 Found 191 hits with Last Name = 'lira' and Initial = 'r'
Found 191 hits with Last Name = 'lira' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552265
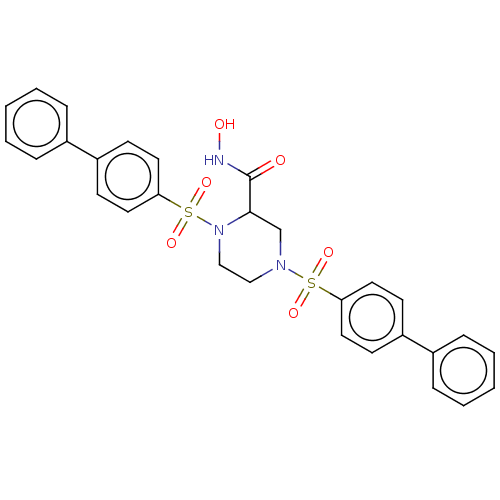
(CHEMBL4799253)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552271
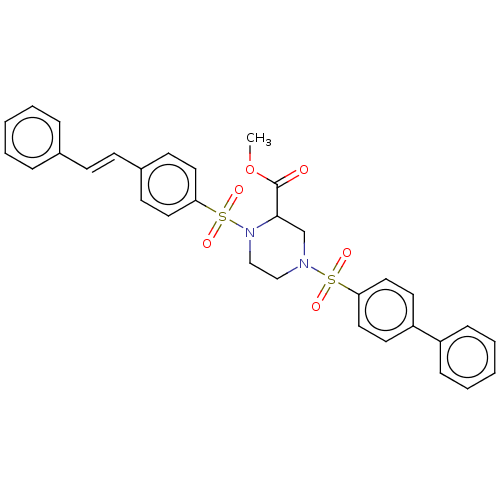
(CHEMBL4743280)Show SMILES COC(=O)C1CN(CCN1S(=O)(=O)c1ccc(\C=C\c2ccccc2)cc1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552264
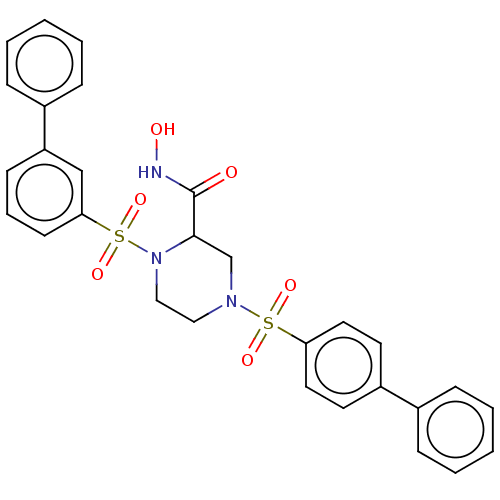
(CHEMBL4782486)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1cccc(c1)-c1ccccc1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552268
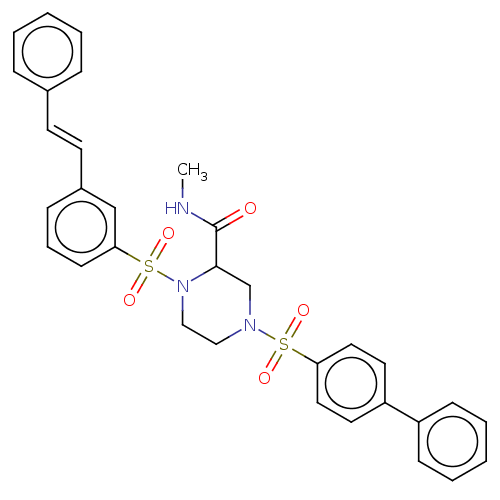
(CHEMBL4799260)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1cccc(\C=C\c2ccccc2)c1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552275
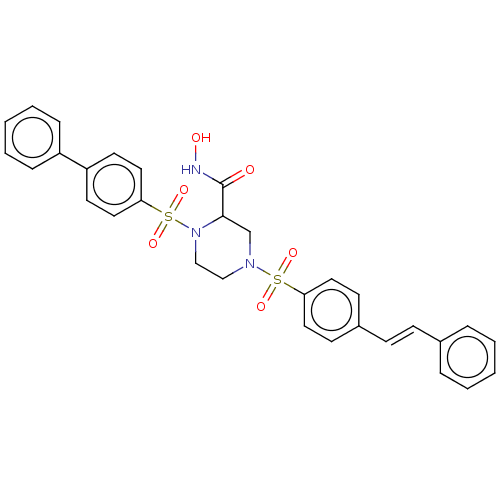
(CHEMBL4797916)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1ccc(\C=C\c2ccccc2)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552272
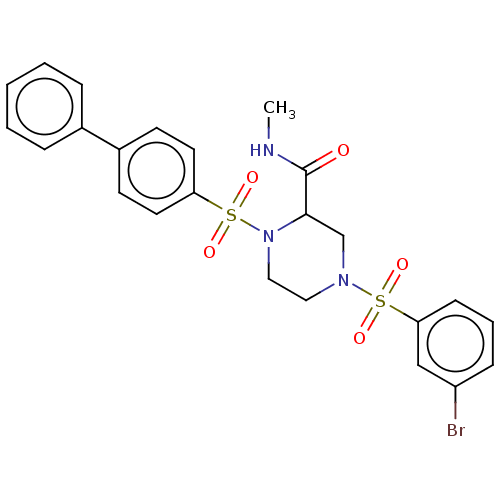
(CHEMBL4794156)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1cccc(Br)c1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552263
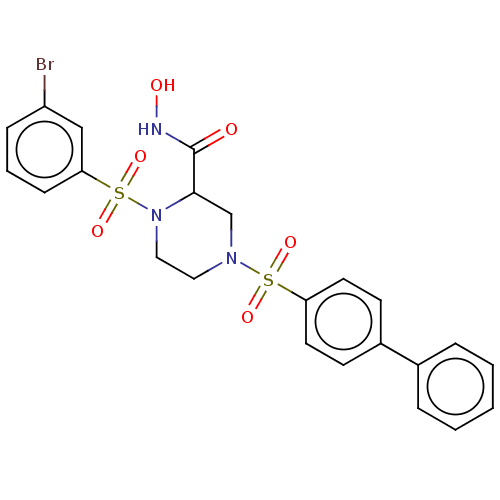
(CHEMBL4749474)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1cccc(Br)c1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552269
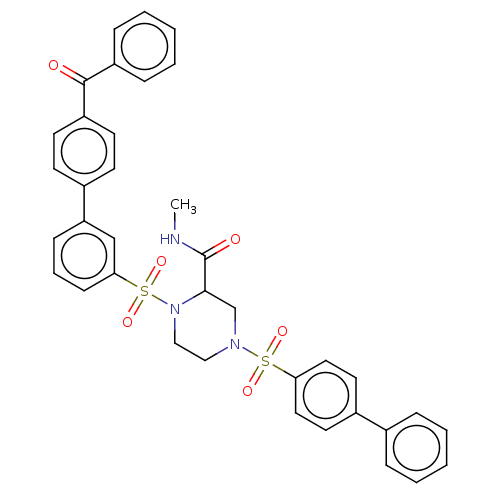
(CHEMBL4785958)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1cccc(c1)-c1ccc(cc1)C(=O)c1ccccc1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552270
(CHEMBL4741390)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1cccc(CCc2ccccc2)c1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552266

(CHEMBL4753948)Show SMILES COC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552273
(CHEMBL4763576)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1ccc(I)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50461717
(CHEMBL4225532)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(Br)cc1)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H22BrN3O5S/c25-20-10-12-21(13-11-20)34(32,33)28-15-14-27(16-22(28)23(29)26-31)24(30)19-8-6-18(7-9-19)17-4-2-1-3-5-17/h1-13,22,31H,14-16H2,(H,26,29) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552258

(CHEMBL4759772)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(Br)cc1)C(=O)c1ccc2ccccc2c1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552261
(CHEMBL4797275)Show SMILES COC(=O)C1CN(Cc2ccc(cc2)-c2ccccc2)CCN1S(=O)(=O)c1ccc(Br)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552252

(CHEMBL4787612)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(I)cc1)C(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552274

(CHEMBL4747594)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)S(=O)(=O)c1ccc(cc1)-c1cccnc1OC | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552267
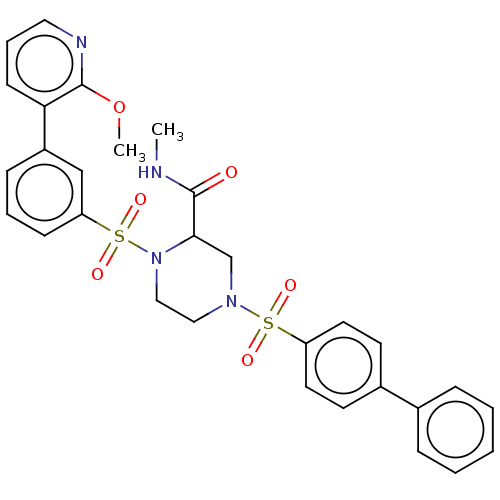
(CHEMBL4749198)Show SMILES CNC(=O)C1CN(CCN1S(=O)(=O)c1cccc(c1)-c1cccnc1OC)S(=O)(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552262
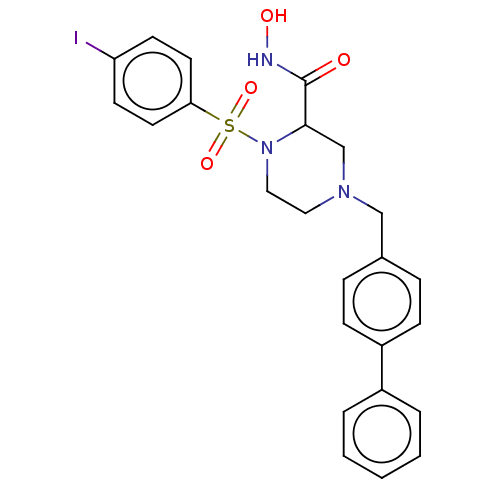
(CHEMBL4746210)Show SMILES ONC(=O)C1CN(Cc2ccc(cc2)-c2ccccc2)CCN1S(=O)(=O)c1ccc(I)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552260
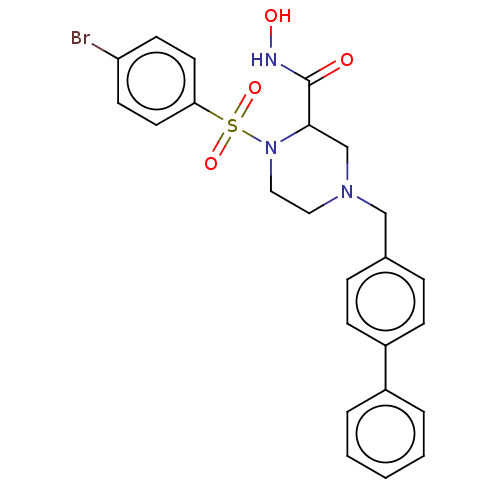
(CHEMBL4786923)Show SMILES ONC(=O)C1CN(Cc2ccc(cc2)-c2ccccc2)CCN1S(=O)(=O)c1ccc(Br)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552257

(CHEMBL4744749)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(Br)cc1)C(=O)c1cccc2ccccc12 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552253
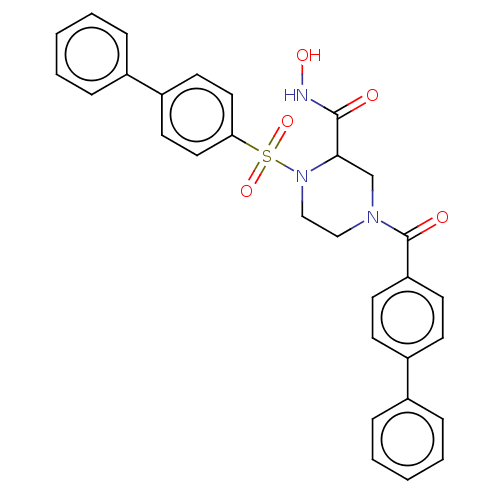
(CHEMBL4748768)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccccc1)C(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552254
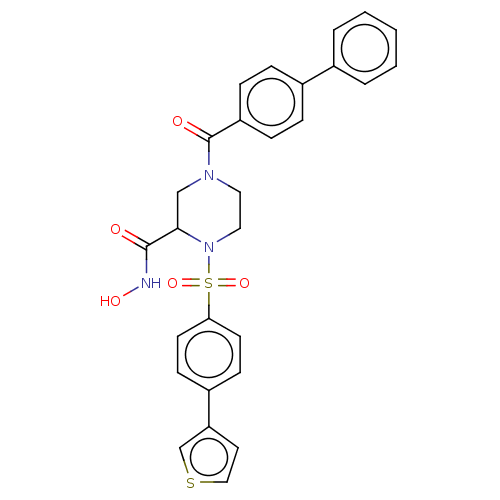
(CHEMBL4760331)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)-c1ccsc1)C(=O)c1ccc(cc1)-c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552256
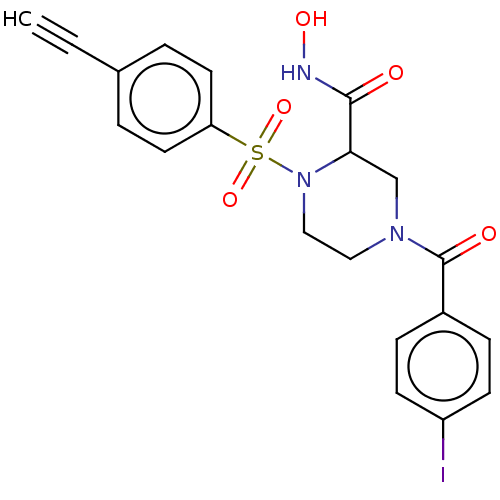
(CHEMBL4743581)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)C#C)C(=O)c1ccc(I)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552276
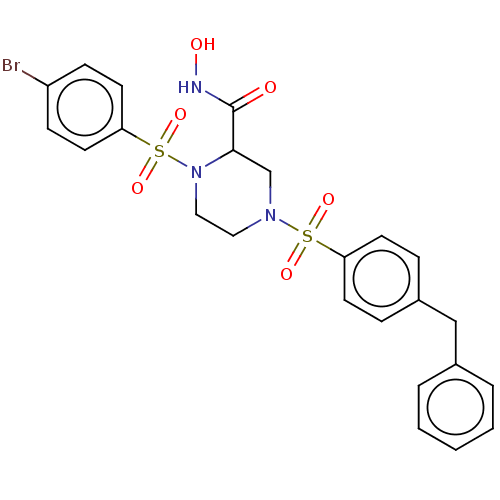
(CHEMBL4779972)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(Br)cc1)S(=O)(=O)c1ccc(Cc2ccccc2)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552277
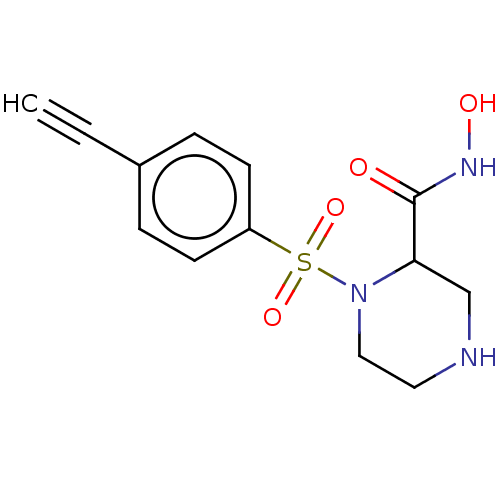
(CHEMBL4795927) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552259
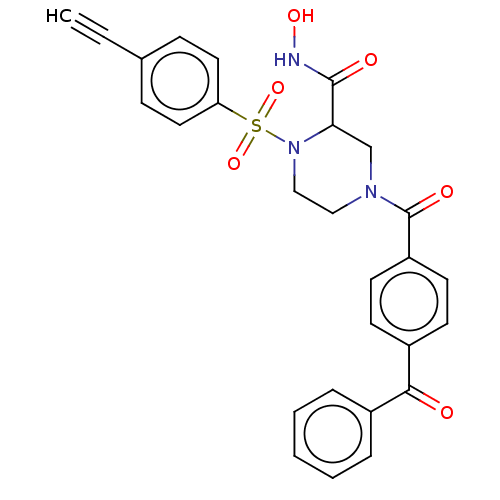
(CHEMBL4793336)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(cc1)C#C)C(=O)c1ccc(cc1)C(=O)c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Falcilysin
(Plasmodium falciparum (isolate 3D7)) | BDBM50552255

(CHEMBL4783383)Show SMILES ONC(=O)C1CN(CCN1S(=O)(=O)c1ccc(Br)cc1)C(=O)c1ccc(I)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum FLN |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127683
BindingDB Entry DOI: 10.7270/Q25B064W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136576
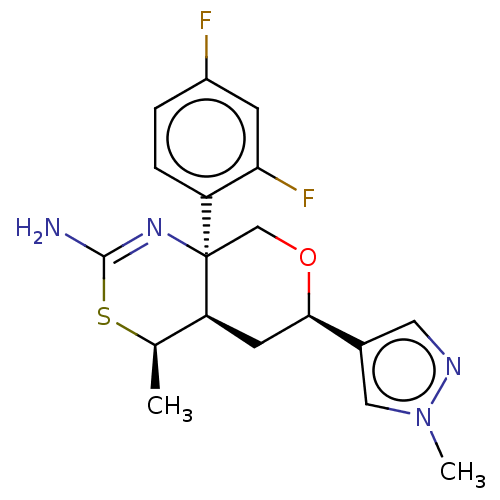
(US8865706, 16)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cnn(C)c1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H20F2N4OS/c1-10-14-6-16(11-7-22-24(2)8-11)25-9-18(14,23-17(21)26-10)13-4-3-12(19)5-15(13)20/h3-5,7-8,10,14,16H,6,9H2,1-2H3,(H2,21,23)/t10-,14+,16-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081645

(CHEMBL3422237)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cc(C)no1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-4-15(24-22-9)14-5-10-7-25-16(20)21-17(10,8-23-14)12-3-2-11(18)6-13(12)19/h2-4,6,10,14H,5,7-8H2,1H3,(H2,20,21)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM41536
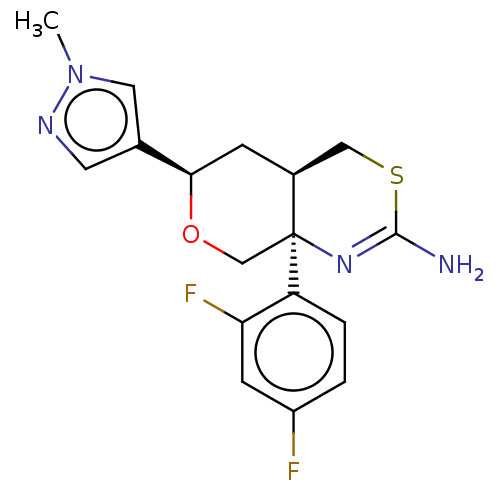
(US8865706, 15)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:13| Show InChI InChI=1S/C19H15F6N5O/c20-12-3-13(21)18(23)17(22)11(12)7-29-6-10(5-26-29)27-16(31)8-30-15(19(24)25)4-14(28-30)9-1-2-9/h3-6,9,19H,1-2,7-8H2,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081646
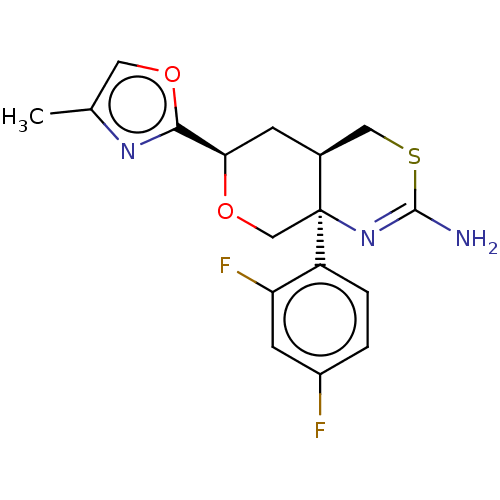
(CHEMBL3422236)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1nc(C)co1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-6-23-15(21-9)14-4-10-7-25-16(20)22-17(10,8-24-14)12-3-2-11(18)5-13(12)19/h2-3,5-6,10,14H,4,7-8H2,1H3,(H2,20,22)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50229129
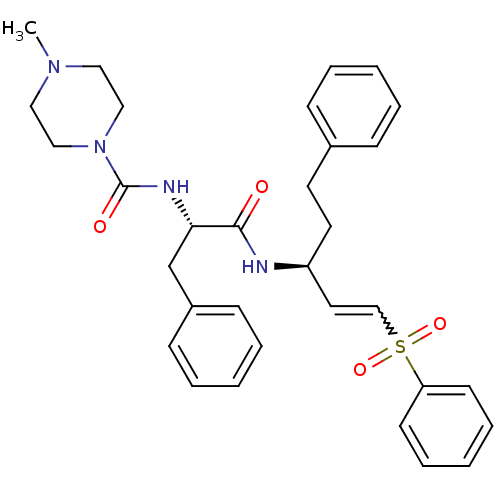
(4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:31.34| Show InChI InChI=1S/C32H38N4O4S/c1-35-20-22-36(23-21-35)32(38)34-30(25-27-13-7-3-8-14-27)31(37)33-28(18-17-26-11-5-2-6-12-26)19-24-41(39,40)29-15-9-4-10-16-29/h2-16,19,24,28,30H,17-18,20-23,25H2,1H3,(H,33,37)(H,34,38)/t28-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain by fluorescence technique |
Bioorg Med Chem Lett 18: 5860-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.012
BindingDB Entry DOI: 10.7270/Q21N80Z2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148176

(US8962616, 22 | US8962616, 4)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1nc(C)co1)c1ccc(F)cc1F |r,c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-7-24-16(22-9)15-6-13-10(2)26-17(21)23-18(13,8-25-15)12-4-3-11(19)5-14(12)20/h3-5,7,10,13,15H,6,8H2,1-2H3,(H2,21,23)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136570
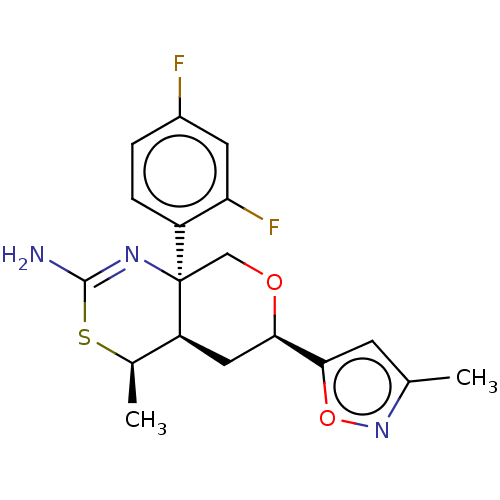
(US8865706, 9)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cc(C)no1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-5-16(25-23-9)15-7-13-10(2)26-17(21)22-18(13,8-24-15)12-4-3-11(19)6-14(12)20/h3-6,10,13,15H,7-8H2,1-2H3,(H2,21,22)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136583

(US8865706, 22)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H19F3N4OS/c1-25-8-10(7-23-25)15-5-13-16(6-19)27-17(22)24-18(13,9-26-15)12-3-2-11(20)4-14(12)21/h2-4,7-8,13,15-16H,5-6,9H2,1H3,(H2,22,24)/t13-,15+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM603960

(US11655252, Compound IC)Show SMILES C[C@H](O)Cn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >8.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081642
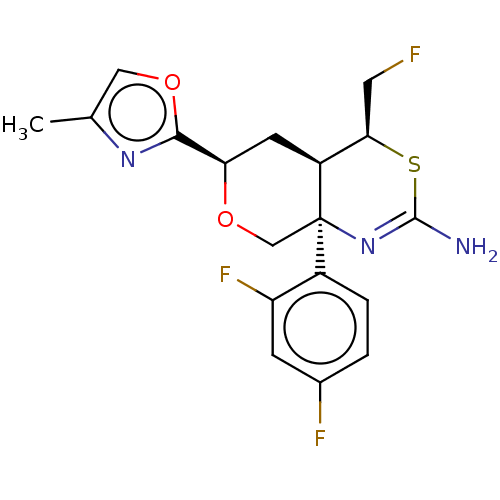
(CHEMBL3422244)Show SMILES [H][C@@]12C[C@@H](OC[C@@]1(N=C(N)S[C@@H]2CF)c1ccc(F)cc1F)c1nc(C)co1 |r,t:8| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-7-25-16(23-9)14-5-12-15(6-19)27-17(22)24-18(12,8-26-14)11-3-2-10(20)4-13(11)21/h2-4,7,12,14-15H,5-6,8H2,1H3,(H2,22,24)/t12-,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136574
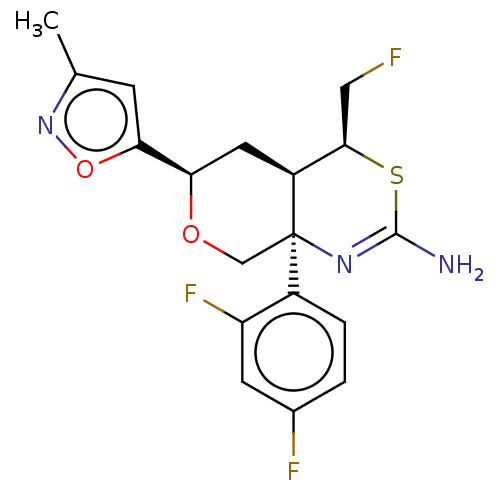
(US8865706, 13)Show SMILES Cc1cc(on1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-4-15(26-24-9)14-6-12-16(7-19)27-17(22)23-18(12,8-25-14)11-3-2-10(20)5-13(11)21/h2-5,12,14,16H,6-8H2,1H3,(H2,22,23)/t12-,14+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50078322
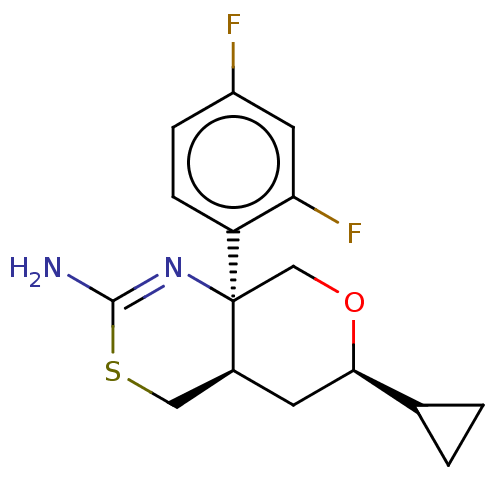
(CHEMBL3414710)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)C1CC1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C16H18F2N2OS/c17-11-3-4-12(13(18)6-11)16-8-21-14(9-1-2-9)5-10(16)7-22-15(19)20-16/h3-4,6,9-10,14H,1-2,5,7-8H2,(H2,19,20)/t10-,14+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA |
J Med Chem 58: 2678-702 (2015)
Article DOI: 10.1021/jm501833t
BindingDB Entry DOI: 10.7270/Q2W37Z1D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50078321

(CHEMBL3414711 | US9260455, 7)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](CC(C)C)C2)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H22F2N2OS/c1-10(2)5-13-6-11-8-23-16(20)21-17(11,9-22-13)14-4-3-12(18)7-15(14)19/h3-4,7,10-11,13H,5-6,8-9H2,1-2H3,(H2,20,21)/t11-,13-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA |
J Med Chem 58: 2678-702 (2015)
Article DOI: 10.1021/jm501833t
BindingDB Entry DOI: 10.7270/Q2W37Z1D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148173
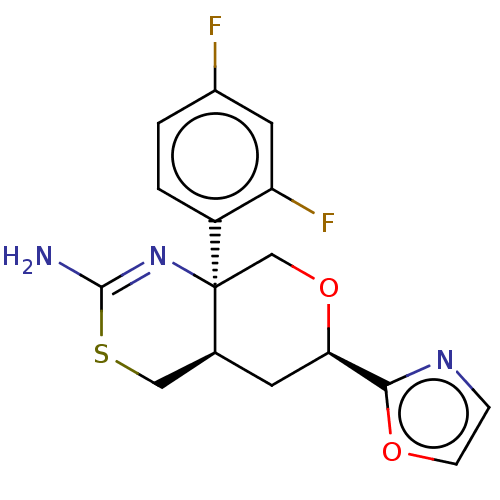
(US8962616, 1)Show SMILES NC1=N[C@]2(CO[C@H](C[C@H]2CS1)c1ncco1)c1ccc(F)cc1F |r,t:1| Show InChI InChI=1S/C16H15F2N3O2S/c17-10-1-2-11(12(18)6-10)16-8-23-13(14-20-3-4-22-14)5-9(16)7-24-15(19)21-16/h1-4,6,9,13H,5,7-8H2,(H2,19,21)/t9-,13+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM603961
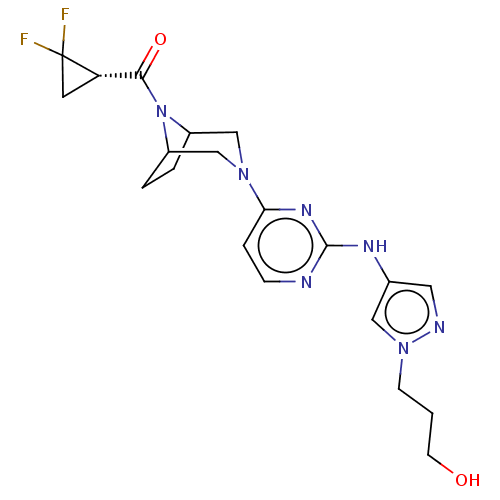
(US11655252, Compound ID)Show SMILES OCCCn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50078324
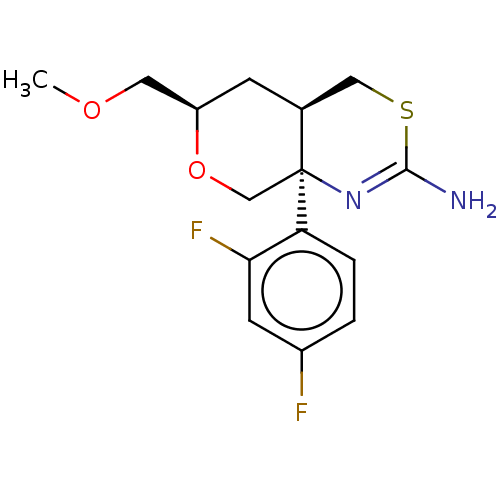
(CHEMBL3414708 | US9260455, 8)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](COC)C2)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C15H18F2N2O2S/c1-20-6-11-4-9-7-22-14(18)19-15(9,8-21-11)12-3-2-10(16)5-13(12)17/h2-3,5,9,11H,4,6-8H2,1H3,(H2,18,19)/t9-,11+,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA |
J Med Chem 58: 2678-702 (2015)
Article DOI: 10.1021/jm501833t
BindingDB Entry DOI: 10.7270/Q2W37Z1D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM603958
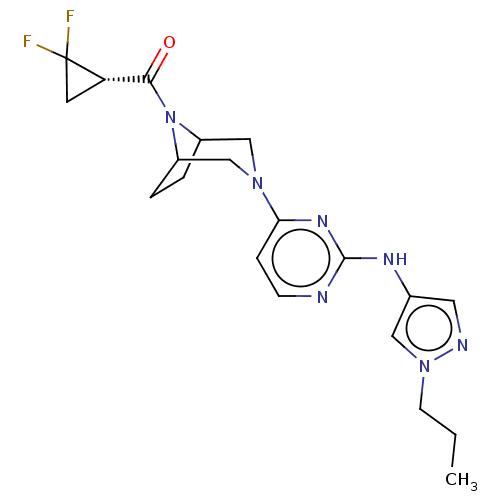
(US11655252, Compound IA)Show SMILES CCCn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136576

(US8865706, 16)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cnn(C)c1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H20F2N4OS/c1-10-14-6-16(11-7-22-24(2)8-11)25-9-18(14,23-17(21)26-10)13-4-3-12(19)5-15(13)20/h3-5,7-8,10,14,16H,6,9H2,1-2H3,(H2,21,23)/t10-,14+,16-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM603958

(US11655252, Compound IA)Show SMILES CCCn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50078349
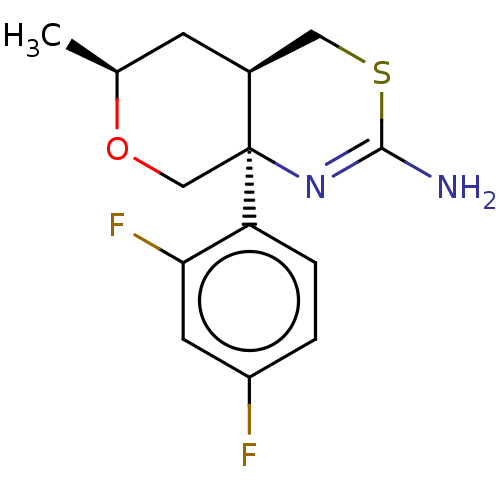
(CHEMBL3414707 | US9260455, 3)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](C)C2)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C14H16F2N2OS/c1-8-4-9-6-20-13(17)18-14(9,7-19-8)11-3-2-10(15)5-12(11)16/h2-3,5,8-9H,4,6-7H2,1H3,(H2,17,18)/t8-,9-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA |
J Med Chem 58: 2678-702 (2015)
Article DOI: 10.1021/jm501833t
BindingDB Entry DOI: 10.7270/Q2W37Z1D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM603960

(US11655252, Compound IC)Show SMILES C[C@H](O)Cn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM603961

(US11655252, Compound ID)Show SMILES OCCCn1cc(Nc2nccc(n2)N2CC3CCC(C2)N3C(=O)[C@@H]2CC2(F)F)cn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1W0G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM41536

(US8865706, 15)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:13| Show InChI InChI=1S/C19H15F6N5O/c20-12-3-13(21)18(23)17(22)11(12)7-29-6-10(5-26-29)27-16(31)8-30-15(19(24)25)4-14(28-30)9-1-2-9/h3-6,9,19H,1-2,7-8H2,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data