 Found 464 hits with Last Name = 'simon' and Initial = 'r'
Found 464 hits with Last Name = 'simon' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039664

(CHEMBL91890 | N-Biphenyl-4-yl-N-[(carbamoylmethyl-...)Show SMILES NC(=O)CN(CCc1ccccc1)C(=O)CN(C(=O)CNCCc1ccccc1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H36N4O3/c35-32(39)25-37(23-21-28-12-6-2-7-13-28)34(41)26-38(33(40)24-36-22-20-27-10-4-1-5-11-27)31-18-16-30(17-19-31)29-14-8-3-9-15-29/h1-19,36H,20-26H2,(H2,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039665
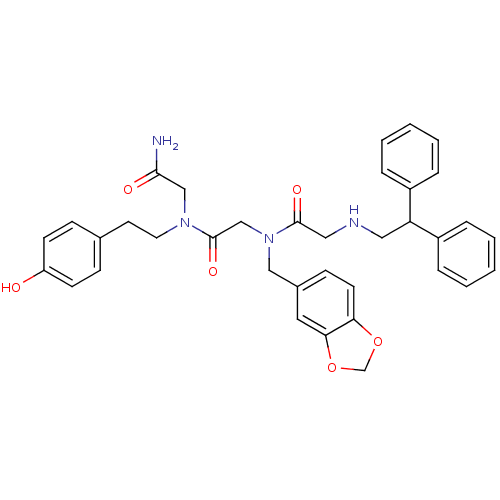
(CHEMBL90649 | CHIR-4531 | N-Benzo[1,3]dioxol-5-ylm...)Show SMILES NC(=O)CN(CCc1ccc(O)cc1)C(=O)CN(Cc1ccc2OCOc2c1)C(=O)CNCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H38N4O6/c37-34(42)23-39(18-17-26-11-14-30(41)15-12-26)36(44)24-40(22-27-13-16-32-33(19-27)46-25-45-32)35(43)21-38-20-31(28-7-3-1-4-8-28)29-9-5-2-6-10-29/h1-16,19,31,38,41H,17-18,20-25H2,(H2,37,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86495
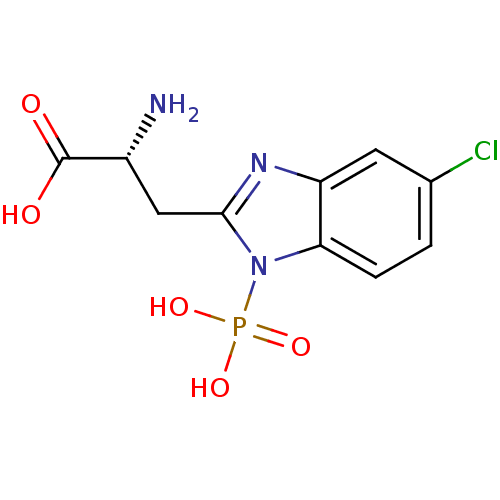
(EAB-318)Show SMILES N[C@H](Cc1nc2cc(Cl)ccc2n1P(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H11ClN3O5P/c11-5-1-2-8-7(3-5)13-9(4-6(12)10(15)16)14(8)20(17,18)19/h1-3,6H,4,12H2,(H,15,16)(H2,17,18,19)/t6-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 563-70 (2004)
Article DOI: 10.1124/jpet.104.066092
BindingDB Entry DOI: 10.7270/Q2DJ5D6D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50019056
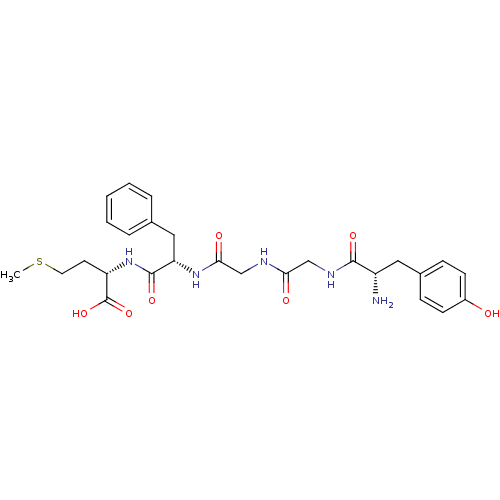
((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C27H35N5O7S/c1-40-12-11-21(27(38)39)32-26(37)22(14-17-5-3-2-4-6-17)31-24(35)16-29-23(34)15-30-25(36)20(28)13-18-7-9-19(33)10-8-18/h2-10,20-22,33H,11-16,28H2,1H3,(H,29,34)(H,30,36)(H,31,35)(H,32,37)(H,38,39)/t20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86494
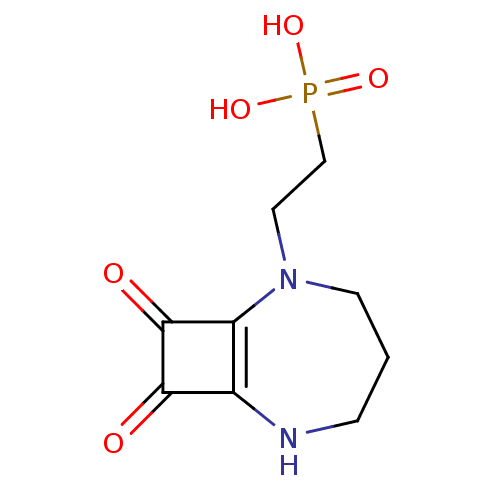
(EAA-090 | Perzinfotel | WAY-126090)Show SMILES [#8][P;v5]([#8])(=O)[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#7]-c2c-1c(=O)c2=O Show InChI InChI=1S/C9H13N2O5P/c12-8-6-7(9(8)13)11(3-1-2-10-6)4-5-17(14,15)16/h10H,1-5H2,(H2,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 563-70 (2004)
Article DOI: 10.1124/jpet.104.066092
BindingDB Entry DOI: 10.7270/Q2DJ5D6D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039661

(CHEMBL327549 | CHIR-4537 | N-({Carbamoylmethyl-[2-...)Show SMILES COc1ccc(CN(CC(=O)N(CCc2ccc(O)cc2)CC(N)=O)C(=O)CNCC(c2ccccc2)c2ccccc2)cc1OC Show InChI InChI=1S/C37H42N4O6/c1-46-33-18-15-28(21-34(33)47-2)24-41(26-37(45)40(25-35(38)43)20-19-27-13-16-31(42)17-14-27)36(44)23-39-22-32(29-9-5-3-6-10-29)30-11-7-4-8-12-30/h3-18,21,32,39,42H,19-20,22-26H2,1-2H3,(H2,38,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039663

(CHEMBL89378 | CHIR-4534 | N-({Carbamoylmethyl-[2-(...)Show SMILES CCCCCN(CC(=O)N(CCc1ccc(O)cc1)CC(N)=O)C(=O)CNCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C33H42N4O4/c1-2-3-10-20-36(25-33(41)37(24-31(34)39)21-19-26-15-17-29(38)18-16-26)32(40)23-35-22-30(27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-18,30,35,38H,2-3,10,19-25H2,1H3,(H2,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85002
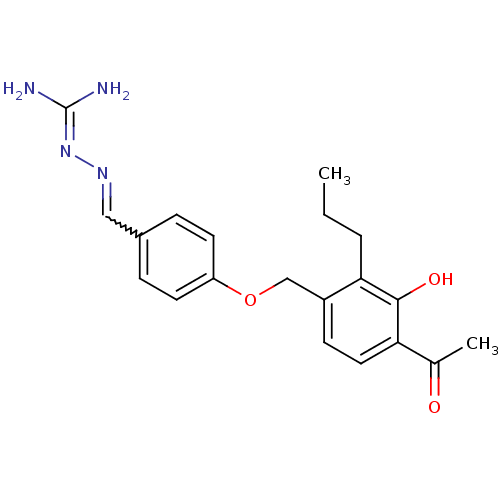
(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85006
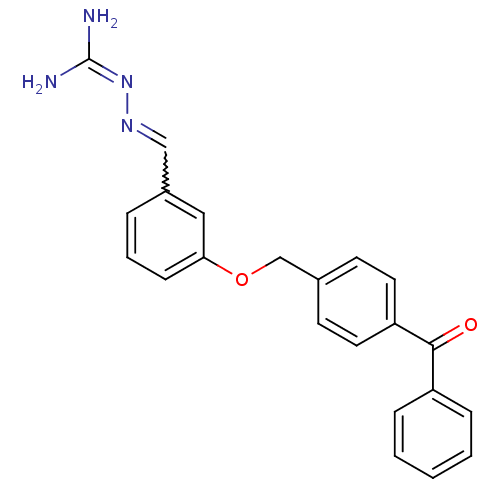
(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85007
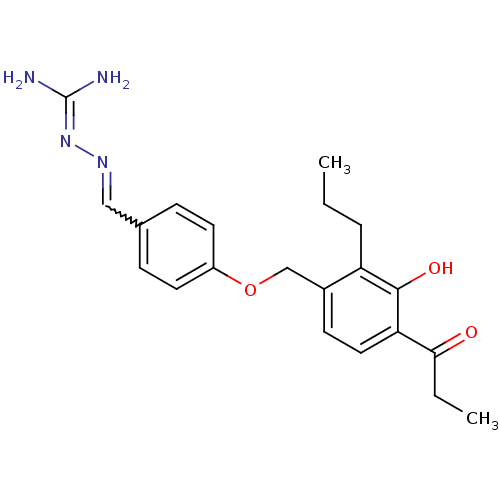
(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85000
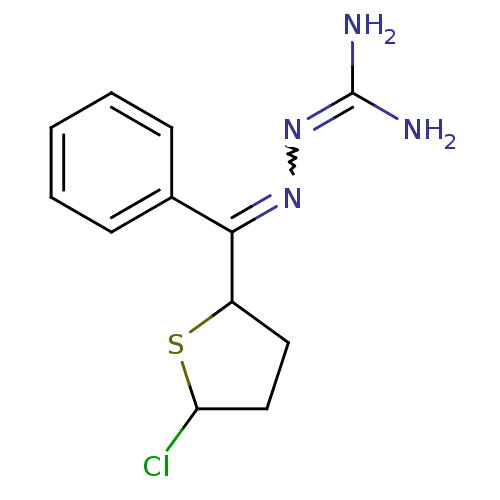
(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85004
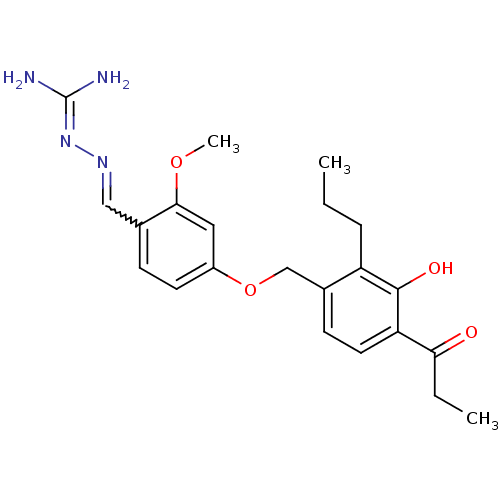
(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85003
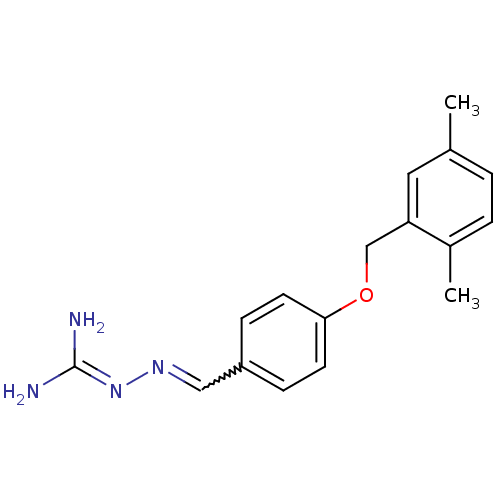
(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of synthetic VMA-tagged p300 (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)-DE3 cells using H4-15 peptid... |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86495

(EAB-318)Show SMILES N[C@H](Cc1nc2cc(Cl)ccc2n1P(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H11ClN3O5P/c11-5-1-2-8-7(3-5)13-9(4-6(12)10(15)16)14(8)20(17,18)19/h1-3,6H,4,12H2,(H,15,16)(H2,17,18,19)/t6-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 719 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 563-70 (2004)
Article DOI: 10.1124/jpet.104.066092
BindingDB Entry DOI: 10.7270/Q2DJ5D6D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85001
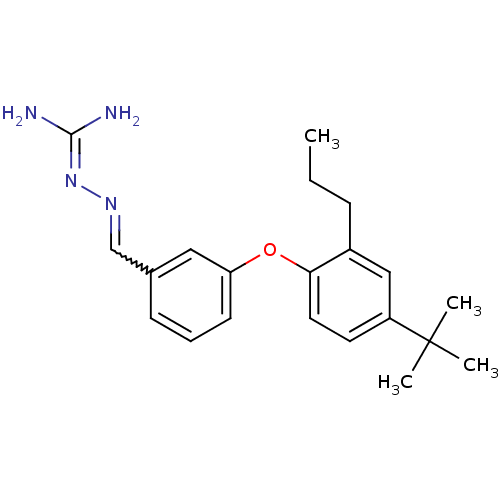
(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50029050
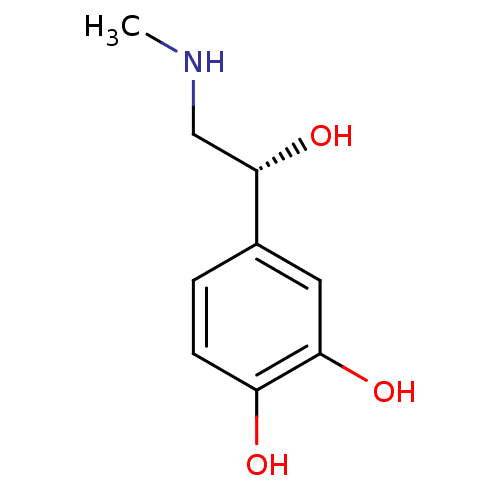
((-)-(R)-epinephrine | (-)-3,4-dihydroxy-alpha-((me...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86494

(EAA-090 | Perzinfotel | WAY-126090)Show SMILES [#8][P;v5]([#8])(=O)[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#7]-c2c-1c(=O)c2=O Show InChI InChI=1S/C9H13N2O5P/c12-8-6-7(9(8)13)11(3-1-2-10-6)4-5-17(14,15)16/h10H,1-5H2,(H2,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 563-70 (2004)
Article DOI: 10.1124/jpet.104.066092
BindingDB Entry DOI: 10.7270/Q2DJ5D6D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85005
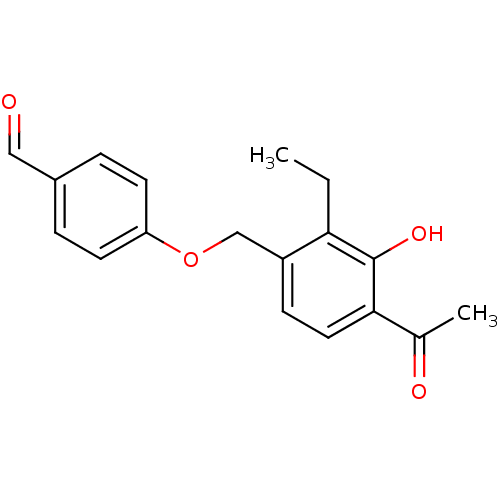
(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85005

(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50004927

(4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...)Show InChI InChI=1S/C7H14NO5P/c9-7(10)6-3-5(1-2-8-6)4-14(11,12)13/h5-6,8H,1-4H2,(H,9,10)(H2,11,12,13)/t5-,6+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 563-70 (2004)
Article DOI: 10.1124/jpet.104.066092
BindingDB Entry DOI: 10.7270/Q2DJ5D6D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50029051
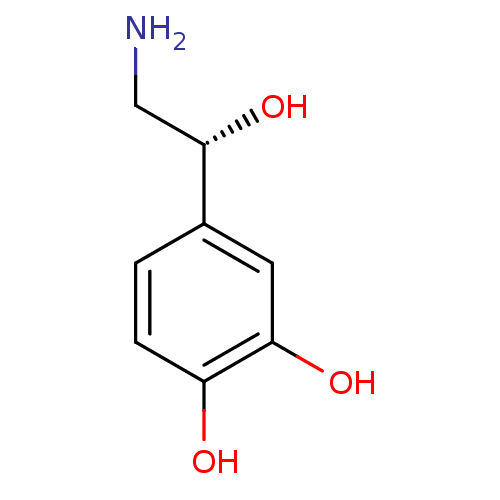
((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175402
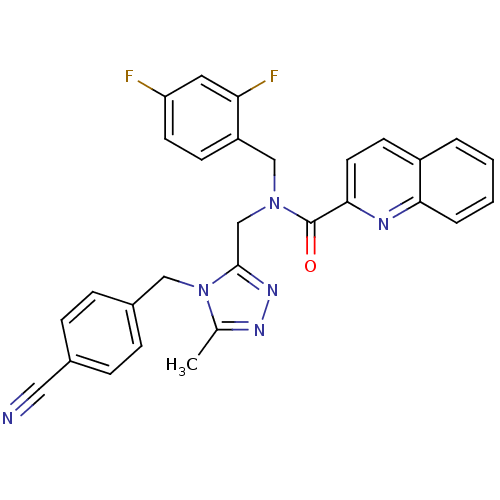
(CHEMBL371435 | N-(2,4-difluorobenzyl)-N-((4-(4-cya...)Show SMILES Cc1nnc(CN(Cc2ccc(F)cc2F)C(=O)c2ccc3ccccc3n2)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C29H22F2N6O/c1-19-34-35-28(37(19)16-21-8-6-20(15-32)7-9-21)18-36(17-23-10-12-24(30)14-25(23)31)29(38)27-13-11-22-4-2-3-5-26(22)33-27/h2-14H,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175406
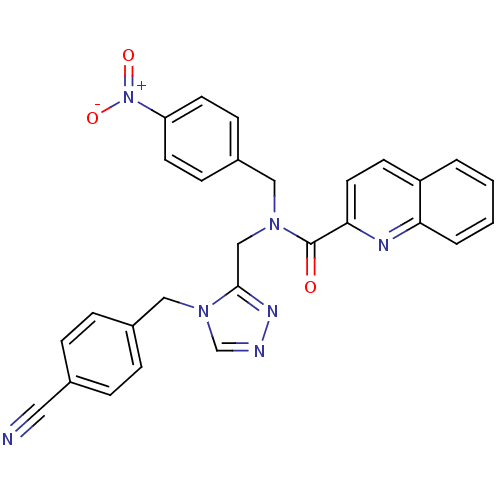
(CHEMBL200291 | N-((4-(4-cyanobenzyl)-4H-1,2,4-tria...)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2nncn2Cc2ccc(cc2)C#N)C(=O)c2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H21N7O3/c29-15-20-5-7-21(8-6-20)17-34-19-30-32-27(34)18-33(16-22-9-12-24(13-10-22)35(37)38)28(36)26-14-11-23-3-1-2-4-25(23)31-26/h1-14,19H,16-18H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175395
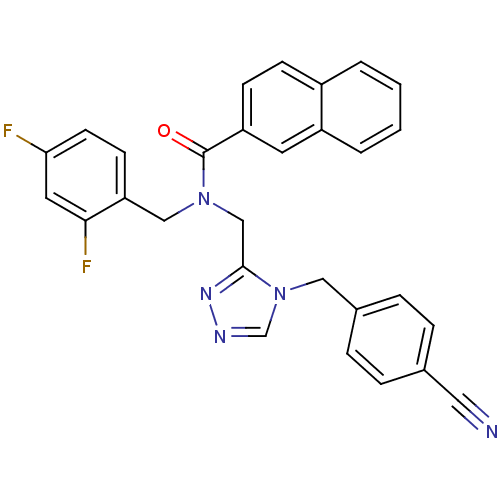
(CHEMBL199965 | N-(2,4-difluorobenzyl)-N-((4-(4-cya...)Show SMILES Fc1ccc(CN(Cc2nncn2Cc2ccc(cc2)C#N)C(=O)c2ccc3ccccc3c2)c(F)c1 Show InChI InChI=1S/C29H21F2N5O/c30-26-12-11-25(27(31)14-26)17-35(29(37)24-10-9-22-3-1-2-4-23(22)13-24)18-28-34-33-19-36(28)16-21-7-5-20(15-32)6-8-21/h1-14,19H,16-18H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50255529
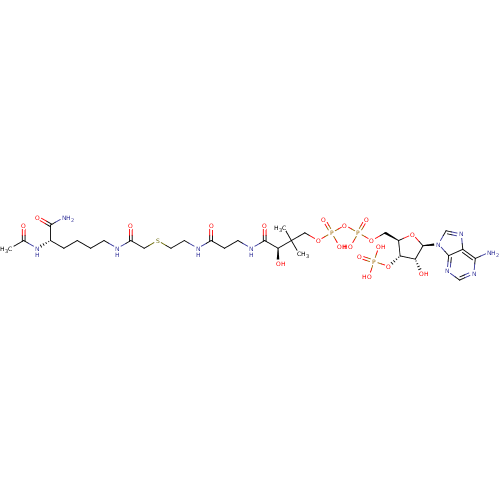
(Ac-Lys(CoA)-NH2 | CHEMBL505121)Show SMILES CC(=O)N[C@@H](CCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)n1cnc2c(N)ncnc12)C(N)=O |r| Show InChI InChI=1S/C31H53N10O19P3S/c1-17(42)40-18(27(33)47)6-4-5-8-34-21(44)13-64-11-10-35-20(43)7-9-36-29(48)25(46)31(2,3)14-57-63(54,55)60-62(52,53)56-12-19-24(59-61(49,50)51)23(45)30(58-19)41-16-39-22-26(32)37-15-38-28(22)41/h15-16,18-19,23-25,30,45-46H,4-14H2,1-3H3,(H2,33,47)(H,34,44)(H,35,43)(H,36,48)(H,40,42)(H,52,53)(H,54,55)(H2,32,37,38)(H2,49,50,51)/t18-,19+,23+,24+,25-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal GST-tagged p300 (1195 to 1673 residues) expressed in competent Escherichia coli DH5alpha cells using histo... |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175407

(CHEMBL371484 | N-(2,4-difluorobenzyl)-N-((4-(4-cya...)Show SMILES Fc1ccc(CN(Cc2nncn2Cc2ccc(cc2)C#N)C(=O)c2ccc3ccccc3n2)c(F)c1 Show InChI InChI=1S/C28H20F2N6O/c29-23-11-9-22(24(30)13-23)16-35(28(37)26-12-10-21-3-1-2-4-25(21)33-26)17-27-34-32-18-36(27)15-20-7-5-19(14-31)6-8-20/h1-13,18H,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175392

(CHEMBL371010 | N-((4-(4-cyanobenzyl)-4H-1,2,4-tria...)Show SMILES Fc1ccc(CN(Cc2nncn2Cc2ccc(cc2)C#N)C(=O)c2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H21FN6O/c29-24-12-9-22(10-13-24)16-34(28(36)26-14-11-23-3-1-2-4-25(23)32-26)18-27-33-31-19-35(27)17-21-7-5-20(15-30)6-8-21/h1-14,19H,16-18H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50175408
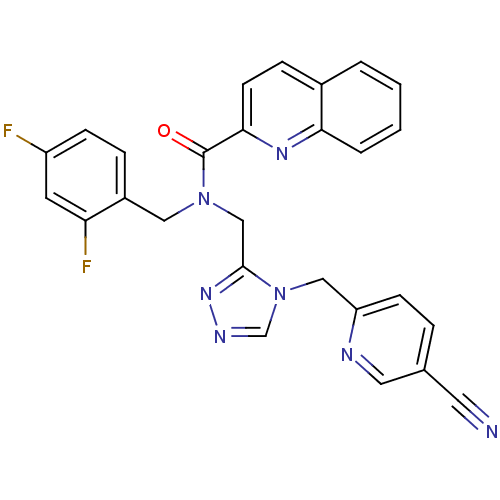
(CHEMBL371423 | N-(2,4-difluorobenzyl)-N-((4-((5-cy...)Show SMILES Fc1ccc(CN(Cc2nncn2Cc2ccc(cn2)C#N)C(=O)c2ccc3ccccc3n2)c(F)c1 Show InChI InChI=1S/C27H19F2N7O/c28-21-8-6-20(23(29)11-21)14-35(27(37)25-10-7-19-3-1-2-4-24(19)33-25)16-26-34-32-17-36(26)15-22-9-5-18(12-30)13-31-22/h1-11,13,17H,14-16H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by ChEMBL
| Assay Description
Inhibitory activity against K-Ras farnesyl transferase |
Bioorg Med Chem Lett 15: 5407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.007
BindingDB Entry DOI: 10.7270/Q2S18239 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312157
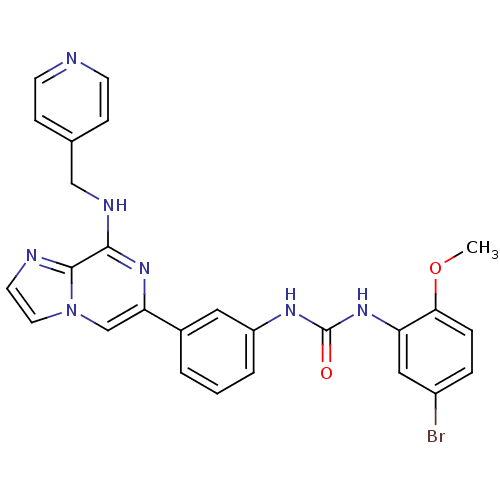
(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data