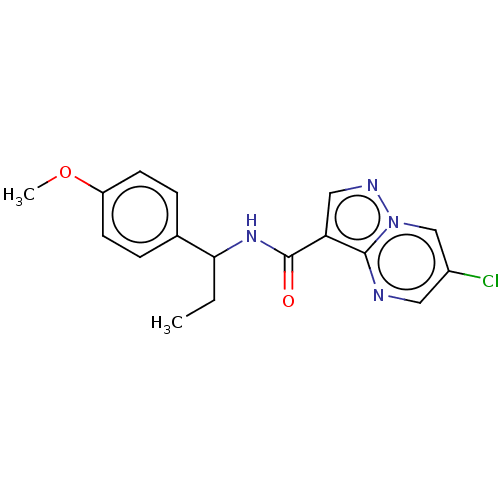 (CHEMBL4085701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50459867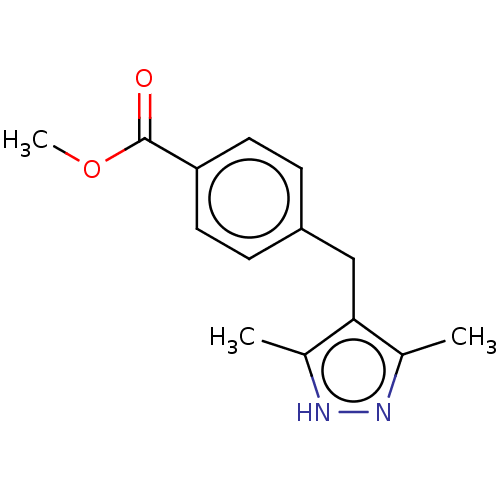 (CHEMBL4226575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Binding affinity to androgen receptor (unknown origin) | J Med Chem 61: 543-575 (2018) Article DOI: 10.1021/acs.jmedchem.7b00168 BindingDB Entry DOI: 10.7270/Q2VT1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50328152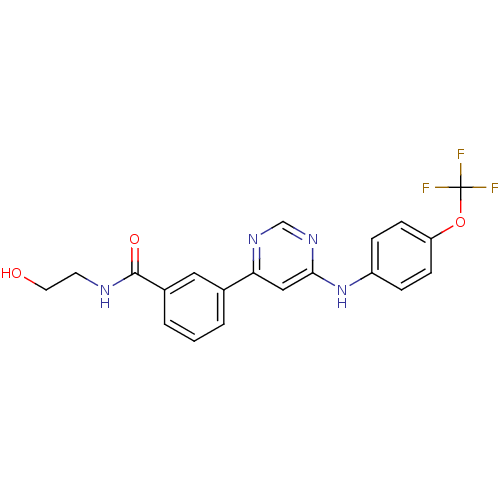 (CHEMBL1257423 | N-(2-hydroxyethyl)-3-(6-(4-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50279274 (CHEMBL4172268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258306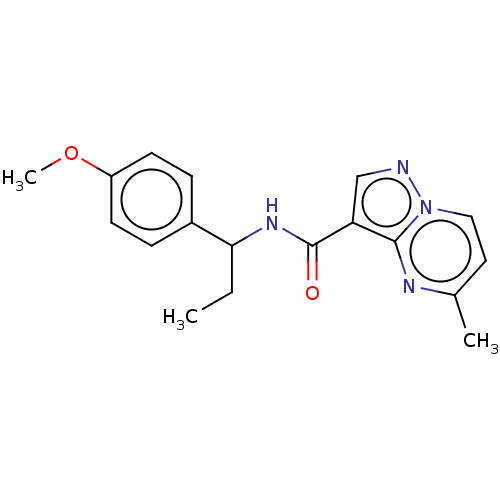 (CHEMBL4094364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50325999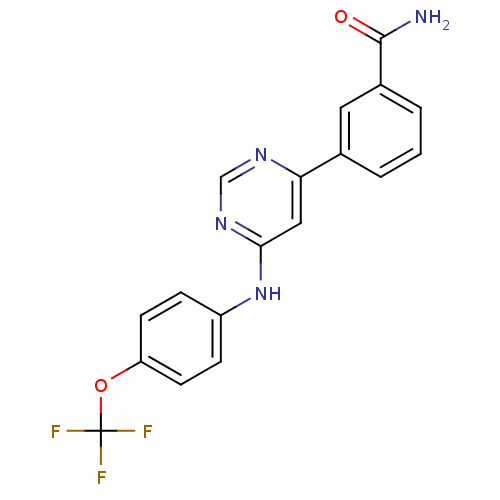 (3-(6-(4-(trifluoromethoxy)phenylamino)pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258305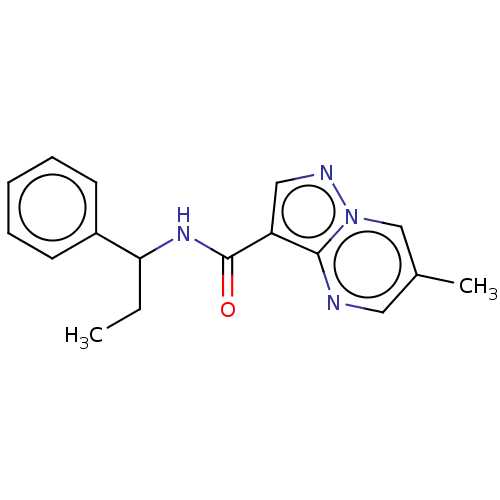 (CHEMBL4102110) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258297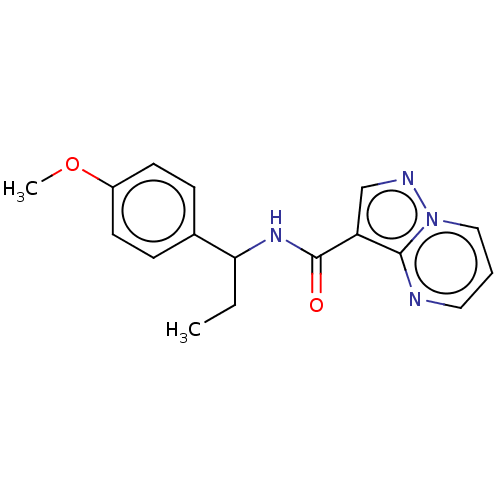 (CHEMBL4076518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged PDE3A (669 to 1141 residues) expressed in baculovirus infected Sf9 cells using [3H]cAMP as subs... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258265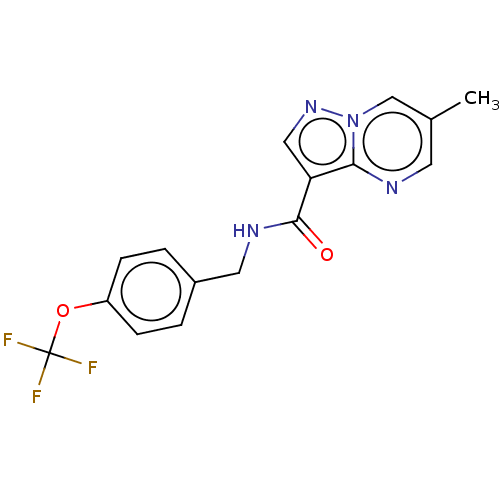 (CHEMBL4068494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50258302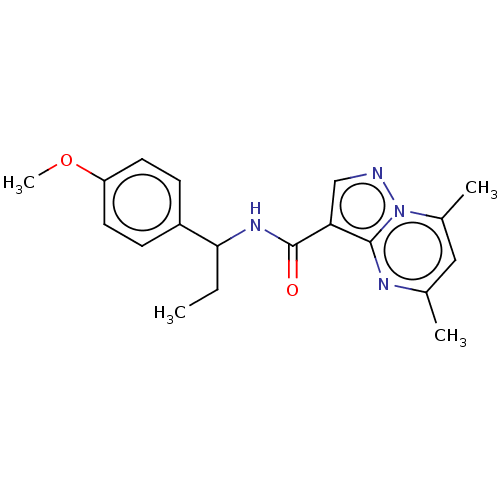 (CHEMBL4086618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal GST-tagged PDE9A2 expressed in baculovirus infected Sf9 cell expression system using [3H]cGMP ... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 7B (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged PDE7B (109 to end residues) expressed in baculovirus infected Sf9 cell ex... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human GST-tagged PDE1A expressed in baculovirus infected Sf9 cell expression system using [3H]cGMP as substrate... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50258268 (CHEMBL4059831 | US11419874, Example 21) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal GST-tagged PDE11A4 expressed in baculovirus infected Sf9 cell expression system using [3H]cGMP... | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |