 Found 3137 hits with Last Name = 'singh' and Initial = 'p'
Found 3137 hits with Last Name = 'singh' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM19461
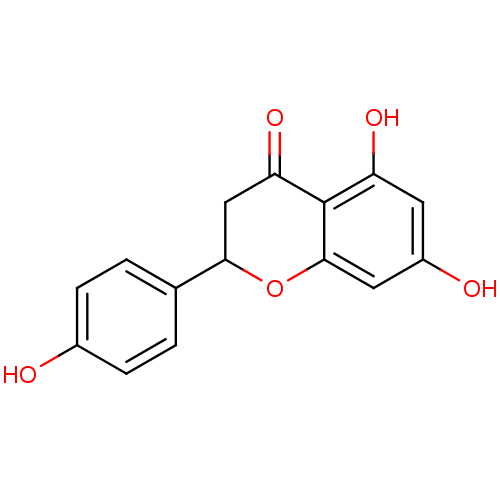
(α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...)Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00110 | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92556
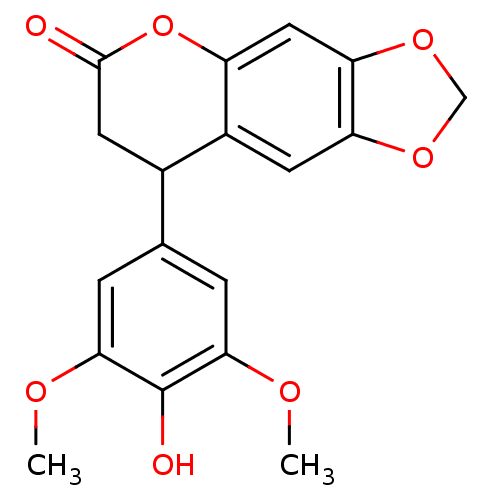
(Neoflavonoid, 8)Show InChI InChI=1S/C18H16O7/c1-21-15-3-9(4-16(22-2)18(15)20)10-6-17(19)25-12-7-14-13(5-11(10)12)23-8-24-14/h3-5,7,10,20H,6,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00182 | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92555
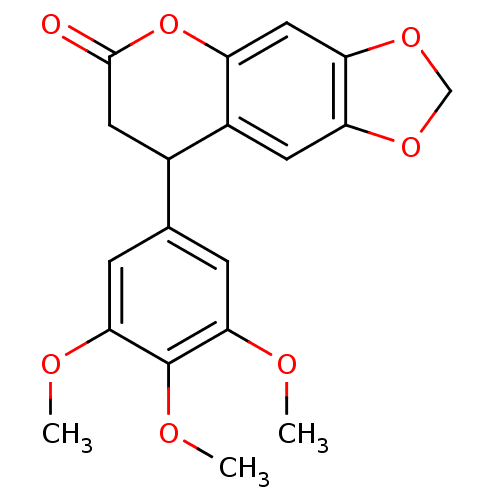
(Neoflavonoid, 7)Show InChI InChI=1S/C19H18O7/c1-21-16-4-10(5-17(22-2)19(16)23-3)11-7-18(20)26-13-8-15-14(6-12(11)13)24-9-25-15/h4-6,8,11H,7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92557
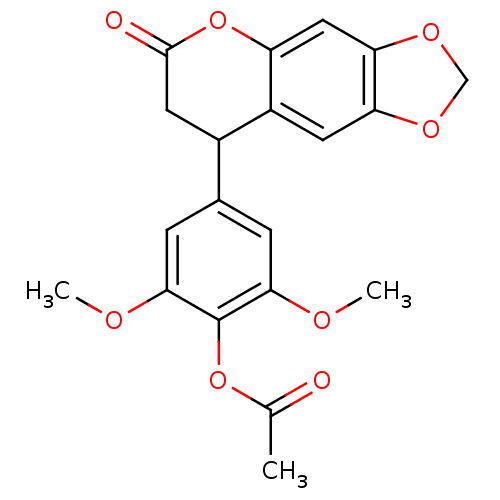
(Neoflavonoid, 9)Show SMILES COc1cc(cc(OC)c1OC(C)=O)C1CC(=O)Oc2cc3OCOc3cc12 Show InChI InChI=1S/C20H18O8/c1-10(21)27-20-17(23-2)4-11(5-18(20)24-3)12-7-19(22)28-14-8-16-15(6-13(12)14)25-9-26-16/h4-6,8,12H,7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92559

(Neoflavonoid, 11)Show SMILES COc1cc(OC)c2C(CC(=O)Oc2c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C20H22O7/c1-22-12-8-14(23-2)19-13(10-18(21)27-15(19)9-12)11-6-16(24-3)20(26-5)17(7-11)25-4/h6-9,13H,10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50033532

(CHEMBL435380 | N-[(3S,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50033536

(CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92560
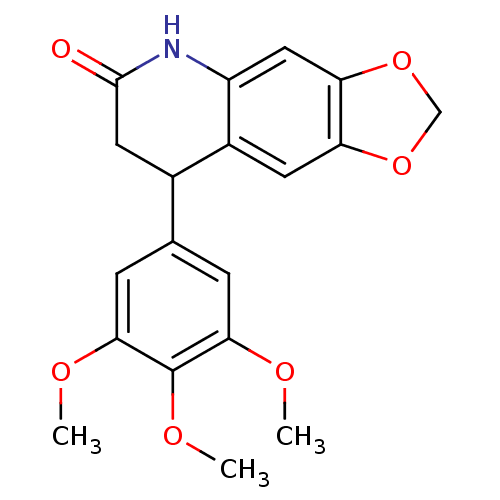
(Neoflavonoid, 19)Show InChI InChI=1S/C19H19NO6/c1-22-16-4-10(5-17(23-2)19(16)24-3)11-7-18(21)20-13-8-15-14(6-12(11)13)25-9-26-15/h4-6,8,11H,7,9H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00597 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92558
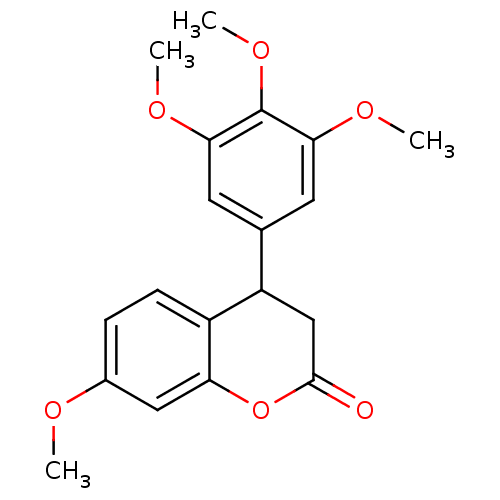
(Neoflavonoid, 10)Show InChI InChI=1S/C19H20O6/c1-21-12-5-6-13-14(10-18(20)25-15(13)9-12)11-7-16(22-2)19(24-4)17(8-11)23-3/h5-9,14H,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92561
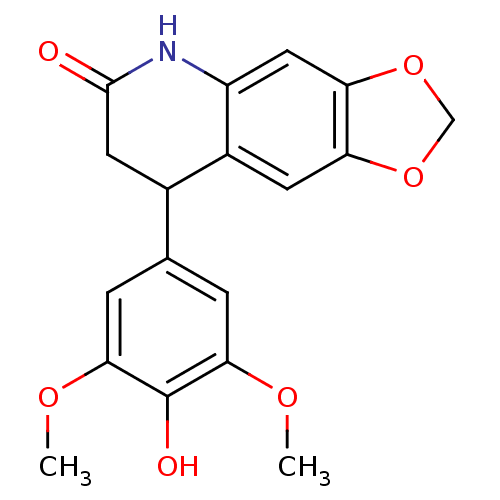
(Neoflavonoid, 20)Show InChI InChI=1S/C18H17NO6/c1-22-15-3-9(4-16(23-2)18(15)21)10-6-17(20)19-12-7-14-13(5-11(10)12)24-8-25-14/h3-5,7,10,21H,6,8H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50033535
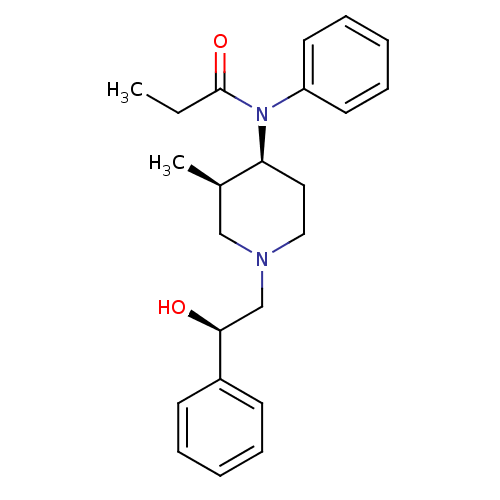
(CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50034623
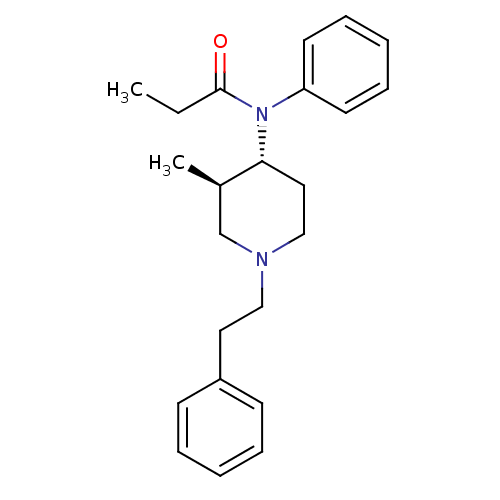
(CHEMBL38685 | N-((3R,4R)-3-Methyl-1-phenethyl-pipe...)Show SMILES CCC(=O)N([C@@H]1CCN(CCc2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 using [3H]DAMGO as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50329836
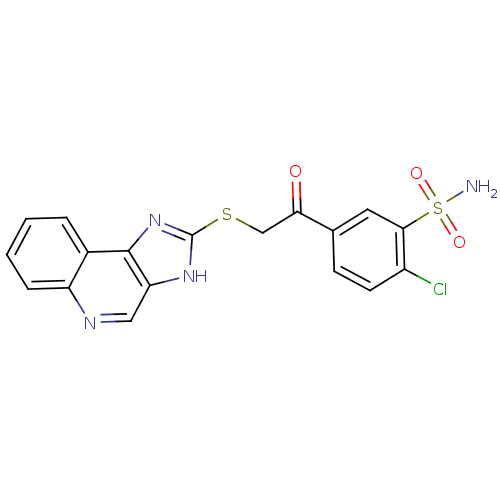
(2-Chloro-5-[(1H-imidazo[4,5-c]quinolin-2-ylsulfany...)Show SMILES NS(=O)(=O)c1cc(ccc1Cl)C(=O)CSc1nc2c(cnc3ccccc23)[nH]1 Show InChI InChI=1S/C18H13ClN4O3S2/c19-12-6-5-10(7-16(12)28(20,25)26)15(24)9-27-18-22-14-8-21-13-4-2-1-3-11(13)17(14)23-18/h1-8H,9H2,(H,22,23)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114247
BindingDB Entry DOI: 10.7270/Q2MC941H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50560609
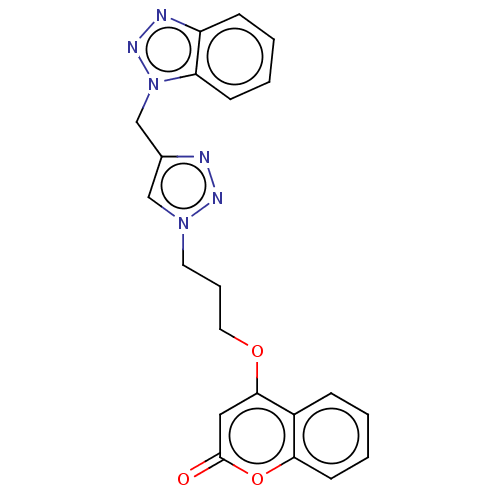
(CHEMBL4749763)Show SMILES O=c1cc(OCCCn2cc(Cn3nnc4ccccc34)nn2)c2ccccc2o1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127477
BindingDB Entry DOI: 10.7270/Q2W099MW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50011851
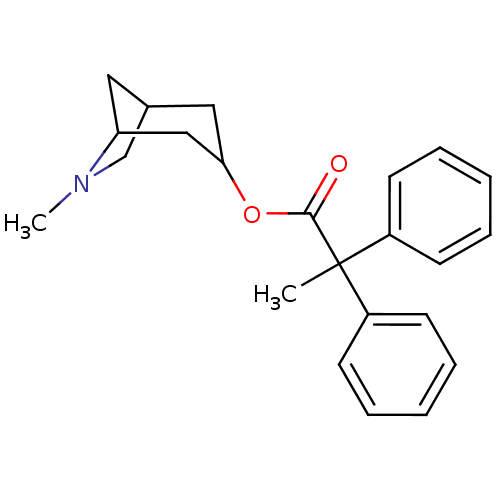
(2,2-Diphenyl-propionic acid 6-methyl-6-aza-bicyclo...)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(C)(c1ccccc1)c1ccccc1 |TLB:9:7:1.2:4,THB:0:1:4:6.7.8| Show InChI InChI=1S/C23H27NO2/c1-23(18-9-5-3-6-10-18,19-11-7-4-8-12-19)22(25)26-21-14-17-13-20(15-21)24(2)16-17/h3-12,17,20-21H,13-16H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding to CHO cells bearing transfected muscarinic acetylcholine receptor M1 |
J Med Chem 34: 1436-40 (1991)
BindingDB Entry DOI: 10.7270/Q2QC043X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50329836

(2-Chloro-5-[(1H-imidazo[4,5-c]quinolin-2-ylsulfany...)Show SMILES NS(=O)(=O)c1cc(ccc1Cl)C(=O)CSc1nc2c(cnc3ccccc23)[nH]1 Show InChI InChI=1S/C18H13ClN4O3S2/c19-12-6-5-10(7-16(12)28(20,25)26)15(24)9-27-18-22-14-8-21-13-4-2-1-3-11(13)17(14)23-18/h1-8H,9H2,(H,22,23)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114247
BindingDB Entry DOI: 10.7270/Q2MC941H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413787
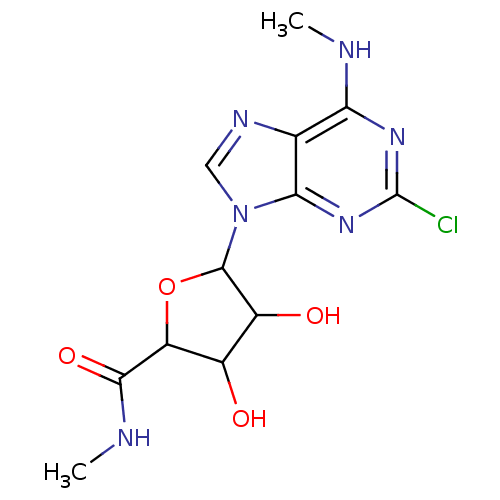
(CHEMBL449380)Show InChI InChI=1S/C12H15ClN6O4/c1-14-8-4-9(18-12(13)17-8)19(3-16-4)11-6(21)5(20)7(23-11)10(22)15-2/h3,5-7,11,20-21H,1-2H3,(H,15,22)(H,14,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 1096-102 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb15923.x
BindingDB Entry DOI: 10.7270/Q2377766 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding to CHO cells bearing transfected muscarinic acetylcholine receptor M3 |
J Med Chem 34: 1436-40 (1991)
BindingDB Entry DOI: 10.7270/Q2QC043X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
muscarinic acetylcholine receptor M1 |
J Med Chem 34: 1436-40 (1991)
BindingDB Entry DOI: 10.7270/Q2QC043X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50594620
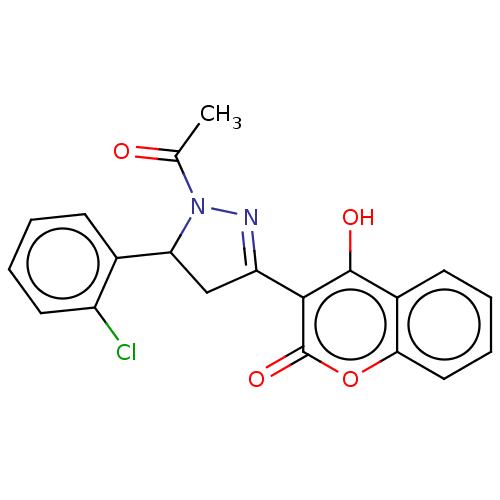
(CHEMBL5176826)Show SMILES CC(=O)N1N=C(CC1c1ccccc1Cl)c1c(O)c2ccccc2oc1=O |c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413786
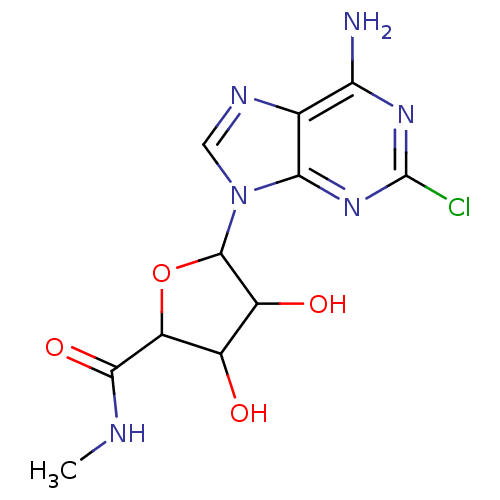
(CHEMBL483954)Show InChI InChI=1S/C11H13ClN6O4/c1-14-9(21)6-4(19)5(20)10(22-6)18-2-15-3-7(13)16-11(12)17-8(3)18/h2,4-6,10,19-20H,1H3,(H,14,21)(H2,13,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50011851

(2,2-Diphenyl-propionic acid 6-methyl-6-aza-bicyclo...)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(C)(c1ccccc1)c1ccccc1 |TLB:9:7:1.2:4,THB:0:1:4:6.7.8| Show InChI InChI=1S/C23H27NO2/c1-23(18-9-5-3-6-10-18,19-11-7-4-8-12-19)22(25)26-21-14-17-13-20(15-21)24(2)16-17/h3-12,17,20-21H,13-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding to CHO cells bearing transfected muscarinic acetylcholine receptor M3 |
J Med Chem 34: 1436-40 (1991)
BindingDB Entry DOI: 10.7270/Q2QC043X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50033537
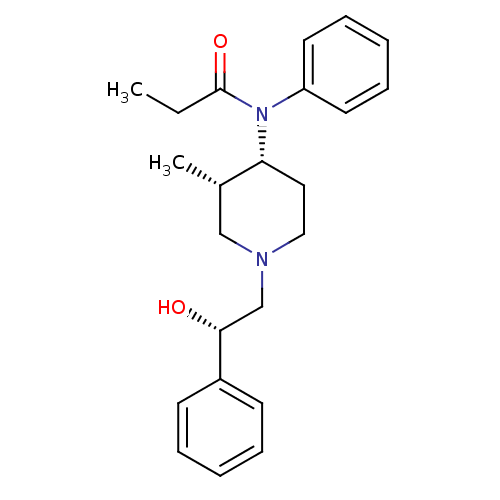
(CHEMBL121060 | N-[(3S,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 using [3H]U-69593 as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50454092
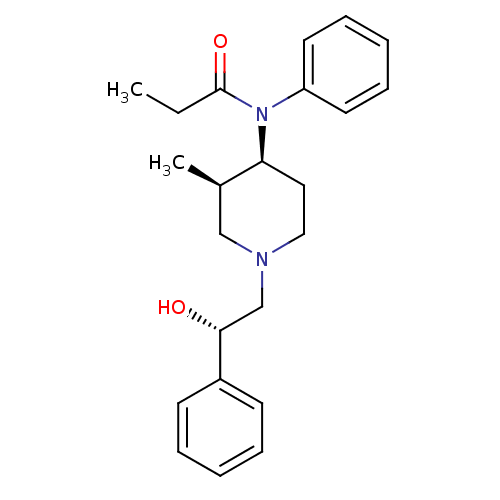
(CHEMBL2373185)Show SMILES Cl.CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 |r| Show InChI InChI=1S/C23H30N2O2.ClH/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19;/h4-13,18,21-22,26H,3,14-17H2,1-2H3;1H/t18-,21+,22-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 using [3H]U-69593 as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413798
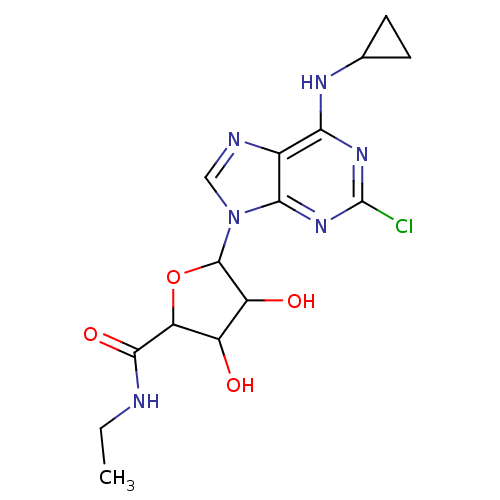
(CHEMBL488149)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NC3CC3)nc(Cl)nc12 Show InChI InChI=1S/C15H19ClN6O4/c1-2-17-13(25)10-8(23)9(24)14(26-10)22-5-18-7-11(19-6-3-4-6)20-15(16)21-12(7)22/h5-6,8-10,14,23-24H,2-4H2,1H3,(H,17,25)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413804
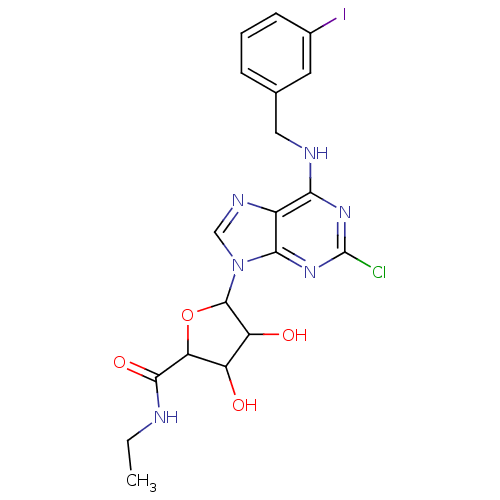
(CHEMBL486171)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C19H20ClIN6O4/c1-2-22-17(30)14-12(28)13(29)18(31-14)27-8-24-11-15(25-19(20)26-16(11)27)23-7-9-4-3-5-10(21)6-9/h3-6,8,12-14,18,28-29H,2,7H2,1H3,(H,22,30)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50034620

(CHEMBL290411 | N-((3S,4S)-3-Methyl-1-phenethyl-pip...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 using [3H]DADLE as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413790
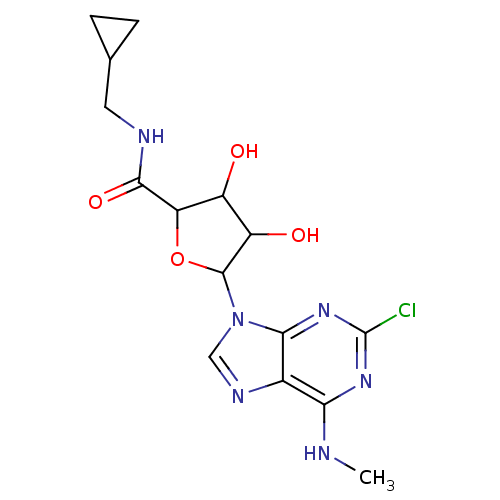
(CHEMBL483969)Show SMILES CNc1nc(Cl)nc2n(cnc12)C1OC(C(O)C1O)C(=O)NCC1CC1 Show InChI InChI=1S/C15H19ClN6O4/c1-17-11-7-12(21-15(16)20-11)22(5-19-7)14-9(24)8(23)10(26-14)13(25)18-4-6-2-3-6/h5-6,8-10,14,23-24H,2-4H2,1H3,(H,18,25)(H,17,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50329836

(2-Chloro-5-[(1H-imidazo[4,5-c]quinolin-2-ylsulfany...)Show SMILES NS(=O)(=O)c1cc(ccc1Cl)C(=O)CSc1nc2c(cnc3ccccc23)[nH]1 Show InChI InChI=1S/C18H13ClN4O3S2/c19-12-6-5-10(7-16(12)28(20,25)26)15(24)9-27-18-22-14-8-21-13-4-2-1-3-11(13)17(14)23-18/h1-8H,9H2,(H,22,23)(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114247
BindingDB Entry DOI: 10.7270/Q2MC941H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50454092

(CHEMBL2373185)Show SMILES Cl.CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 |r| Show InChI InChI=1S/C23H30N2O2.ClH/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19;/h4-13,18,21-22,26H,3,14-17H2,1-2H3;1H/t18-,21+,22-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 using [3H]DADLE as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413791
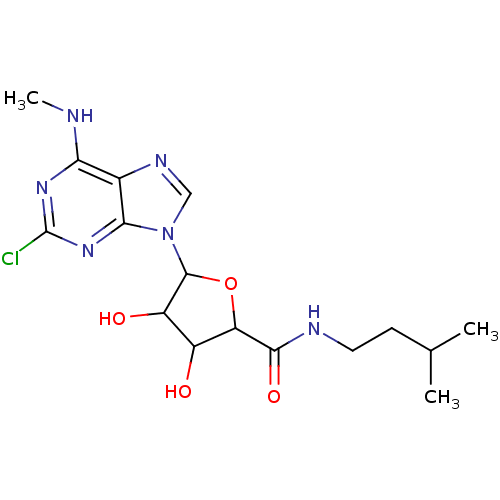
(CHEMBL483975)Show SMILES CNc1nc(Cl)nc2n(cnc12)C1OC(C(O)C1O)C(=O)NCCC(C)C Show InChI InChI=1S/C16H23ClN6O4/c1-7(2)4-5-19-14(26)11-9(24)10(25)15(27-11)23-6-20-8-12(18-3)21-16(17)22-13(8)23/h6-7,9-11,15,24-25H,4-5H2,1-3H3,(H,19,26)(H,18,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50055832
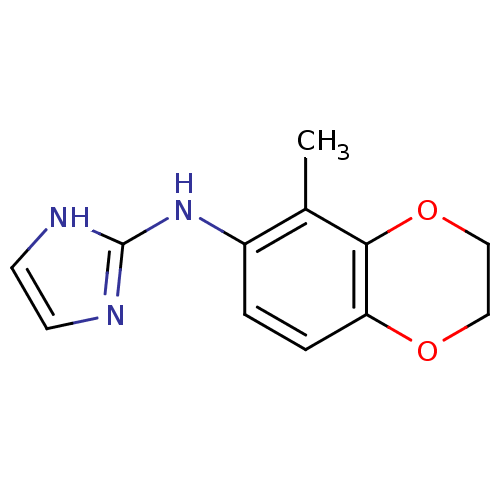
((1H-Imidazol-2-yl)-(5-methyl-2,3-dihydro-benzo[1,4...)Show InChI InChI=1S/C12H13N3O2/c1-8-9(15-12-13-4-5-14-12)2-3-10-11(8)17-7-6-16-10/h2-5H,6-7H2,1H3,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50030650
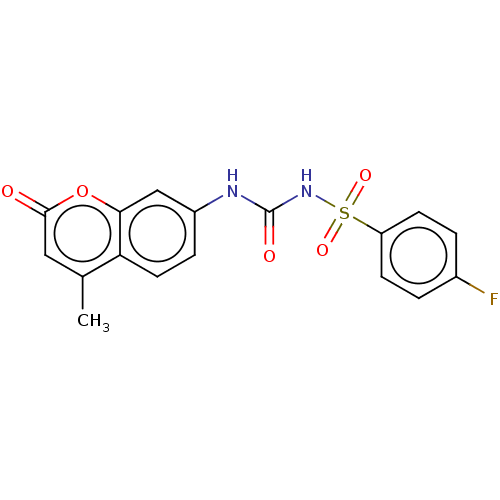
(CHEMBL3354141)Show SMILES Cc1cc(=O)oc2cc(NC(=O)NS(=O)(=O)c3ccc(F)cc3)ccc12 Show InChI InChI=1S/C17H13FN2O5S/c1-10-8-16(21)25-15-9-12(4-7-14(10)15)19-17(22)20-26(23,24)13-5-2-11(18)3-6-13/h2-9H,1H3,(H2,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as inhibition constant preincubated with enzyme for 10 mins by phenol red dye based st... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115586
BindingDB Entry DOI: 10.7270/Q2NK3JQJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50052880

(CHEMBL49137 | Imidazolidin-2-ylidene-(5-methyl-qui...)Show SMILES [#6]-c1c(ccc2nccnc12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C12H13N5/c1-8-9(17-12-15-6-7-16-12)2-3-10-11(8)14-5-4-13-10/h2-5H,6-7H2,1H3,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50453718
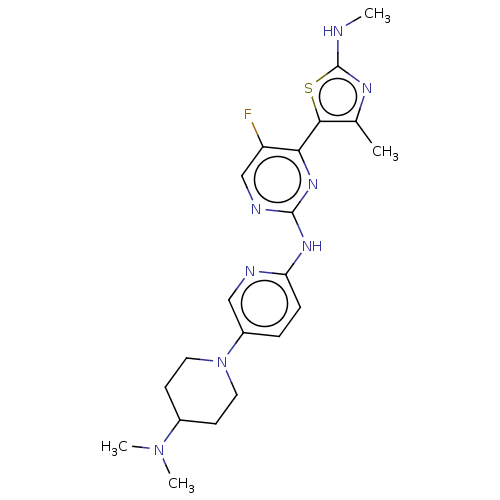
(CHEMBL5287062)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6](=O)-[#7]-[#6@H](-[#6]P([#8])([#8])=O)-[#6](-[#8])=O Show InChI InChI=1S/C19H32NO6P/c1-14(2)7-5-8-15(3)9-6-10-16(4)11-12-18(21)20-17(19(22)23)13-27(24,25)26/h7,9,11,17H,5-6,8,10,12-13H2,1-4H3,(H,20,21)(H,22,23)(H2,24,25,26)/b15-9+,16-11+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413816
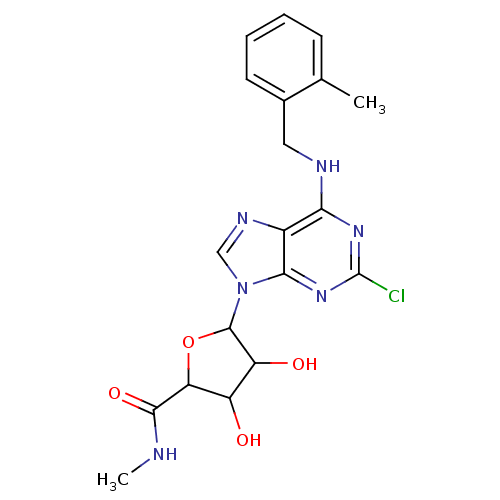
(CHEMBL516346)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3ccccc3C)nc(Cl)nc12 Show InChI InChI=1S/C19H21ClN6O4/c1-9-5-3-4-6-10(9)7-22-15-11-16(25-19(20)24-15)26(8-23-11)18-13(28)12(27)14(30-18)17(29)21-2/h3-6,8,12-14,18,27-28H,7H2,1-2H3,(H,21,29)(H,22,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413797

(CHEMBL488148)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NC3CC3)nc(Cl)nc12 Show InChI InChI=1S/C14H17ClN6O4/c1-16-12(24)9-7(22)8(23)13(25-9)21-4-17-6-10(18-5-2-3-5)19-14(15)20-11(6)21/h4-5,7-9,13,22-23H,2-3H2,1H3,(H,16,24)(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50596811

(CHEMBL5181231)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Cn2cc(\C=C\C(=O)c3cccc(c3)C(F)(F)F)c3ccccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114247
BindingDB Entry DOI: 10.7270/Q2MC941H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413817
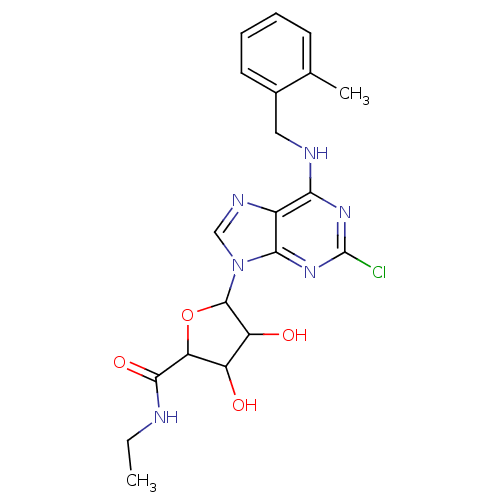
(CHEMBL473502)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3ccccc3C)nc(Cl)nc12 Show InChI InChI=1S/C20H23ClN6O4/c1-3-22-18(30)15-13(28)14(29)19(31-15)27-9-24-12-16(25-20(21)26-17(12)27)23-8-11-7-5-4-6-10(11)2/h4-7,9,13-15,19,28-29H,3,8H2,1-2H3,(H,22,30)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM34572
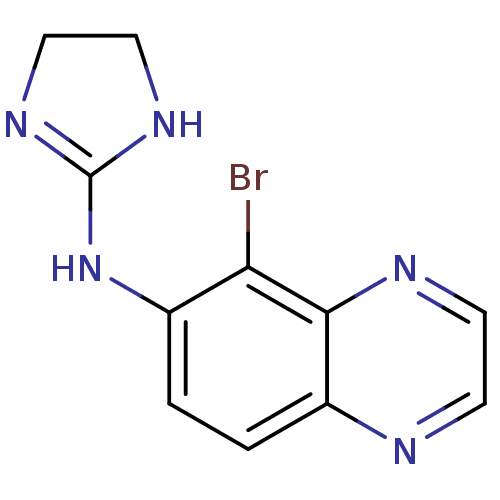
(BRIMONIDINE | CHEMBL844 | MLS000069370 | SMR000058...)Show InChI InChI=1S/C11H10BrN5/c12-9-7(17-11-15-5-6-16-11)1-2-8-10(9)14-4-3-13-8/h1-4H,5-6H2,(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of rauwolscine from human Alpha-2A adrenergic receptor expressed in CHO cells |
J Med Chem 40: 18-23 (1997)
Article DOI: 10.1021/jm9605142
BindingDB Entry DOI: 10.7270/Q2RV0MST |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413789
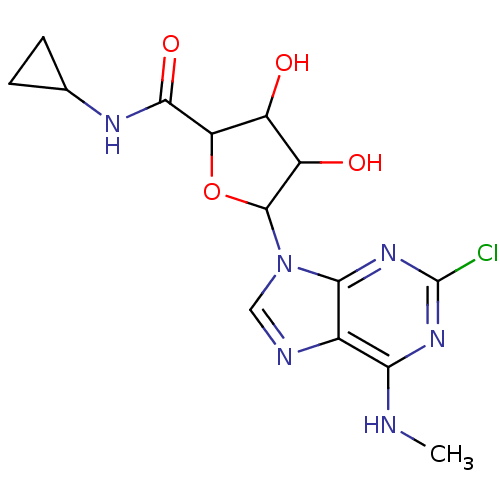
(CHEMBL521312)Show SMILES CNc1nc(Cl)nc2n(cnc12)C1OC(C(O)C1O)C(=O)NC1CC1 Show InChI InChI=1S/C14H17ClN6O4/c1-16-10-6-11(20-14(15)19-10)21(4-17-6)13-8(23)7(22)9(25-13)12(24)18-5-2-3-5/h4-5,7-9,13,22-23H,2-3H2,1H3,(H,18,24)(H,16,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413803

(CHEMBL487754)Show SMILES CCNC(=O)C1OC(C(O)C1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 Show InChI InChI=1S/C17H23ClN6O4/c1-2-19-15(27)12-10(25)11(26)16(28-12)24-7-20-9-13(21-8-5-3-4-6-8)22-17(18)23-14(9)24/h7-8,10-12,16,25-26H,2-6H2,1H3,(H,19,27)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50413806
(CHEMBL486374)Show SMILES OC1C(O)C(OC1C(=O)NC1CC1)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C20H20ClIN6O4/c21-20-26-16(23-7-9-2-1-3-10(22)6-9)12-17(27-20)28(8-24-12)19-14(30)13(29)15(32-19)18(31)25-11-4-5-11/h1-3,6,8,11,13-15,19,29-30H,4-5,7H2,(H,25,31)(H,23,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells |
Eur J Med Chem 44: 1377-82 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.022
BindingDB Entry DOI: 10.7270/Q20C4X03 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50033537

(CHEMBL121060 | N-[(3S,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 using [3H]DADLE as radioligand. |
J Med Chem 38: 1547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2GQ6ZDF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM13543
(3,4,5-Trimethoxy-N-(8-oxo-8-(2-(1,2,3,4-tetrahydro...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)NCCCCCCCC(=O)NNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H40N4O5/c1-38-26-19-21(20-27(39-2)30(26)40-3)31(37)32-18-12-6-4-5-7-17-28(36)34-35-29-22-13-8-10-15-24(22)33-25-16-11-9-14-23(25)29/h8,10,13,15,19-20H,4-7,9,11-12,14,16-18H2,1-3H3,(H,32,37)(H,33,35)(H,34,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23 | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... |
J Med Chem 49: 7540-4 (2006)
Article DOI: 10.1021/jm060742o
BindingDB Entry DOI: 10.7270/Q21Z42NF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data