 Found 1800 hits with Last Name = 'xu' and Initial = 'p'
Found 1800 hits with Last Name = 'xu' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50021655
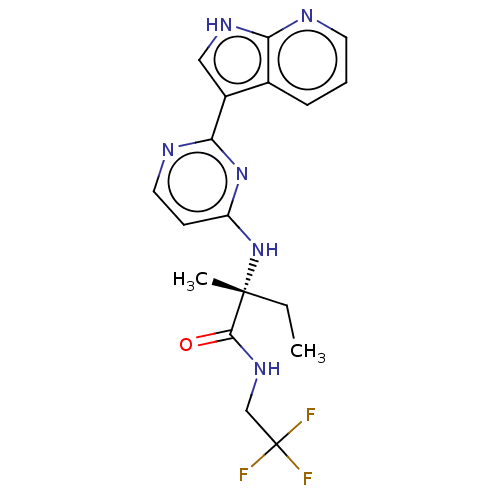
(DECERNOTINIB | US10112907, Example 00017 | US10766...)Show SMILES CC[C@@](C)(Nc1ccnc(n1)-c1c[nH]c2ncccc12)C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600600
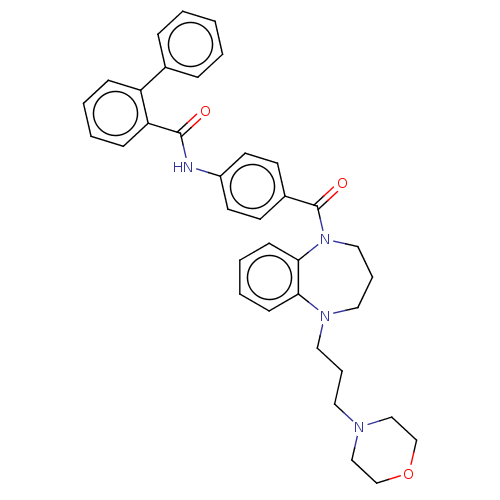
(CHEMBL5207885)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531924
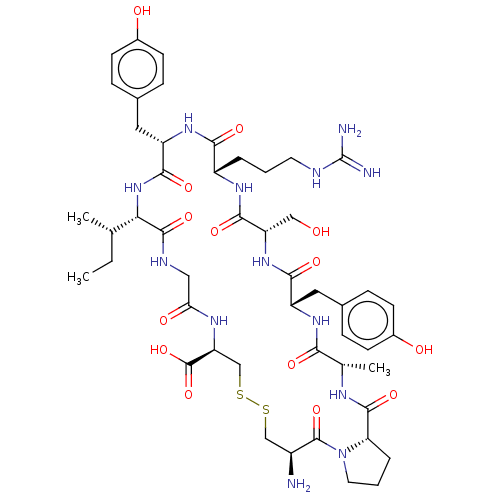
(CHEMBL4464577)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)CSSC[C@H](NC(=O)CNC1=O)C(O)=O |r| Show InChI InChI=1S/C49H71N13O14S2/c1-4-25(2)39-46(73)54-21-38(66)56-36(48(75)76)24-78-77-23-31(50)47(74)62-18-6-8-37(62)45(72)55-26(3)40(67)58-33(19-27-9-13-29(64)14-10-27)42(69)60-35(22-63)44(71)57-32(7-5-17-53-49(51)52)41(68)59-34(43(70)61-39)20-28-11-15-30(65)16-12-28/h9-16,25-26,31-37,39,63-65H,4-8,17-24,50H2,1-3H3,(H,54,73)(H,55,72)(H,56,66)(H,57,71)(H,58,67)(H,59,68)(H,60,69)(H,61,70)(H,75,76)(H4,51,52,53)/t25-,26-,31-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499241

(CHEMBL3735513)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C56H84N16O11S2/c1-31(2)25-39-49(77)69-42(27-35-15-9-6-10-16-35)51(79)70-43(54(82)83)30-85-84-29-37(57)53(81)72-22-12-18-44(72)52(80)64-33(4)45(73)65-38(17-11-21-62-55(58)59)47(75)67-41(26-34-13-7-5-8-14-34)50(78)68-40(48(76)63-32(3)46(74)66-39)28-36-19-23-71(24-20-36)56(60)61/h5-10,13-16,31-33,36-44H,11-12,17-30,57H2,1-4H3,(H3,60,61)(H,63,76)(H,64,80)(H,65,73)(H,66,74)(H,67,75)(H,68,78)(H,69,77)(H,70,79)(H,82,83)(H4,58,59,62)/t32-,33-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600605
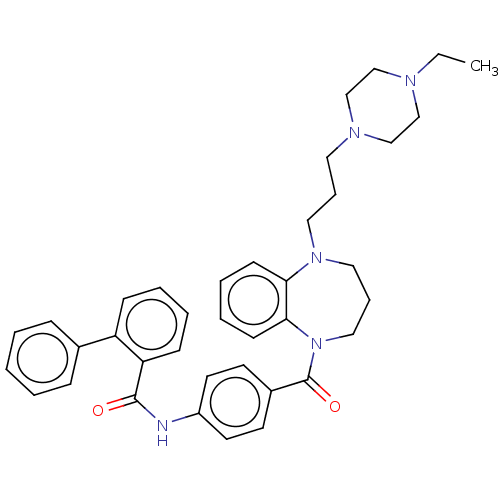
(CHEMBL5208751)Show SMILES CCN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499238
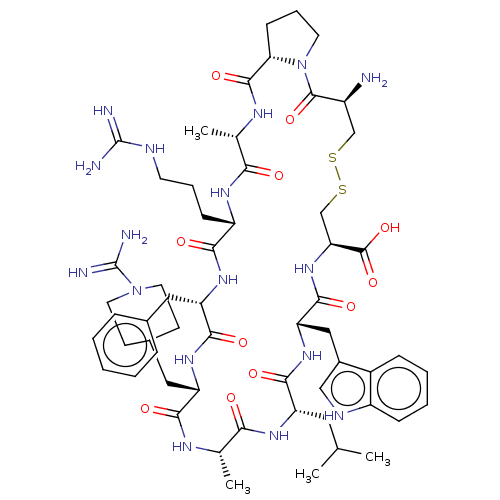
(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human wild-type plasma kallikrein catalytic domain after 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600607

(CHEMBL5207405)Show SMILES CCN(CC)CCCN1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50021655

(DECERNOTINIB | US10112907, Example 00017 | US10766...)Show SMILES CC[C@@](C)(Nc1ccnc(n1)-c1c[nH]c2ncccc12)C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50021655

(DECERNOTINIB | US10112907, Example 00017 | US10766...)Show SMILES CC[C@@](C)(Nc1ccnc(n1)-c1c[nH]c2ncccc12)C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Isochorismatase family protein
(Streptococcus pneumoniae) | BDBM50026863
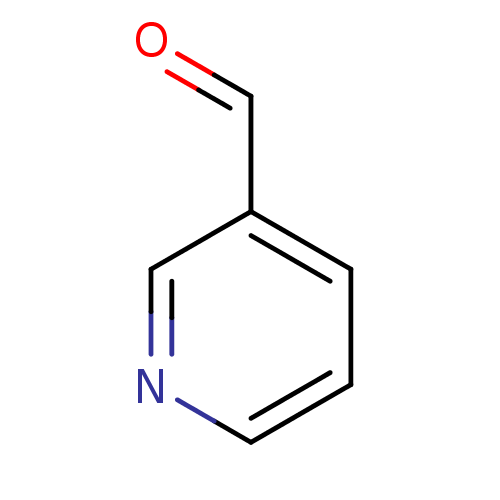
(CHEMBL268493 | Nicotinamidase Inhibitor, 16 | PncA...)Show InChI InChI=1S/C6H5NO/c8-5-6-2-1-3-7-4-6/h1-5H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600603

(CHEMBL5192961)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCCCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600611
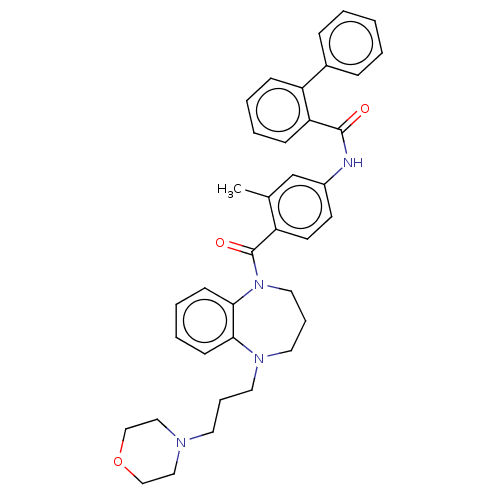
(CHEMBL5199628)Show SMILES Cc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50021655

(DECERNOTINIB | US10112907, Example 00017 | US10766...)Show SMILES CC[C@@](C)(Nc1ccnc(n1)-c1c[nH]c2ncccc12)C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600604
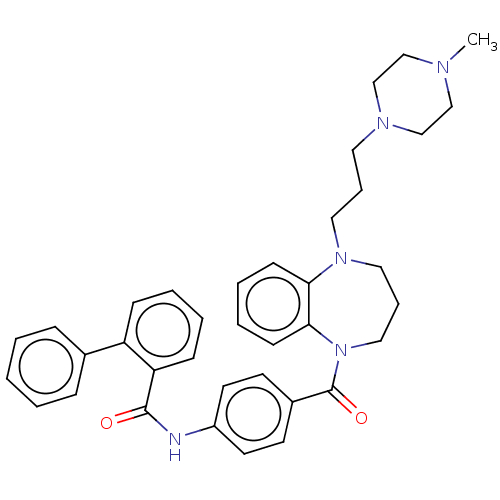
(CHEMBL5193621)Show SMILES CN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499238

(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531924

(CHEMBL4464577)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)CSSC[C@H](NC(=O)CNC1=O)C(O)=O |r| Show InChI InChI=1S/C49H71N13O14S2/c1-4-25(2)39-46(73)54-21-38(66)56-36(48(75)76)24-78-77-23-31(50)47(74)62-18-6-8-37(62)45(72)55-26(3)40(67)58-33(19-27-9-13-29(64)14-10-27)42(69)60-35(22-63)44(71)57-32(7-5-17-53-49(51)52)41(68)59-34(43(70)61-39)20-28-11-15-30(65)16-12-28/h9-16,25-26,31-37,39,63-65H,4-8,17-24,50H2,1-3H3,(H,54,73)(H,55,72)(H,56,66)(H,57,71)(H,58,67)(H,59,68)(H,60,69)(H,61,70)(H,75,76)(H4,51,52,53)/t25-,26-,31-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA serine protease domain preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407800
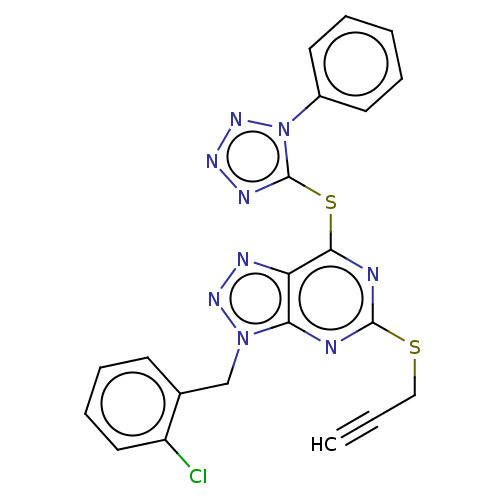
(CHEMBL5291138)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C38H53N11O5S/c39-37(40)43-15-5-12-26-33(51)47-28(20-25-11-7-17-55-25)35(53)48-21-24-10-2-1-8-22(24)18-31(48)36(54)49-29-14-4-3-9-23(29)19-30(49)34(52)46-27(32(50)45-26)13-6-16-44-38(41)42/h1-2,7-8,10-11,17,23,26-31H,3-6,9,12-16,18-21H2,(H,45,50)(H,46,52)(H,47,51)(H4,39,40,43)(H4,41,42,44)/t23?,26-,27-,28-,29?,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600601
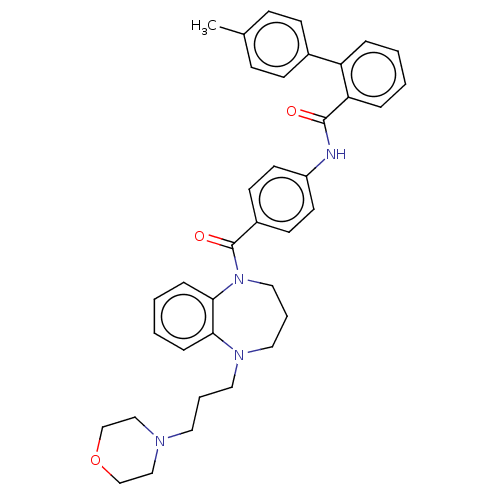
(CHEMBL5181318)Show SMILES Cc1ccc(cc1)-c1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531926

(CHEMBL4550668)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)CNC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C51H74N14O14S2/c1-5-26(2)41-49(78)56-22-40(70)59-37(42(52)71)24-80-81-25-38(58-28(4)67)50(79)65-19-7-9-39(65)48(77)57-27(3)43(72)61-34(20-29-10-14-31(68)15-11-29)45(74)63-36(23-66)47(76)60-33(8-6-18-55-51(53)54)44(73)62-35(46(75)64-41)21-30-12-16-32(69)17-13-30/h10-17,26-27,33-39,41,66,68-69H,5-9,18-25H2,1-4H3,(H2,52,71)(H,56,78)(H,57,77)(H,58,67)(H,59,70)(H,60,76)(H,61,72)(H,62,73)(H,63,74)(H,64,75)(H4,53,54,55)/t26-,27-,33-,34-,35-,36-,37-,38-,39-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531926

(CHEMBL4550668)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)CNC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C51H74N14O14S2/c1-5-26(2)41-49(78)56-22-40(70)59-37(42(52)71)24-80-81-25-38(58-28(4)67)50(79)65-19-7-9-39(65)48(77)57-27(3)43(72)61-34(20-29-10-14-31(68)15-11-29)45(74)63-36(23-66)47(76)60-33(8-6-18-55-51(53)54)44(73)62-35(46(75)64-41)21-30-12-16-32(69)17-13-30/h10-17,26-27,33-39,41,66,68-69H,5-9,18-25H2,1-4H3,(H2,52,71)(H,56,78)(H,57,77)(H,58,67)(H,59,70)(H,60,76)(H,61,72)(H,62,73)(H,63,74)(H,64,75)(H4,53,54,55)/t26-,27-,33-,34-,35-,36-,37-,38-,39-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Isochorismatase domain-containing protein
(Caenorhabditis elegans) | BDBM50026863

(CHEMBL268493 | Nicotinamidase Inhibitor, 16 | PncA...)Show InChI InChI=1S/C6H5NO/c8-5-6-2-1-3-7-4-6/h1-5H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499237

(CHEMBL3735217)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC2=O)C(O)=O |r| Show InChI InChI=1S/C59H91N17O11S2/c1-34(2)28-42-52(81)73-45(30-37-16-8-5-9-17-37)54(83)74-46(57(86)87)33-89-88-32-39(61)56(85)76-25-13-20-47(76)55(84)69-40(18-10-11-23-60)49(78)68-41(19-12-24-66-58(62)63)50(79)71-44(29-36-14-6-4-7-15-36)53(82)72-43(51(80)67-35(3)48(77)70-42)31-38-21-26-75(27-22-38)59(64)65/h4-9,14-17,34-35,38-47H,10-13,18-33,60-61H2,1-3H3,(H3,64,65)(H,67,80)(H,68,78)(H,69,84)(H,70,77)(H,71,79)(H,72,82)(H,73,81)(H,74,83)(H,86,87)(H4,62,63,66)/t35-,39-,40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600610
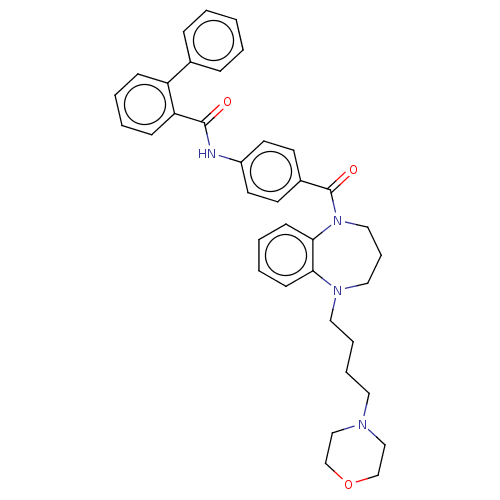
(CHEMBL5179440)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600609

(CHEMBL5172250)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50499241

(CHEMBL3735513)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C56H84N16O11S2/c1-31(2)25-39-49(77)69-42(27-35-15-9-6-10-16-35)51(79)70-43(54(82)83)30-85-84-29-37(57)53(81)72-22-12-18-44(72)52(80)64-33(4)45(73)65-38(17-11-21-62-55(58)59)47(75)67-41(26-34-13-7-5-8-14-34)50(78)68-40(48(76)63-32(3)46(74)66-39)28-36-19-23-71(24-20-36)56(60)61/h5-10,13-16,31-33,36-44H,11-12,17-30,57H2,1-4H3,(H3,60,61)(H,63,76)(H,64,80)(H,65,73)(H,66,74)(H,67,75)(H,68,78)(H,69,77)(H,70,79)(H,82,83)(H4,58,59,62)/t32-,33-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499238

(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein catalytic domain E217A mutant after 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600612

(CHEMBL5187046)Show SMILES Clc1ccc2N(CCCN3CCOCC3)CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
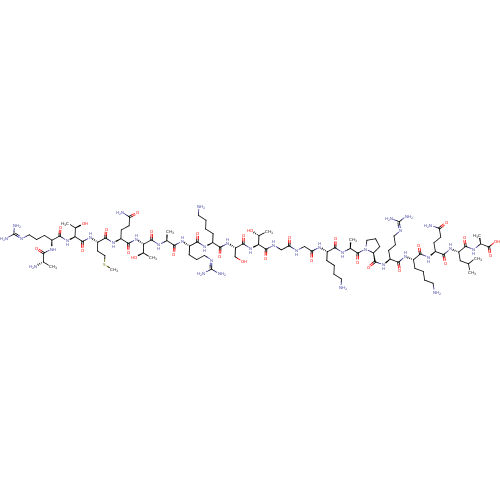
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50499237

(CHEMBL3735217)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC2=O)C(O)=O |r| Show InChI InChI=1S/C59H91N17O11S2/c1-34(2)28-42-52(81)73-45(30-37-16-8-5-9-17-37)54(83)74-46(57(86)87)33-89-88-32-39(61)56(85)76-25-13-20-47(76)55(84)69-40(18-10-11-23-60)49(78)68-41(19-12-24-66-58(62)63)50(79)71-44(29-36-14-6-4-7-15-36)53(82)72-43(51(80)67-35(3)48(77)70-42)31-38-21-26-75(27-22-38)59(64)65/h4-9,14-17,34-35,38-47H,10-13,18-33,60-61H2,1-3H3,(H3,64,65)(H,67,80)(H,68,78)(H,69,84)(H,70,77)(H,71,79)(H,72,82)(H,73,81)(H,74,83)(H,86,87)(H4,62,63,66)/t35-,39-,40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499240

(CHEMBL3735080)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C54H77N13O16S2/c1-27(2)19-35-45(74)63-39(23-43(71)72)49(78)65-41(53(82)83)26-85-84-25-34(55)52(81)67-16-4-5-42(67)51(80)58-28(3)44(73)59-36(20-29-6-10-32(69)11-7-29)48(77)64-40(24-68)50(79)62-38(22-31-14-17-66(18-15-31)54(56)57)47(76)61-37(46(75)60-35)21-30-8-12-33(70)13-9-30/h6-13,27-28,31,34-42,68-70H,4-5,14-26,55H2,1-3H3,(H3,56,57)(H,58,80)(H,59,73)(H,60,75)(H,61,76)(H,62,79)(H,63,74)(H,64,77)(H,65,78)(H,71,72)(H,82,83)/t28-,34-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346870

(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Mus musculus) | BDBM50499238

(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600608
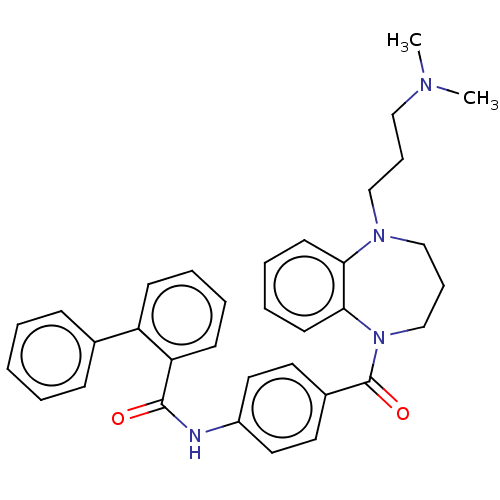
(CHEMBL5192496)Show SMILES CN(C)CCCN1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499238

(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein catalytic domain G99Y mutant after 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Isochorismatase family protein
(Streptococcus pneumoniae) | BDBM92852
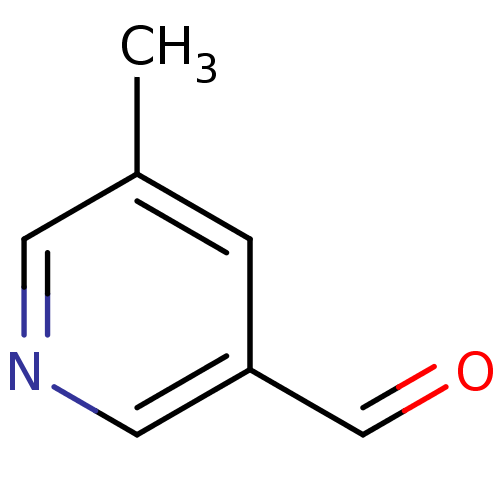
(Nicotinamidase Inhibitor, 19)Show InChI InChI=1S/C7H7NO/c1-6-2-7(5-9)4-8-3-6/h2-5H,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600593

(CHEMBL5188374)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600594
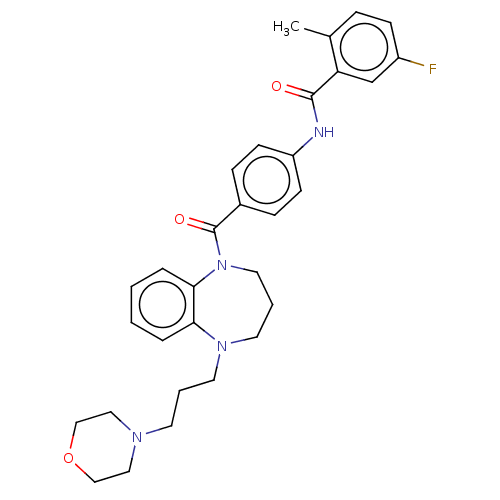
(CHEMBL5172734)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499242
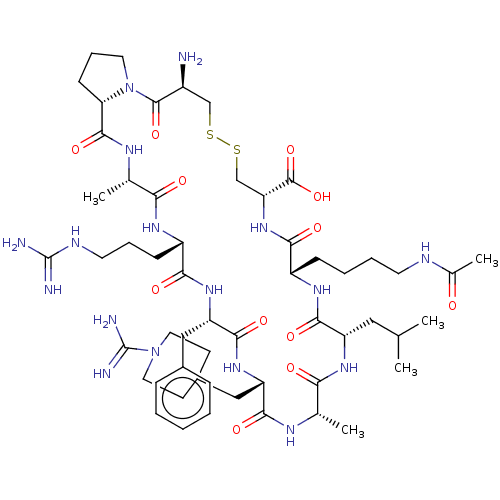
(CHEMBL3736473)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@@H](NC(=O)[C@H](CCCCNC(C)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C55H89N17O12S2/c1-30(2)25-39-49(79)66-37(15-9-10-20-61-33(5)73)47(77)70-42(53(83)84)29-86-85-28-36(56)52(82)72-22-12-17-43(72)51(81)64-32(4)44(74)65-38(16-11-21-62-54(57)58)46(76)68-41(26-34-13-7-6-8-14-34)50(80)69-40(48(78)63-31(3)45(75)67-39)27-35-18-23-71(24-19-35)55(59)60/h6-8,13-14,30-32,35-43H,9-12,15-29,56H2,1-5H3,(H3,59,60)(H,61,73)(H,63,78)(H,64,81)(H,65,74)(H,66,79)(H,67,75)(H,68,76)(H,69,80)(H,70,77)(H,83,84)(H4,57,58,62)/t31-,32-,36-,37-,38-,39-,40-,41-,42+,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein for 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600606
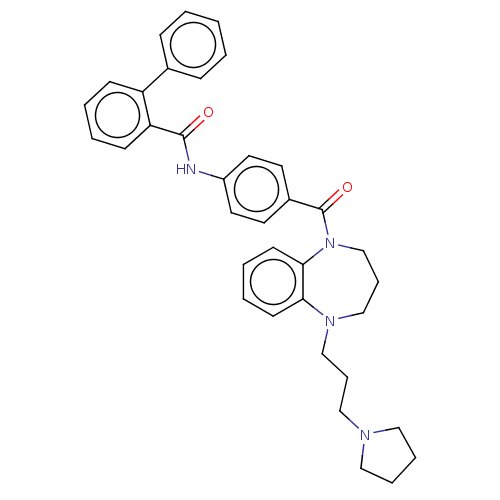
(CHEMBL5181341)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Isochorismatase family protein
(Streptococcus pneumoniae) | BDBM92850

(Nicotinamidase Inhibitor, 17)Show InChI InChI=1S/C6H4BrNO/c7-6-1-5(4-9)2-8-3-6/h1-4H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529667

(CHEMBL4444904 | US10806720, Compound 11 | US112305...)Show SMILES C[C@](O)(Cn1ccc2cc(F)ccc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H15F4N3O2/c1-19(29,11-27-7-6-12-8-14(21)3-5-17(12)27)18(28)26-15-4-2-13(10-25)16(9-15)20(22,23)24/h2-9,29H,11H2,1H3,(H,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-mibolerone from GST-tagged human AR-LBD expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115554
BindingDB Entry DOI: 10.7270/Q2PK0KVX |
More data for this
Ligand-Target Pair | |
Isochorismatase domain-containing protein
(Caenorhabditis elegans) | BDBM92850

(Nicotinamidase Inhibitor, 17)Show InChI InChI=1S/C6H4BrNO/c7-6-1-5(4-9)2-8-3-6/h1-4H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600596
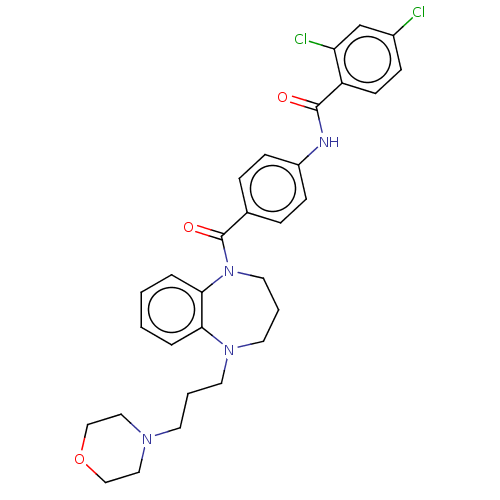
(CHEMBL5207214)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2CCCN(CCCN3CCOCC3)c3ccccc23)c(Cl)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600595
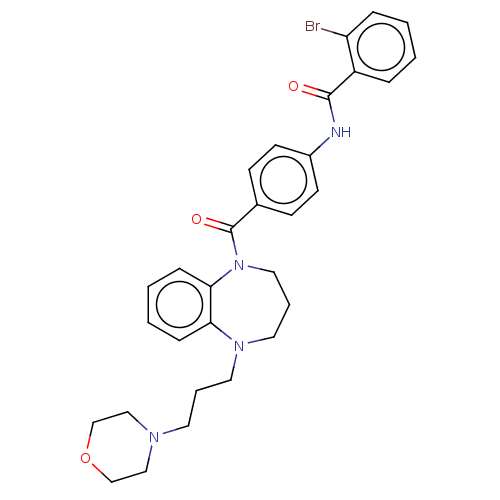
(CHEMBL5207597)Show SMILES Brc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Pyrazinamidase/nicotinamidase, PncA protein
(Borrelia burgdorferi) | BDBM50026863

(CHEMBL268493 | Nicotinamidase Inhibitor, 16 | PncA...)Show InChI InChI=1S/C6H5NO/c8-5-6-2-1-3-7-4-6/h1-5H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Isochorismatase domain-containing protein
(Caenorhabditis elegans) | BDBM50026863

(CHEMBL268493 | Nicotinamidase Inhibitor, 16 | PncA...)Show InChI InChI=1S/C6H5NO/c8-5-6-2-1-3-7-4-6/h1-5H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50499240

(CHEMBL3735080)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C54H77N13O16S2/c1-27(2)19-35-45(74)63-39(23-43(71)72)49(78)65-41(53(82)83)26-85-84-25-34(55)52(81)67-16-4-5-42(67)51(80)58-28(3)44(73)59-36(20-29-6-10-32(69)11-7-29)48(77)64-40(24-68)50(79)62-38(22-31-14-17-66(18-15-31)54(56)57)47(76)61-37(46(75)60-35)21-30-8-12-33(70)13-9-30/h6-13,27-28,31,34-42,68-70H,4-5,14-26,55H2,1-3H3,(H3,56,57)(H,58,80)(H,59,73)(H,60,75)(H,61,76)(H,62,79)(H,63,74)(H,64,77)(H,65,78)(H,71,72)(H,82,83)/t28-,34-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein catalytic domain G99Y mutant after 15 mins using H-D-Pro-Phe-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Isochorismatase family protein
(Streptococcus pneumoniae) | BDBM92851
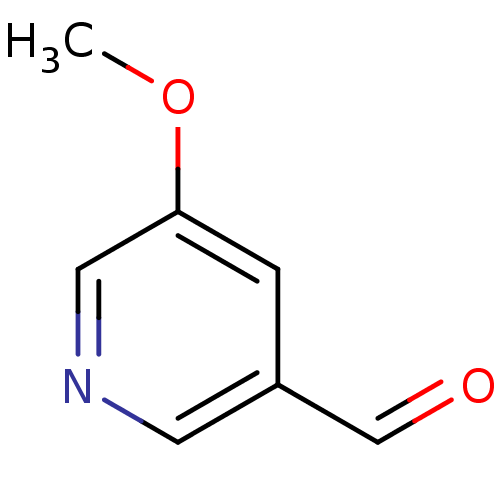
(Nicotinamidase Inhibitor, 18)Show InChI InChI=1S/C7H7NO2/c1-10-7-2-6(5-9)3-8-4-7/h2-5H,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |
Isochorismatase domain-containing protein
(Caenorhabditis elegans) | BDBM92851

(Nicotinamidase Inhibitor, 18)Show InChI InChI=1S/C7H7NO2/c1-10-7-2-6(5-9)3-8-4-7/h2-5H,1H3 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
| Assay Description
Nicotinamidase activity was monitored by coupling the production of ammonia with the consumption of NAD(P)H by the enzyme bovine glutamate dehydrogen... |
Biochemistry 49: 10421-39 (2010)
Article DOI: 10.1021/bi1012518
BindingDB Entry DOI: 10.7270/Q2G44NWC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data