 Found 12210 hits with Last Name = 'mal' and Initial = 'r'
Found 12210 hits with Last Name = 'mal' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514220
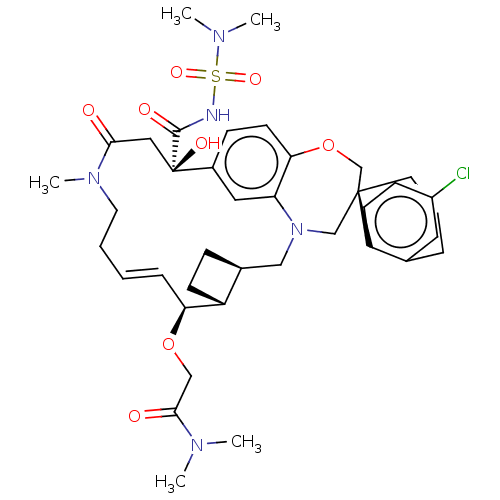
(CHEMBL4535151 | US11274105, Example 188)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCC(=O)N(C)C)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:15| Show InChI InChI=1S/C39H52ClN5O8S/c1-42(2)36(47)23-52-33-10-6-7-18-44(5)35(46)21-39(49,37(48)41-54(50,51)43(3)4)28-12-16-34-32(20-28)45(22-27-11-14-30(27)33)24-38(25-53-34)17-8-9-26-19-29(40)13-15-31(26)38/h6,10,12-13,15-16,19-20,27,30,33,49H,7-9,11,14,17-18,21-25H2,1-5H3,(H,41,48)/b10-6+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514203
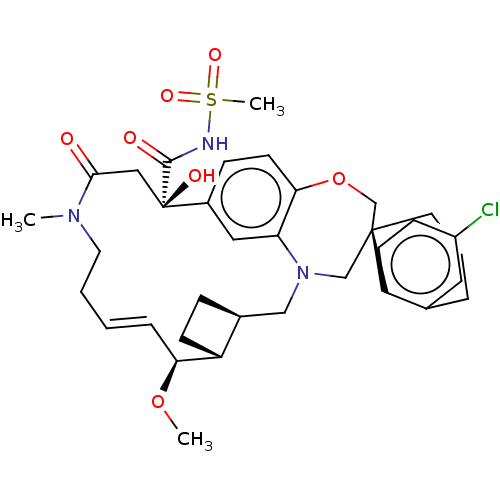
(CHEMBL4593361 | US11274105, Example 6)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(C)(=O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C35H44ClN3O7S/c1-38-16-5-4-8-30(45-2)27-12-9-24(27)20-39-21-34(15-6-7-23-17-26(36)11-13-28(23)34)22-46-31-14-10-25(18-29(31)39)35(42,19-32(38)40)33(41)37-47(3,43)44/h4,8,10-11,13-14,17-18,24,27,30,42H,5-7,9,12,15-16,19-22H2,1-3H3,(H,37,41)/b8-4+/t24-,27+,30-,34-,35+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514222
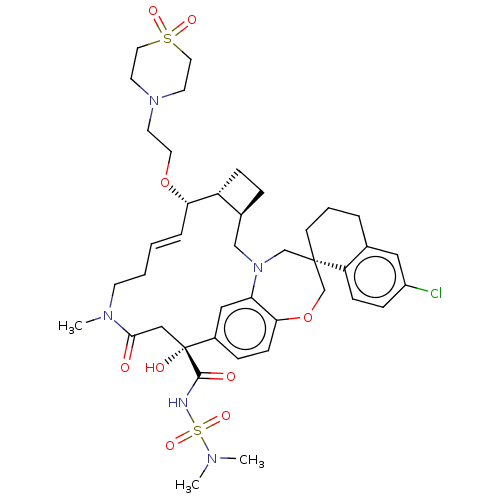
(CHEMBL4580244 | US11274105, Example 193)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCS(=O)(=O)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C41H56ClN5O9S2/c1-44(2)58(53,54)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(55-20-17-46-18-21-57(51,52)22-19-46)33-12-9-30(33)26-47-27-40(28-56-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514196
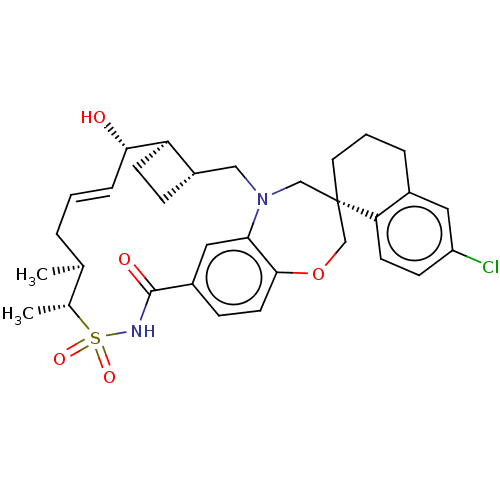
(CHEMBL4476472)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H39ClN2O5S/c1-20-5-3-7-29(36)26-11-8-24(26)17-35-18-32(14-4-6-22-15-25(33)10-12-27(22)32)19-40-30-13-9-23(16-28(30)35)31(37)34-41(38,39)21(20)2/h3,7,9-10,12-13,15-16,20-21,24,26,29,36H,4-6,8,11,14,17-19H2,1-2H3,(H,34,37)/b7-3+/t20-,21+,24-,26+,29-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0558 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514202

(CHEMBL4446369 | US11274105, Example 179)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C40H52ClF2N5O7S/c1-45(2)56(52,53)44-37(50)40(51)21-36(49)46(3)16-5-4-8-34(54-18-17-47-24-39(42,43)25-47)31-12-9-28(31)22-48-23-38(26-55-35-14-10-29(40)20-33(35)48)15-6-7-27-19-30(41)11-13-32(27)38/h4,8,10-11,13-14,19-20,28,31,34,51H,5-7,9,12,15-18,21-26H2,1-3H3,(H,44,50)/b8-4+/t28-,31+,34-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514199
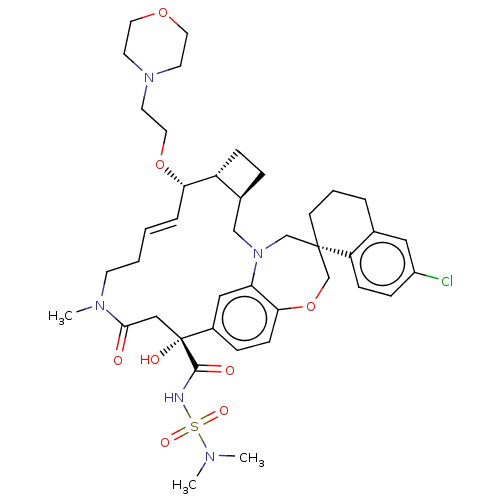
(CHEMBL4553660 | US11274105, Example 182)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCOCC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(54-22-19-46-17-20-53-21-18-46)33-12-9-30(33)26-47-27-40(28-55-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031895
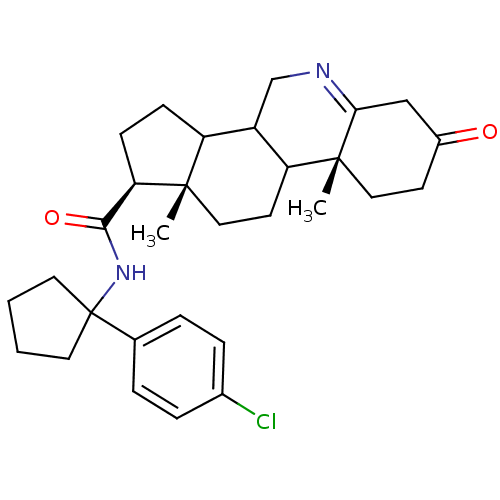
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514200
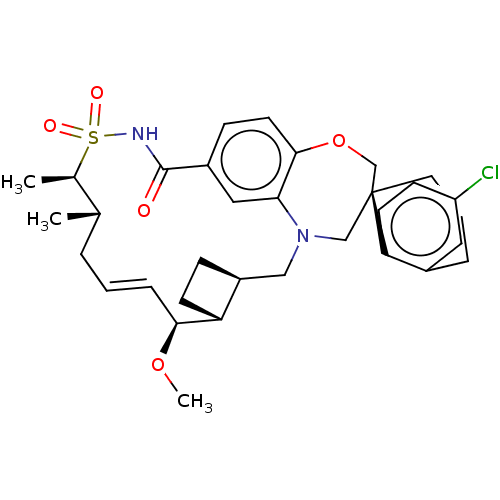
(CHEMBL4446378 | US10703733, Comparative Example 1)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194072

(CHEMBL220476 | syn-7-(6-chloro-pyridin-3-yl)-2-aza...)Show SMILES Clc1ccc(cn1)C1C2CCC1NC2 |THB:4:7:10.9:13.12| Show InChI InChI=1S/C11H13ClN2/c12-10-4-2-8(6-14-10)11-7-1-3-9(11)13-5-7/h2,4,6-7,9,11,13H,1,3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514215
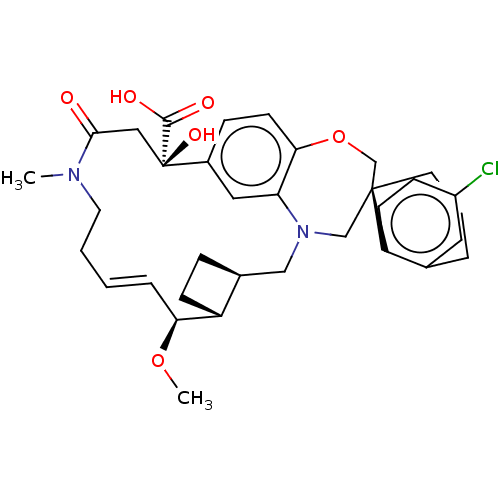
(CHEMBL4577379 | US11274105, Example 4)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(42-2)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(41,32(39)40)18-31(36)38/h3,7,9-10,12-13,16-17,23,26,29,41H,4-6,8,11,14-15,18-21H2,1-2H3,(H,39,40)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514216
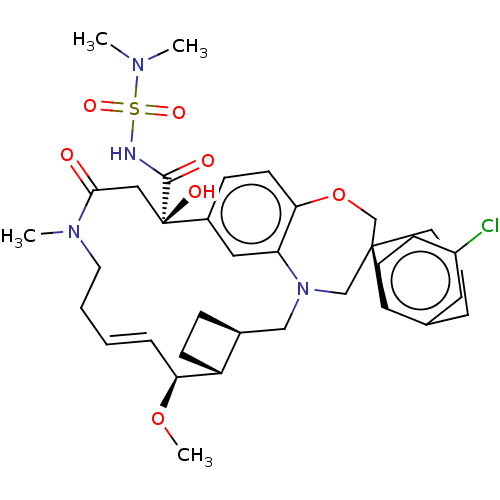
(CHEMBL4528051 | US11274105, Example 5)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C36H47ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h5,9,11-12,14-15,18-19,25,28,31,44H,6-8,10,13,16-17,20-23H2,1-4H3,(H,38,43)/b9-5+/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031877
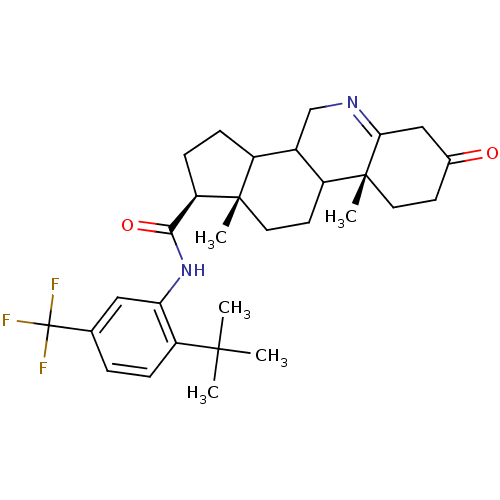
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514219

(CHEMBL4438074 | US11274105, Example 181)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:17| Show InChI InChI=1S/C40H53ClFN5O7S/c1-44(2)55(51,52)43-38(49)40(50)21-37(48)45(3)16-5-4-8-35(53-18-17-46-23-31(42)24-46)32-12-9-28(32)22-47-25-39(26-54-36-14-10-29(40)20-34(36)47)15-6-7-27-19-30(41)11-13-33(27)39/h4,8,10-11,13-14,19-20,28,31-32,35,50H,5-7,9,12,15-18,21-26H2,1-3H3,(H,43,49)/b8-4+/t28-,32+,35-,39-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514201

(CHEMBL4547370 | US11274105, Example 191)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCOCC(F)F)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:16| Show InChI InChI=1S/C39H51ClF2N4O8S/c1-44(2)55(50,51)43-37(48)39(49)21-36(47)45(3)16-5-4-8-33(53-18-17-52-23-35(41)42)30-12-9-27(30)22-46-24-38(25-54-34-14-10-28(39)20-32(34)46)15-6-7-26-19-29(40)11-13-31(26)38/h4,8,10-11,13-14,19-20,27,30,33,35,49H,5-7,9,12,15-18,21-25H2,1-3H3,(H,43,48)/b8-4+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514214

(CHEMBL4542646 | US11274105, Example 41)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C33H39ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h2,6,8-9,11-12,15-16,22,25,28,37,41H,3-5,7,10,13-14,17-20H2,1H3,(H,39,40)/b6-2+/t22-,25+,28-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514218
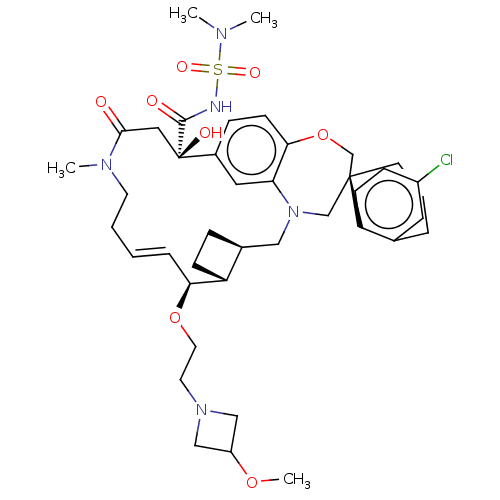
(CHEMBL4539543 | US11274105, Example 197)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(C1)OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)22-38(48)45(3)17-6-5-9-36(54-19-18-46-24-32(25-46)53-4)33-13-10-29(33)23-47-26-40(27-55-37-15-11-30(41)21-35(37)47)16-7-8-28-20-31(42)12-14-34(28)40/h5,9,11-12,14-15,20-21,29,32-33,36,50H,6-8,10,13,16-19,22-27H2,1-4H3,(H,43,49)/b9-5+/t29-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514206
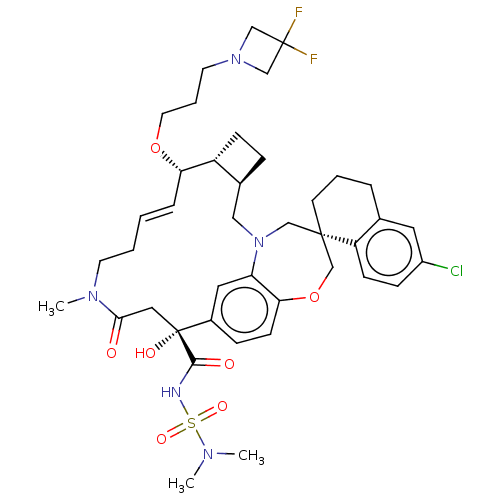
(CHEMBL4588330 | US11274105, Example 187)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:19| Show InChI InChI=1S/C41H54ClF2N5O7S/c1-46(2)57(53,54)45-38(51)41(52)22-37(50)47(3)17-5-4-9-35(55-19-7-18-48-25-40(43,44)26-48)32-13-10-29(32)23-49-24-39(27-56-36-15-11-30(41)21-34(36)49)16-6-8-28-20-31(42)12-14-33(28)39/h4,9,11-12,14-15,20-21,29,32,35,52H,5-8,10,13,16-19,22-27H2,1-3H3,(H,45,51)/b9-4+/t29-,32+,35-,39-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514204
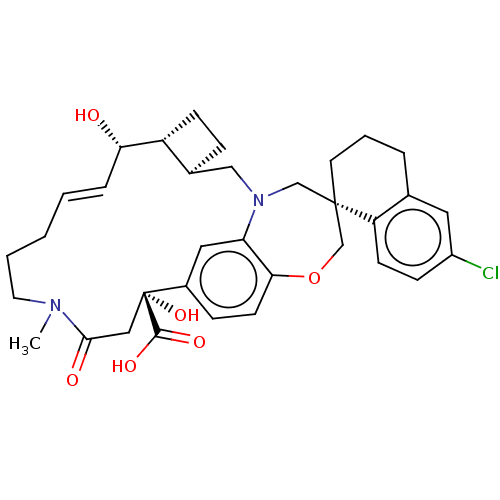
(CHEMBL4437832)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-2-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38,42H,2,4-6,8,11,14-15,18-21H2,1H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031895

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50339236
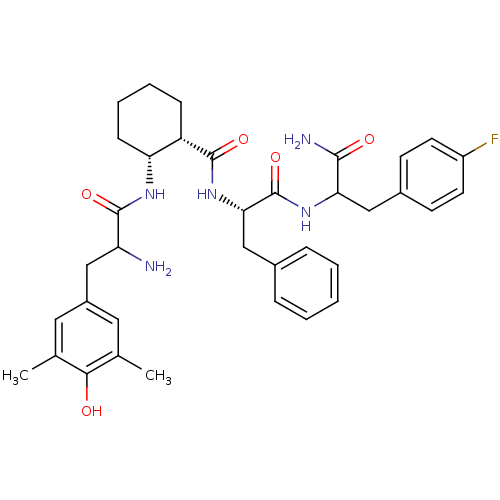
((1S,2R)-N-((2S)-1-(1-amino-3-(4-fluorophenyl)-1-ox...)Show SMILES Cc1cc(CC(N)C(=O)N[C@@H]2CCCC[C@@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)NC(Cc2ccc(F)cc2)C(N)=O)cc(C)c1O |r| Show InChI InChI=1S/C36H44FN5O5/c1-21-16-25(17-22(2)32(21)43)18-28(38)35(46)40-29-11-7-6-10-27(29)34(45)42-31(20-23-8-4-3-5-9-23)36(47)41-30(33(39)44)19-24-12-14-26(37)15-13-24/h3-5,8-9,12-17,27-31,43H,6-7,10-11,18-20,38H2,1-2H3,(H2,39,44)(H,40,46)(H,41,47)(H,42,45)/t27-,28?,29+,30?,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane homogenate by liquid scintillation counting |
J Med Chem 54: 1462-72 (2011)
Article DOI: 10.1021/jm101515v
BindingDB Entry DOI: 10.7270/Q26T0MX9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031874
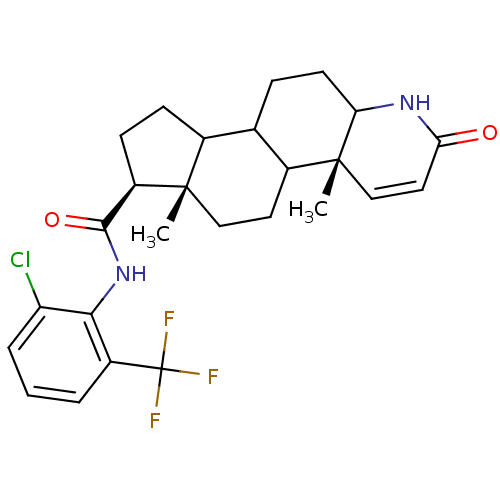
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CC[C@@H]2C(=O)Nc1c(Cl)cccc1C(F)(F)F |c:12| Show InChI InChI=1S/C26H30ClF3N2O2/c1-24-12-10-16-14(6-9-20-25(16,2)13-11-21(33)31-20)15(24)7-8-18(24)23(34)32-22-17(26(28,29)30)4-3-5-19(22)27/h3-5,11,13-16,18,20H,6-10,12H2,1-2H3,(H,31,33)(H,32,34)/t14?,15?,16?,18-,20?,24+,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031877

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405
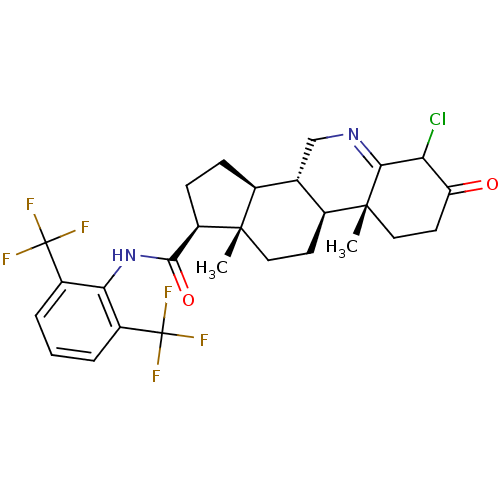
(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514217

(CHEMBL4452794 | US11274105, Example 196)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCC(F)(F)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C42H56ClF2N5O7S/c1-47(2)58(54,55)46-39(52)42(53)25-38(51)48(3)18-5-4-8-36(56-22-21-49-19-16-41(44,45)17-20-49)33-12-9-30(33)26-50-27-40(28-57-37-14-10-31(42)24-35(37)50)15-6-7-29-23-32(43)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,53H,5-7,9,12,15-22,25-28H2,1-3H3,(H,46,52)/b8-4+/t30-,33+,36-,40-,42+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031883
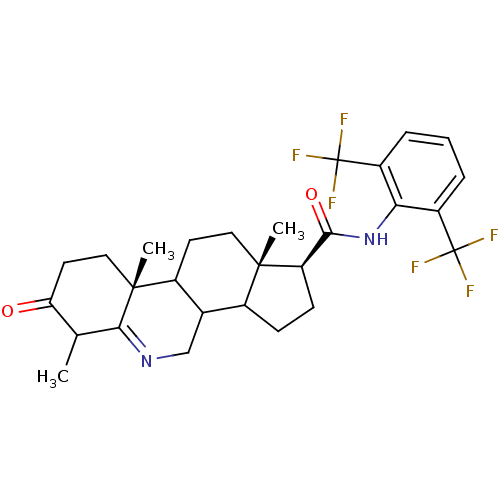
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(F)(F)F)C3CN=C12 |t:39| Show InChI InChI=1S/C28H32F6N2O2/c1-14-21(37)10-12-26(3)17-9-11-25(2)16(15(17)13-35-23(14)26)7-8-20(25)24(38)36-22-18(27(29,30)31)5-4-6-19(22)28(32,33)34/h4-6,14-17,20H,7-13H2,1-3H3,(H,36,38)/t14?,15?,16?,17?,20-,25+,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405

(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031896
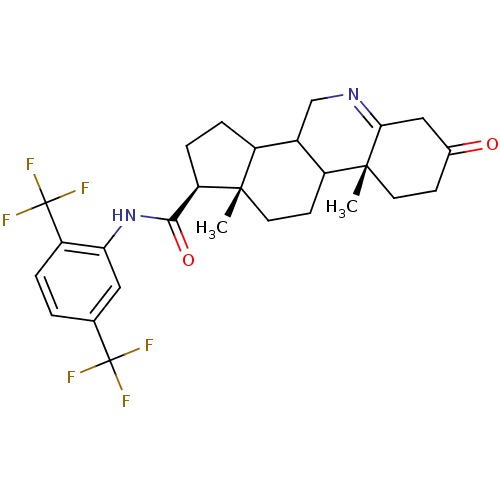
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-10-8-18-16(13-34-22-12-15(36)7-9-25(18,22)2)17(24)5-6-20(24)23(37)35-21-11-14(26(28,29)30)3-4-19(21)27(31,32)33/h3-4,11,16-18,20H,5-10,12-13H2,1-2H3,(H,35,37)/t16?,17?,18?,20-,24+,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514208
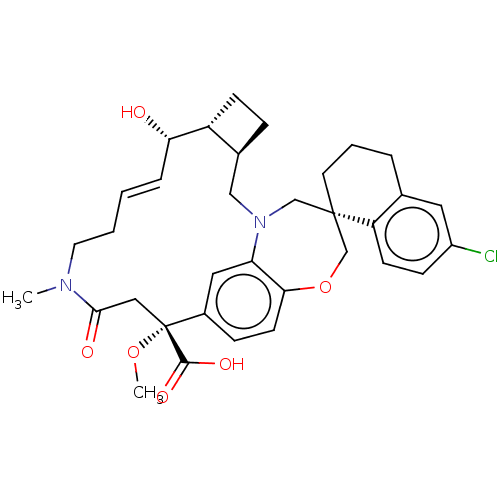
(CHEMBL4469850 | US11274105, Example 61)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](OC)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42-2,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38H,4-6,8,11,14-15,18-21H2,1-2H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031889
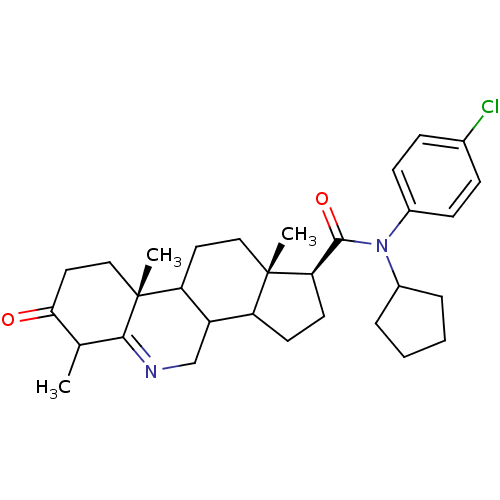
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)N(C4CCCC4)c4ccc(Cl)cc4)C3CN=C12 |t:38| Show InChI InChI=1S/C31H41ClN2O2/c1-19-27(35)15-17-31(3)25-14-16-30(2)24(23(25)18-33-28(19)31)12-13-26(30)29(36)34(21-6-4-5-7-21)22-10-8-20(32)9-11-22/h8-11,19,21,23-26H,4-7,12-18H2,1-3H3/t19?,23?,24?,25?,26-,30+,31-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194070

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES Clc1ccc(cn1)C1CC2CCCC1N2 |THB:4:7:14:12.11.10| Show InChI InChI=1S/C12H15ClN2/c13-12-5-4-8(7-14-12)10-6-9-2-1-3-11(10)15-9/h4-5,7,9-11,15H,1-3,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194070

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES Clc1ccc(cn1)C1CC2CCCC1N2 |THB:4:7:14:12.11.10| Show InChI InChI=1S/C12H15ClN2/c13-12-5-4-8(7-14-12)10-6-9-2-1-3-11(10)15-9/h4-5,7,9-11,15H,1-3,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514207

(CHEMBL4562159)Show SMILES [H][C@@]12CC[C@@]1([H])[C@H](CCCCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1)OC |r| Show InChI InChI=1S/C36H49ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h11-12,14-15,18-19,25,28,31,44H,5-10,13,16-17,20-23H2,1-4H3,(H,38,43)/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50021325

(4-methyl-(1S,5R,13R,14S)-12-oxa-4-azapentacyclo[9....)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1C3CC[C@@H]4O)ccc5O |TLB:13:12:8.9.10:1.3.2| Show InChI InChI=1S/C17H21NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2,4,10-11,13,16,19-20H,3,5-8H2,1H3/t10?,11-,13+,16+,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368883

(CHEMBL1159458)Show SMILES COc1ccc(CCC(=O)c2c(O)cc(O[C@@H]3O[C@](C)(CO)[C@@](C)(O)[C@](C)(O)[C@@]3(C)O[C@]3(C)OC(C)(C)[C@](C)(O)[C@@](C)(O)[C@H]3O)cc2O)cc1O |r| Show InChI InChI=1S/C36H52O15/c1-29(2)34(7,44)31(4,43)27(42)33(6,50-29)51-32(5)28(49-30(3,18-37)35(8,45)36(32,9)46)48-20-16-23(40)26(24(41)17-20)21(38)13-11-19-12-14-25(47-10)22(39)15-19/h12,14-17,27-28,37,39-46H,11,13,18H2,1-10H3/t27-,28-,30-,31+,32+,33+,34+,35-,36-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 1 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50339240
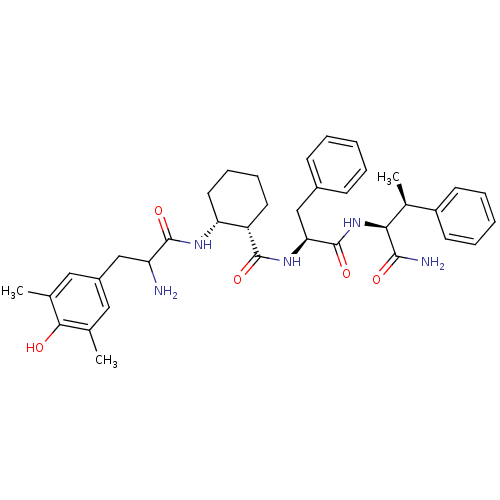
((1S,2R)-N-((S)-1-((2S,3S)-1-amino-1-oxo-3-phenylbu...)Show SMILES C[C@H]([C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCC[C@H]1NC(=O)C(N)Cc1cc(C)c(O)c(C)c1)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H47N5O5/c1-22-18-26(19-23(2)33(22)43)20-29(38)36(46)40-30-17-11-10-16-28(30)35(45)41-31(21-25-12-6-4-7-13-25)37(47)42-32(34(39)44)24(3)27-14-8-5-9-15-27/h4-9,12-15,18-19,24,28-32,43H,10-11,16-17,20-21,38H2,1-3H3,(H2,39,44)(H,40,46)(H,41,45)(H,42,47)/t24-,28-,29?,30+,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane homogenate by liquid scintillation counting |
J Med Chem 54: 1462-72 (2011)
Article DOI: 10.1021/jm101515v
BindingDB Entry DOI: 10.7270/Q26T0MX9 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514197
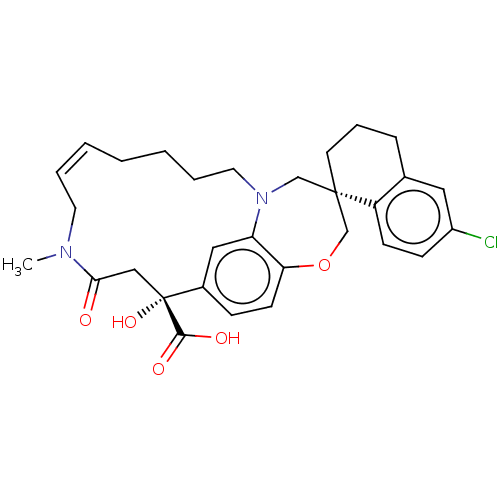
(CHEMBL4561691 | US11274105, Example 63)Show SMILES CN1C\C=C\CCCCN2C[C@@]3(CCCc4cc(Cl)ccc34)COc3ccc(cc23)[C@](O)(CC1=O)C(O)=O |r,t:3| Show InChI InChI=1S/C30H35ClN2O5/c1-32-14-5-3-2-4-6-15-33-19-29(13-7-8-21-16-23(31)10-11-24(21)29)20-38-26-12-9-22(17-25(26)33)30(37,28(35)36)18-27(32)34/h3,5,9-12,16-17,37H,2,4,6-8,13-15,18-20H2,1H3,(H,35,36)/b5-3+/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031878
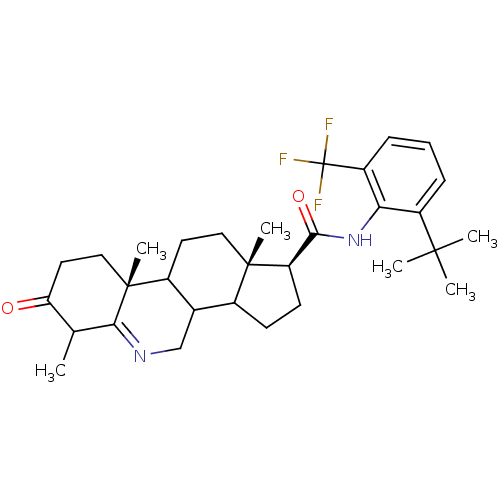
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(C)(C)C)C3CN=C12 |t:39| Show InChI InChI=1S/C31H41F3N2O2/c1-17-24(37)13-15-30(6)20-12-14-29(5)19(18(20)16-35-26(17)30)10-11-23(29)27(38)36-25-21(28(2,3)4)8-7-9-22(25)31(32,33)34/h7-9,17-20,23H,10-16H2,1-6H3,(H,36,38)/t17?,18?,19?,20?,23-,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... |
J Med Chem 62: 11416-11422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00937
BindingDB Entry DOI: 10.7270/Q2736V9G |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031897
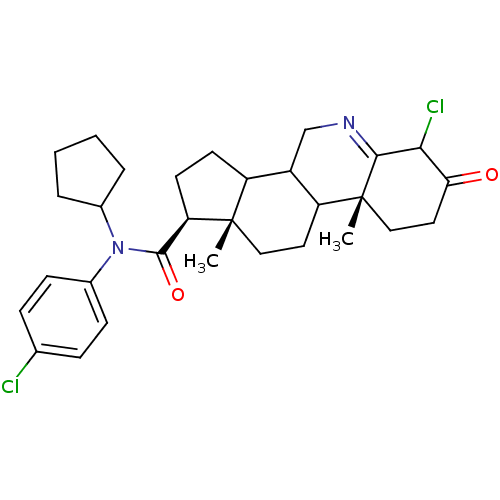
((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...)Show SMILES C[C@]12CCC3C(CN=C4C(Cl)C(=O)CC[C@]34C)C1CC[C@@H]2C(=O)N(C1CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H38Cl2N2O2/c1-29-15-13-23-21(17-33-27-26(32)25(35)14-16-30(23,27)2)22(29)11-12-24(29)28(36)34(19-5-3-4-6-19)20-9-7-18(31)8-10-20/h7-10,19,21-24,26H,3-6,11-17H2,1-2H3/t21?,22?,23?,24-,26?,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NPA from rat brain Dopamine receptor D2 |
J Med Chem 39: 143-8 (1996)
Article DOI: 10.1021/jm950625l
BindingDB Entry DOI: 10.7270/Q2ZG6RB0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50194068
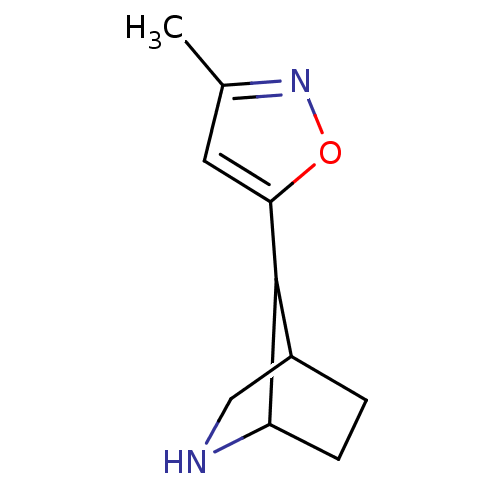
(7-(3-methylisoxazol-5-yl)-6-aza-bicyclo[2.2.1]hept...)Show InChI InChI=1S/C10H14N2O/c1-6-4-9(13-12-6)10-7-2-3-8(10)11-5-7/h4,7-8,10-11H,2-3,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.763 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
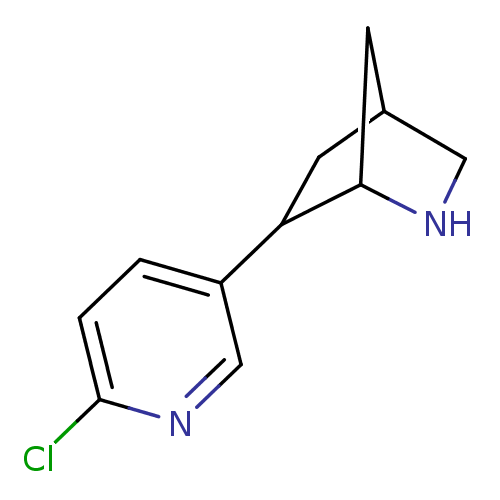
(Homo sapiens (Human)) | BDBM50194069
(2-(6-chloropyridin-3-yl)-6-aza-bicyclo[2.2.1]hepta...)Show InChI InChI=1S/C11H13ClN2/c12-11-2-1-8(6-14-11)9-3-7-4-10(9)13-5-7/h1-2,6-7,9-10,13H,3-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay |
Bioorg Med Chem Lett 16: 5493-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.049
BindingDB Entry DOI: 10.7270/Q2D79C7V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
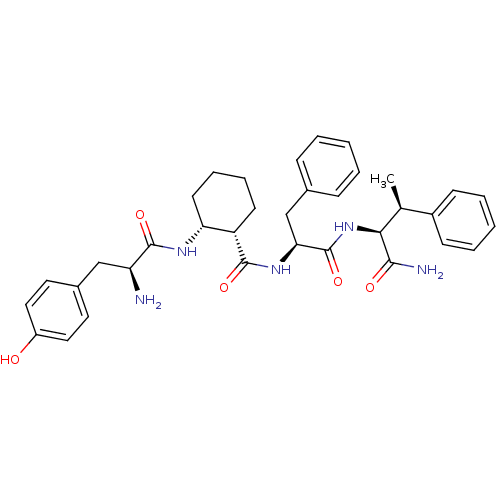
(Rattus norvegicus (rat)) | BDBM50339242
((1S,2R)-N-((S)-1-((2S,3S)-1-amino-1-oxo-3-phenylbu...)Show SMILES C[C@H]([C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCC[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C35H43N5O5/c1-22(25-12-6-3-7-13-25)31(32(37)42)40-35(45)30(21-23-10-4-2-5-11-23)39-33(43)27-14-8-9-15-29(27)38-34(44)28(36)20-24-16-18-26(41)19-17-24/h2-7,10-13,16-19,22,27-31,41H,8-9,14-15,20-21,36H2,1H3,(H2,37,42)(H,38,44)(H,39,43)(H,40,45)/t22-,27-,28-,29+,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane homogenate by liquid scintillation counting |
J Med Chem 54: 1462-72 (2011)
Article DOI: 10.1021/jm101515v
BindingDB Entry DOI: 10.7270/Q26T0MX9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50339233

((1S,2R)-N-((S)-1-((2S,3S)-1-amino-1-oxo-3-phenylbu...)Show SMILES C[C@H]([C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCC[C@H]1NC(=O)C(N)Cc1cc(C)c(O)c(C)c1)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C39H48N6O5/c1-22-17-25(18-23(2)35(22)46)19-30(40)38(49)43-32-16-10-8-14-29(32)37(48)44-33(20-27-21-42-31-15-9-7-13-28(27)31)39(50)45-34(36(41)47)24(3)26-11-5-4-6-12-26/h4-7,9,11-13,15,17-18,21,24,29-30,32-34,42,46H,8,10,14,16,19-20,40H2,1-3H3,(H2,41,47)(H,43,49)(H,44,48)(H,45,50)/t24-,29-,30?,32+,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane homogenate by liquid scintillation counting |
J Med Chem 54: 1462-72 (2011)
Article DOI: 10.1021/jm101515v
BindingDB Entry DOI: 10.7270/Q26T0MX9 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514209

(CHEMBL4460664)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)CCCCN(C)C(=O)C[C@@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r| Show InChI InChI=1S/C33H41ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h8-9,11-12,15-16,22,25,28,37,41H,2-7,10,13-14,17-20H2,1H3,(H,39,40)/t22-,25+,28-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
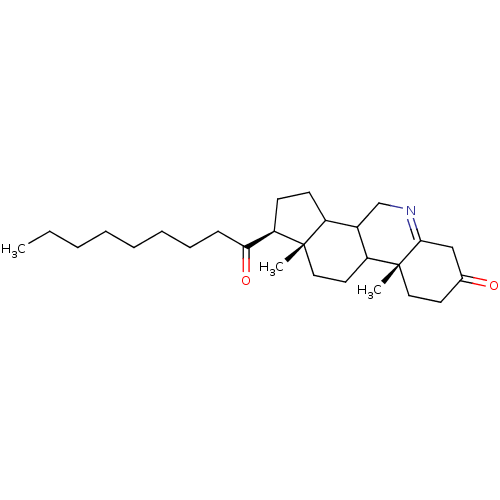
(Homo sapiens (Human)) | BDBM50039276
((1S,9aR,11aS)-9a,11a-Dimethyl-1-nonanoyl-1,2,3,3a,...)Show SMILES CCCCCCCCC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:16| Show InChI InChI=1S/C27H43NO2/c1-4-5-6-7-8-9-10-24(30)23-12-11-21-20-18-28-25-17-19(29)13-15-27(25,3)22(20)14-16-26(21,23)2/h20-23H,4-18H2,1-3H3/t20?,21?,22?,23-,26+,27-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 1 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514205

(CHEMBL4452403)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H37ClN2O6/c1-34-13-3-5-27(36)24-9-6-21(24)17-35-18-31(12-2-4-20-14-23(33)8-10-25(20)31)19-41-28-11-7-22(15-26(28)35)32(40,30(38)39)16-29(34)37/h3,5,7-8,10-11,14-15,21,24,27,36,40H,2,4,6,9,12-13,16-19H2,1H3,(H,38,39)/b5-3+/t21-,24+,27-,31-,32+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data