 Found 104 hits with Last Name = 'grant' and Initial = 'p'
Found 104 hits with Last Name = 'grant' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517230

(CHEMBL4467984)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C64H83Cl3N10O8/c1-5-38-76(51-28-29-52-47(40-51)15-14-18-57(52)80)39-33-45-19-26-50(27-20-45)74-59(82)32-31-58(81)68-34-12-8-16-54(72-43(3)78)63(84)70-37-13-9-17-55(73-44(4)79)62(83)69-35-10-6-7-11-36-71-64(85)60-42(2)61(46-21-23-48(65)24-22-46)77(75-60)56-30-25-49(66)41-53(56)67/h14-15,18-27,30,41,51,54-55,80H,5-13,16-17,28-29,31-40H2,1-4H3,(H,68,81)(H,69,83)(H,70,84)(H,71,85)(H,72,78)(H,73,79)(H,74,82)/t51?,54-,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517232
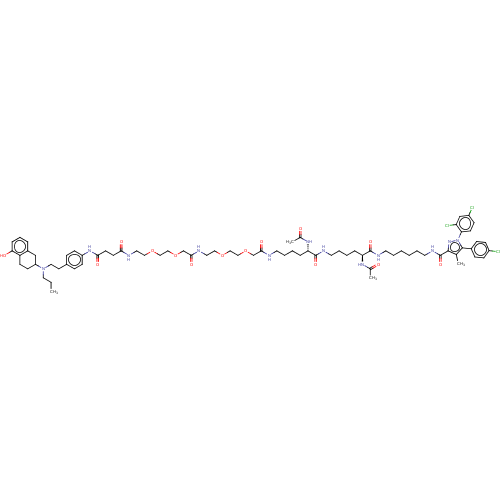
(CHEMBL4546839)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C76H105Cl3N12O14/c1-5-40-90(61-28-29-62-57(48-61)15-14-18-67(62)94)41-33-55-19-26-60(27-20-55)88-69(96)32-31-68(95)81-38-42-102-44-47-105-51-71(98)82-39-43-103-45-46-104-50-70(97)80-34-12-8-16-64(86-53(3)92)75(100)84-37-13-9-17-65(87-54(4)93)74(99)83-35-10-6-7-11-36-85-76(101)72-52(2)73(56-21-23-58(77)24-22-56)91(89-72)66-30-25-59(78)49-63(66)79/h14-15,18-27,30,49,61,64-65,94H,5-13,16-17,28-29,31-48,50-51H2,1-4H3,(H,80,97)(H,81,95)(H,82,98)(H,83,99)(H,84,100)(H,85,101)(H,86,92)(H,87,93)(H,88,96)/t61?,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517229
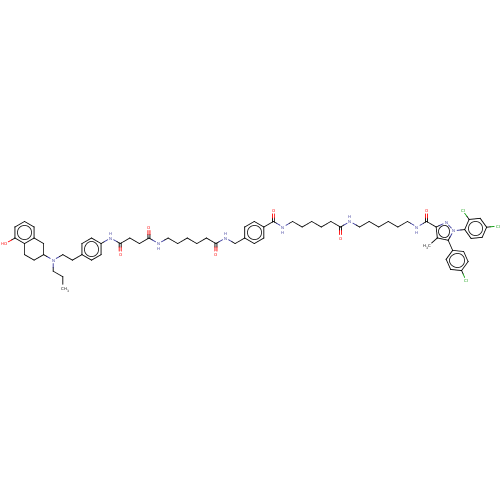
(CHEMBL4541515)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C68H84Cl3N9O7/c1-3-42-79(56-32-33-57-52(44-56)15-14-16-60(57)81)43-37-48-21-30-55(31-22-48)77-64(85)36-35-63(84)73-39-12-6-9-18-62(83)76-46-49-19-23-51(24-20-49)67(86)74-40-13-7-8-17-61(82)72-38-10-4-5-11-41-75-68(87)65-47(2)66(50-25-27-53(69)28-26-50)80(78-65)59-34-29-54(70)45-58(59)71/h14-16,19-31,34,45,56,81H,3-13,17-18,32-33,35-44,46H2,1-2H3,(H,72,82)(H,73,84)(H,74,86)(H,75,87)(H,76,83)(H,77,85) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517236

(CHEMBL4465127)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCOCCOCC(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCC[C@H](NC(C)=O)C(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 |r| Show InChI InChI=1S/C70H94Cl3N11O11/c1-5-39-83(56-28-29-57-52(44-56)15-14-18-62(57)87)40-33-50-19-26-55(27-20-50)81-64(89)32-31-63(88)75-38-41-94-42-43-95-46-65(90)74-34-12-8-16-59(79-48(3)85)69(92)77-37-13-9-17-60(80-49(4)86)68(91)76-35-10-6-7-11-36-78-70(93)66-47(2)67(51-21-23-53(71)24-22-51)84(82-66)61-30-25-54(72)45-58(61)73/h14-15,18-27,30,45,56,59-60,87H,5-13,16-17,28-29,31-44,46H2,1-4H3,(H,74,90)(H,75,88)(H,76,91)(H,77,92)(H,78,93)(H,79,85)(H,80,86)(H,81,89)/t56?,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50517222
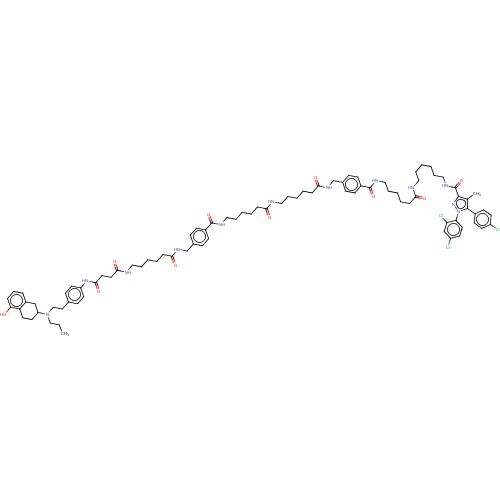
(CHEMBL4579585)Show SMILES CCCN(CCc1ccc(NC(=O)CCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCC(=O)NCc2ccc(cc2)C(=O)NCCCCCC(=O)NCCCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C88H113Cl3N12O10/c1-3-56-102(73-44-45-74-69(58-73)21-20-22-77(74)104)57-49-63-31-42-72(43-32-63)100-83(110)48-47-82(109)94-52-17-7-13-26-81(108)99-61-65-29-35-68(36-30-65)87(112)96-54-19-9-11-24-79(106)93-51-16-6-12-25-80(107)98-60-64-27-33-67(34-28-64)86(111)95-53-18-8-10-23-78(105)92-50-14-4-5-15-55-97-88(113)84-62(2)85(66-37-39-70(89)40-38-66)103(101-84)76-46-41-71(90)59-75(76)91/h20-22,27-43,46,59,73,104H,3-19,23-26,44-45,47-58,60-61H2,1-2H3,(H,92,105)(H,93,106)(H,94,109)(H,95,111)(H,96,112)(H,97,113)(H,98,107)(H,99,108)(H,100,110) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Binding affinity to human D2R |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517233

(CHEMBL4568756)Show SMILES CCOC(=O)c1ccc(CNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C27H22Cl3N3O3/c1-3-36-27(35)19-6-4-17(5-7-19)15-31-26(34)24-16(2)25(18-8-10-20(28)11-9-18)33(32-24)23-13-12-21(29)14-22(23)30/h4-14H,3,15H2,1-2H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517221
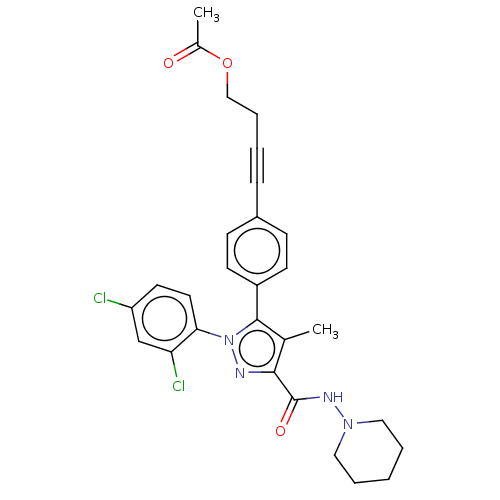
(CHEMBL4554135)Show SMILES CC(=O)OCCC#Cc1ccc(cc1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C28H28Cl2N4O3/c1-19-26(28(36)32-33-15-5-3-6-16-33)31-34(25-14-13-23(29)18-24(25)30)27(19)22-11-9-21(10-12-22)8-4-7-17-37-20(2)35/h9-14,18H,3,5-7,15-17H2,1-2H3,(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289
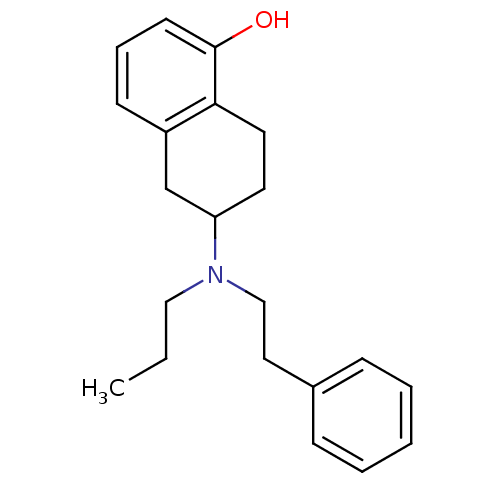
((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H] spiperone from human D2 dopamine receptor expressed in monkey caudate-putamen membranes |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517223
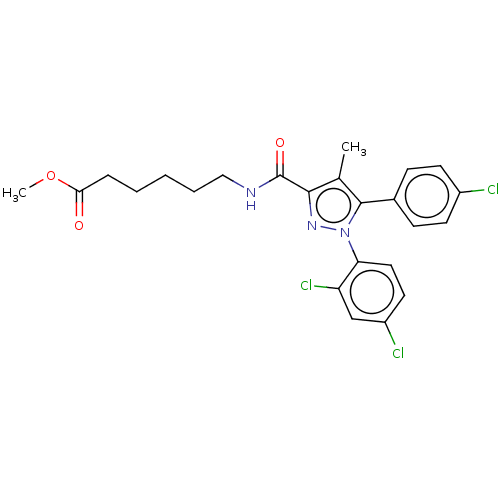
(CHEMBL4471116)Show SMILES COC(=O)CCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H24Cl3N3O3/c1-15-22(24(32)28-13-5-3-4-6-21(31)33-2)29-30(20-12-11-18(26)14-19(20)27)23(15)16-7-9-17(25)10-8-16/h7-12,14H,3-6,13H2,1-2H3,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517226
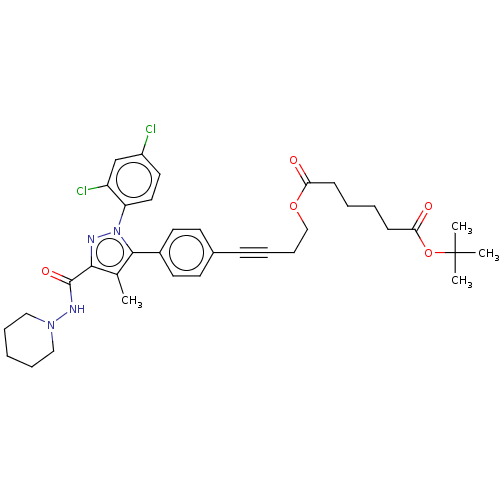
(CHEMBL4449666)Show SMILES Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCC(=O)OC(C)(C)C)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C36H42Cl2N4O5/c1-25-33(35(45)40-41-21-9-5-10-22-41)39-42(30-20-19-28(37)24-29(30)38)34(25)27-17-15-26(16-18-27)12-8-11-23-46-31(43)13-6-7-14-32(44)47-36(2,3)4/h15-20,24H,5-7,9-11,13-14,21-23H2,1-4H3,(H,40,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517227
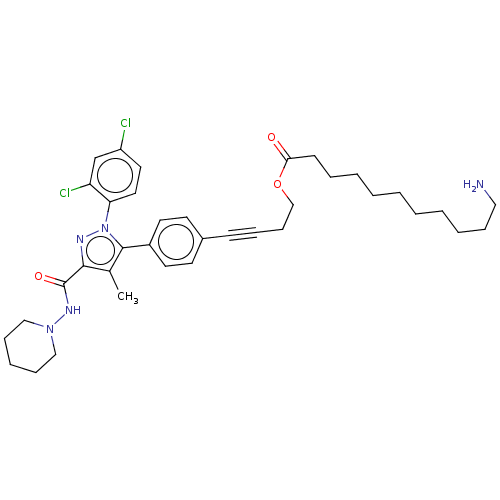
(CHEMBL4562705)Show SMILES OC(=O)C(F)(F)F.Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCCCCCCCN)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C37H47Cl2N5O3/c1-28-35(37(46)42-43-24-12-8-13-25-43)41-44(33-22-21-31(38)27-32(33)39)36(28)30-19-17-29(18-20-30)15-10-14-26-47-34(45)16-9-6-4-2-3-5-7-11-23-40/h17-22,27H,2-9,11-14,16,23-26,40H2,1H3,(H,42,46) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517225
(CHEMBL4453578)Show SMILES Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCCNC(=O)OC(C)(C)C)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C37H45Cl2N5O5/c1-26-33(35(46)42-43-22-10-6-11-23-43)41-44(31-20-19-29(38)25-30(31)39)34(26)28-17-15-27(16-18-28)13-8-12-24-48-32(45)14-7-5-9-21-40-36(47)49-37(2,3)4/h15-20,25H,5-7,9-12,14,21-24H2,1-4H3,(H,40,47)(H,42,46) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517224
(CHEMBL4443273)Show SMILES COC(=O)c1ccc(cc1)-c1ccc(cc1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C30H28Cl2N4O3/c1-19-27(29(37)34-35-16-4-3-5-17-35)33-36(26-15-14-24(31)18-25(26)32)28(19)22-10-6-20(7-11-22)21-8-12-23(13-9-21)30(38)39-2/h6-15,18H,3-5,16-17H2,1-2H3,(H,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517234
(CHEMBL4446228)Show SMILES Cc1c(nn(c1-c1ccc(cc1)C#CCCO)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H26Cl2N4O2/c1-18-24(26(34)30-31-14-4-2-5-15-31)29-32(23-13-12-21(27)17-22(23)28)25(18)20-10-8-19(9-11-20)7-3-6-16-33/h8-13,17,33H,2,4-6,14-16H2,1H3,(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517235
(CHEMBL4444520)Show SMILES Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCCNC(=S)c1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C53H47Cl2N5O8S/c1-32-48(50(64)58-59-25-7-3-8-26-59)57-60(44-23-17-36(54)29-43(44)55)49(32)34-14-12-33(13-15-34)10-5-9-27-66-47(63)11-4-2-6-24-56-51(69)35-16-20-40-39(28-35)52(65)68-53(40)41-21-18-37(61)30-45(41)67-46-31-38(62)19-22-42(46)53/h12-23,28-31,61-62H,2-4,6-9,11,24-27H2,1H3,(H,56,69)(H,58,64) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517231
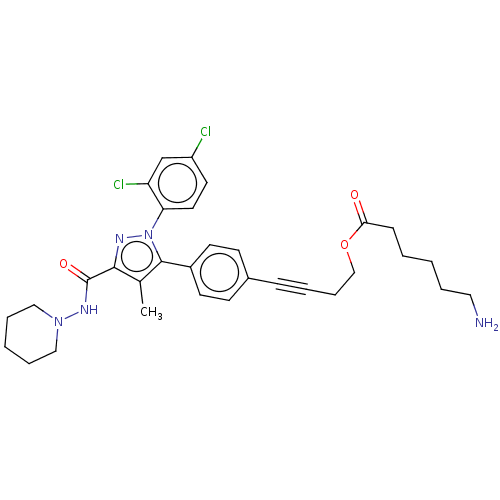
(CHEMBL4517197)Show SMILES OC(=O)C(F)(F)F.Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCCN)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C32H37Cl2N5O3/c1-23-30(32(41)37-38-19-7-3-8-20-38)36-39(28-17-16-26(33)22-27(28)34)31(23)25-14-12-24(13-15-25)10-5-9-21-42-29(40)11-4-2-6-18-35/h12-17,22H,2-4,6-9,11,18-21,35H2,1H3,(H,37,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50517228
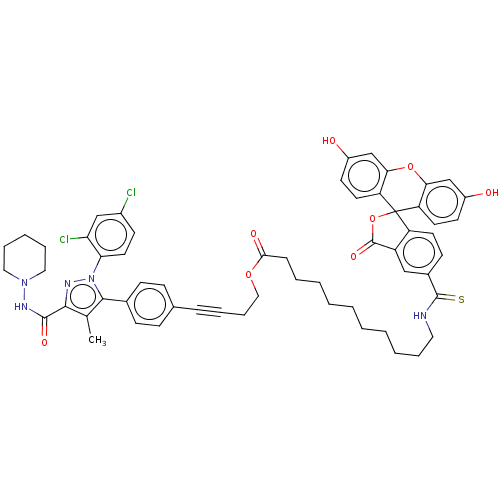
(CHEMBL4461338)Show SMILES Cc1c(nn(c1-c1ccc(cc1)C#CCCOC(=O)CCCCCCCCCCNC(=S)c1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C58H57Cl2N5O8S/c1-37-53(55(69)63-64-30-12-8-13-31-64)62-65(49-28-22-41(59)34-48(49)60)54(37)39-19-17-38(18-20-39)15-10-14-32-71-52(68)16-9-6-4-2-3-5-7-11-29-61-56(74)40-21-25-45-44(33-40)57(70)73-58(45)46-26-23-42(66)35-50(46)72-51-36-43(67)24-27-47(51)58/h17-28,33-36,66-67H,2-9,11-14,16,29-32H2,1H3,(H,61,74)(H,63,69) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126644
BindingDB Entry DOI: 10.7270/Q2QV3QW7 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1
(Homo sapiens (Human)) | BDBM50545573
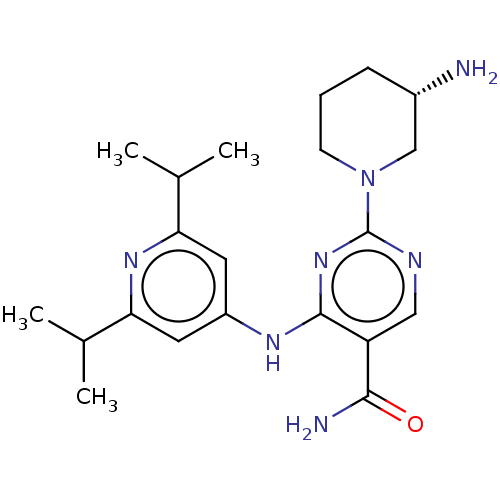
(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1G
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1G using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1
(Homo sapiens (Human)) | BDBM50545576
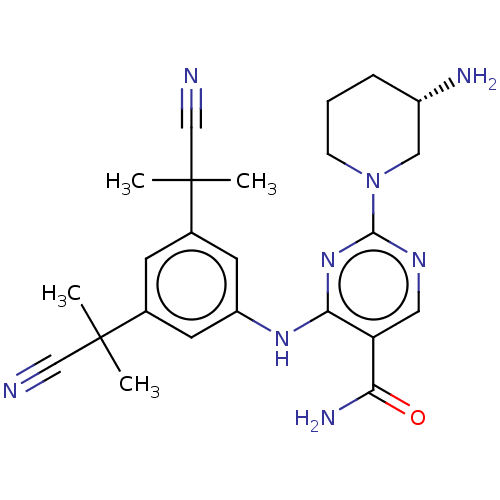
(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1B
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1B using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083722
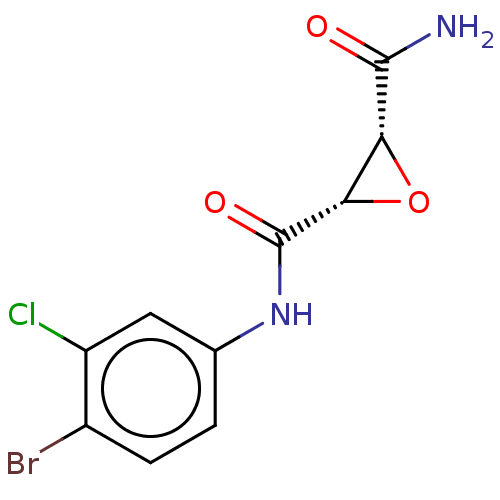
(CHEMBL3423198)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)c(Cl)c1 |r| Show InChI InChI=1S/C10H8BrClN2O3/c11-5-2-1-4(3-6(5)12)14-10(16)8-7(17-8)9(13)15/h1-3,7-8H,(H2,13,15)(H,14,16)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083725
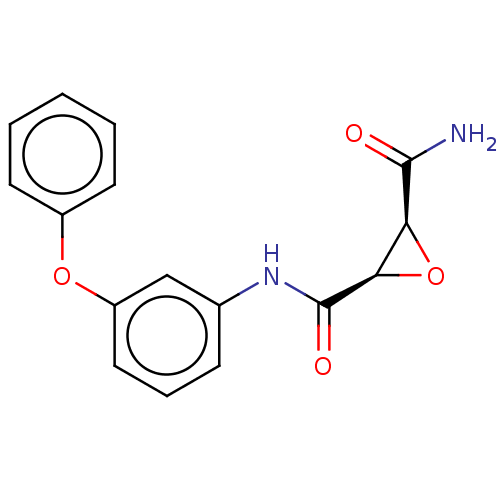
(CHEMBL3423195)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-5-4-8-12(9-10)21-11-6-2-1-3-7-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249319
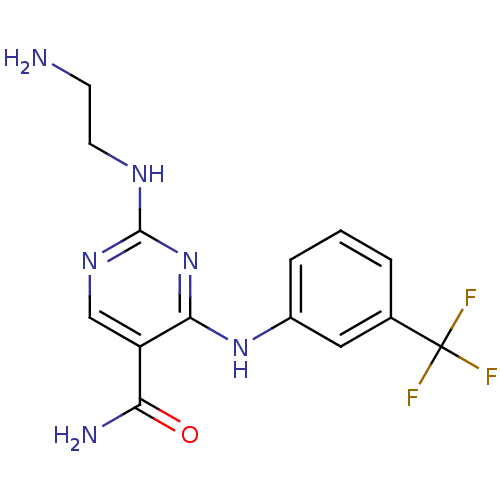
(2-(2-aminoethylamino)-4-(3-(trifluoromethyl)phenyl...)Show InChI InChI=1S/C14H15F3N6O/c15-14(16,17)8-2-1-3-9(6-8)22-12-10(11(19)24)7-21-13(23-12)20-5-4-18/h1-3,6-7H,4-5,18H2,(H2,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1D using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545576

(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083722

(CHEMBL3423198)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)c(Cl)c1 |r| Show InChI InChI=1S/C10H8BrClN2O3/c11-5-2-1-4(3-6(5)12)14-10(16)8-7(17-8)9(13)15/h1-3,7-8H,(H2,13,15)(H,14,16)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1B
(Homo sapiens (Human)) | BDBM50545576

(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1B using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545587

(CHEMBL4633229 | US11530193, Example 135)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H29N7O/c1-26(2,16-27)19-11-18(17-7-4-3-5-8-17)12-21(13-19)31-24-22(23(29)34)14-30-25(32-24)33-10-6-9-20(28)15-33/h3-5,7-8,11-14,20H,6,9-10,15,28H2,1-2H3,(H2,29,34)(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545587

(CHEMBL4633229 | US11530193, Example 135)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H29N7O/c1-26(2,16-27)19-11-18(17-7-4-3-5-8-17)12-21(13-19)31-24-22(23(29)34)14-30-25(32-24)33-10-6-9-20(28)15-33/h3-5,7-8,11-14,20H,6,9-10,15,28H2,1-2H3,(H2,29,34)(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 5
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human MEK5 using ERK5 as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545576

(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1D using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545586

(CHEMBL4632457 | US11530193, Example 106)Show SMILES CC(C)(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H30N6O3S/c1-21(2,3)13-8-15(10-16(9-13)31(4,29)30)25-19-17(18(23)28)11-24-20(26-19)27-7-5-6-14(22)12-27/h8-11,14H,5-7,12,22H2,1-4H3,(H2,23,28)(H,24,25,26)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545586

(CHEMBL4632457 | US11530193, Example 106)Show SMILES CC(C)(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H30N6O3S/c1-21(2,3)13-8-15(10-16(9-13)31(4,29)30)25-19-17(18(23)28)11-24-20(26-19)27-7-5-6-14(22)12-27/h8-11,14H,5-7,12,22H2,1-4H3,(H2,23,28)(H,24,25,26)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545576

(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083728

(CHEMBL3423193)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-6-8-12(9-7-10)21-11-4-2-1-3-5-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545583

(CHEMBL4644948)Show SMILES N[C@H]1CCCN(C1)c1ncc(C(N)=O)c(Nc2cccc(c2)-c2ccccc2)n1 |r| Show InChI InChI=1S/C22H24N6O/c23-17-9-5-11-28(14-17)22-25-13-19(20(24)29)21(27-22)26-18-10-4-8-16(12-18)15-6-2-1-3-7-15/h1-4,6-8,10,12-13,17H,5,9,11,14,23H2,(H2,24,29)(H,25,26,27)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083718
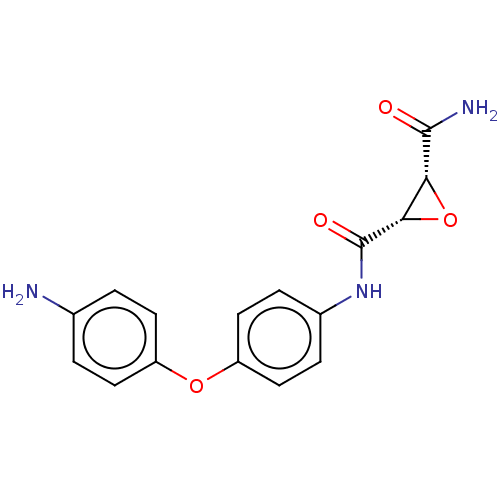
(CHEMBL3423202)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(N)cc2)cc1 |r| Show InChI InChI=1S/C16H15N3O4/c17-9-1-5-11(6-2-9)22-12-7-3-10(4-8-12)19-16(21)14-13(23-14)15(18)20/h1-8,13-14H,17H2,(H2,18,20)(H,19,21)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083726
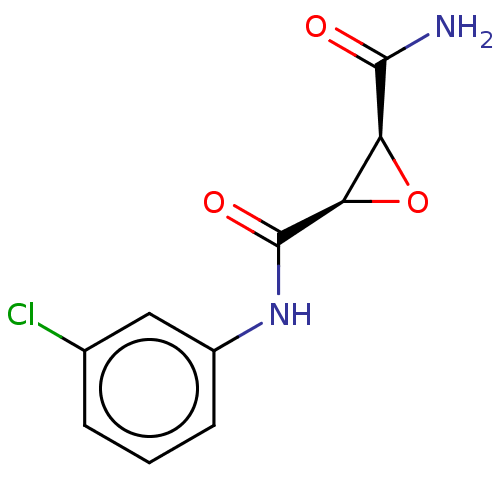
(CHEMBL3423194)Show InChI InChI=1S/C10H9ClN2O3/c11-5-2-1-3-6(4-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083726

(CHEMBL3423194)Show InChI InChI=1S/C10H9ClN2O3/c11-5-2-1-3-6(4-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083725

(CHEMBL3423195)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-5-4-8-12(9-10)21-11-6-2-1-3-7-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM50419254

(CHEMBL1835063)Show SMILES N[C@H]1CCCN(C1)c1ncc(C(N)=O)c(Nc2cccc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C17H19F3N6O/c18-17(19,20)10-3-1-5-12(7-10)24-15-13(14(22)27)8-23-16(25-15)26-6-2-4-11(21)9-26/h1,3,5,7-8,11H,2,4,6,9,21H2,(H2,22,27)(H,23,24,25)/t11-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human MLK3 using myelin basic protein as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50419254

(CHEMBL1835063)Show SMILES N[C@H]1CCCN(C1)c1ncc(C(N)=O)c(Nc2cccc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C17H19F3N6O/c18-17(19,20)10-3-1-5-12(7-10)24-15-13(14(22)27)8-23-16(25-15)26-6-2-4-11(21)9-26/h1,3,5,7-8,11H,2,4,6,9,21H2,(H2,22,27)(H,23,24,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using KKRNRTLTK as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083728

(CHEMBL3423193)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-6-8-12(9-7-10)21-11-4-2-1-3-5-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50419254

(CHEMBL1835063)Show SMILES N[C@H]1CCCN(C1)c1ncc(C(N)=O)c(Nc2cccc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C17H19F3N6O/c18-17(19,20)10-3-1-5-12(7-10)24-15-13(14(22)27)8-23-16(25-15)26-6-2-4-11(21)9-26/h1,3,5,7-8,11H,2,4,6,9,21H2,(H2,22,27)(H,23,24,25)/t11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083723

(CHEMBL3423197)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)cc1 |r| Show InChI InChI=1S/C10H9BrN2O3/c11-5-1-3-6(4-2-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083718

(CHEMBL3423202)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(N)cc2)cc1 |r| Show InChI InChI=1S/C16H15N3O4/c17-9-1-5-11(6-2-9)22-12-7-3-10(4-8-12)19-16(21)14-13(23-14)15(18)20/h1-8,13-14H,17H2,(H2,18,20)(H,19,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50419254

(CHEMBL1835063)Show SMILES N[C@H]1CCCN(C1)c1ncc(C(N)=O)c(Nc2cccc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C17H19F3N6O/c18-17(19,20)10-3-1-5-12(7-10)24-15-13(14(22)27)8-23-16(25-15)26-6-2-4-11(21)9-26/h1,3,5,7-8,11H,2,4,6,9,21H2,(H2,22,27)(H,23,24,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data