

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527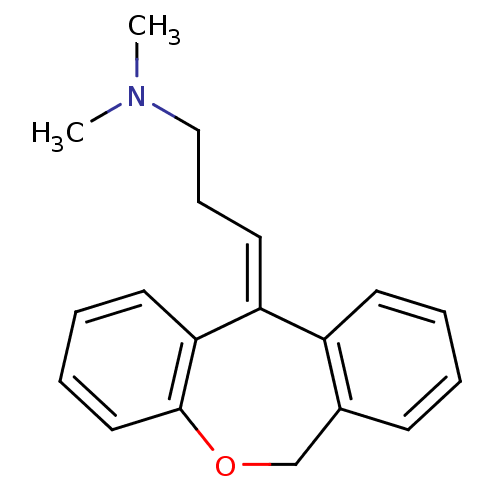 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50079527 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50079527 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423169 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423183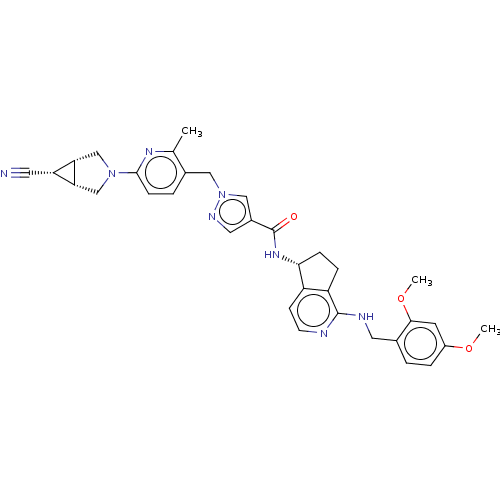 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423170 (US10501440, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423171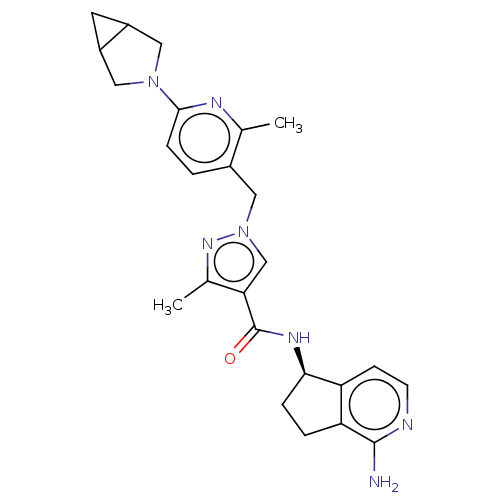 (US10501440, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50079527 ((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423180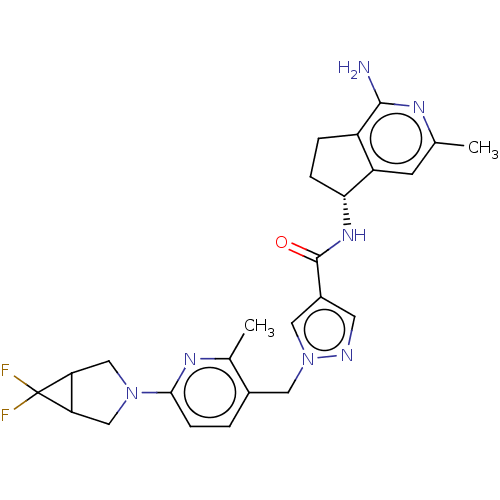 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423185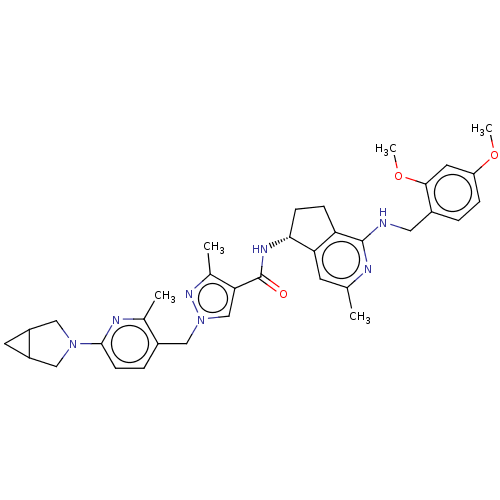 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423174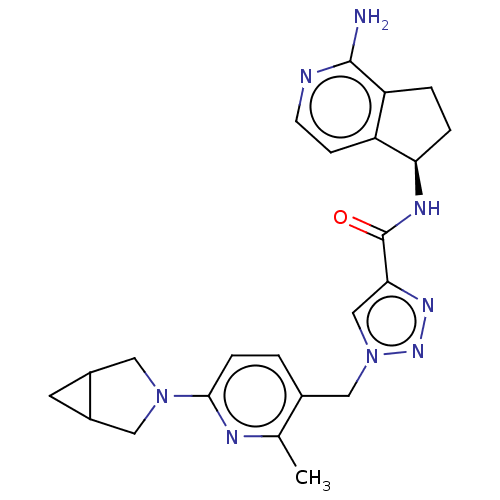 (US10501440, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423173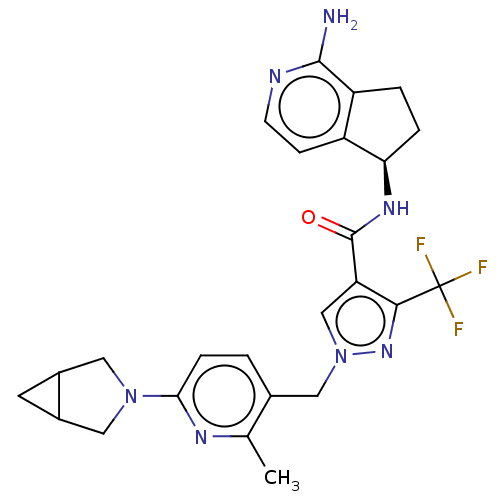 (US10501440, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423179 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.407 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423187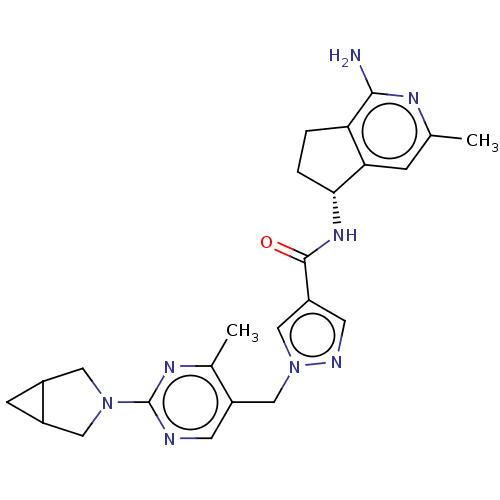 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423188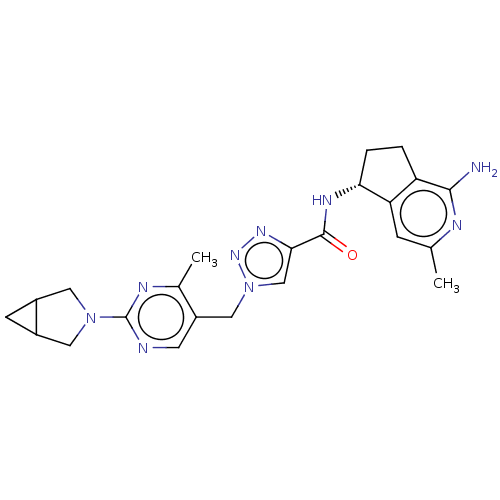 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50150945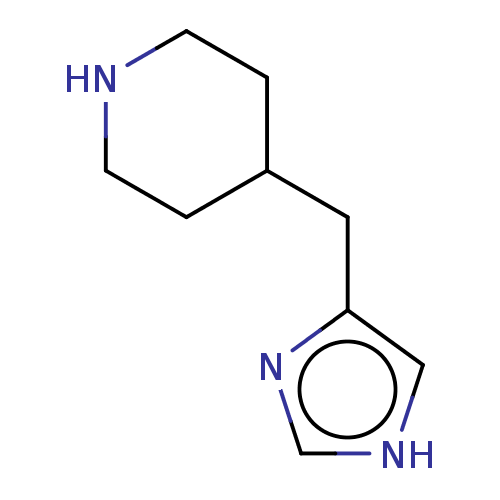 (CHEBI:81390 | Immepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-R-methylhistamine binding to SK-N-MC cell membranes expressing human H3 receptor | J Med Chem 48: 2100-7 (2005) Article DOI: 10.1021/jm049475h BindingDB Entry DOI: 10.7270/Q28K7CVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86032 (trans-H2-PAT(-) | trans-PAT) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423182 (US10501440, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916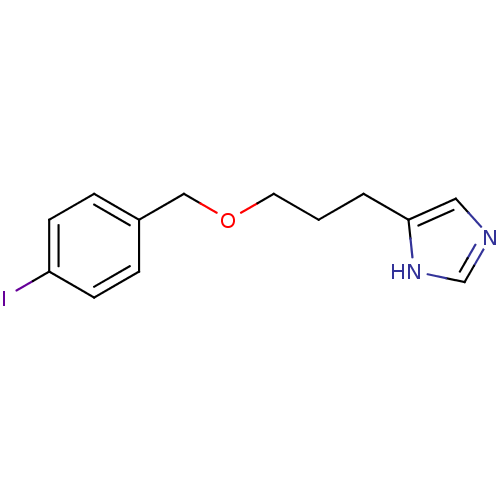 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50292411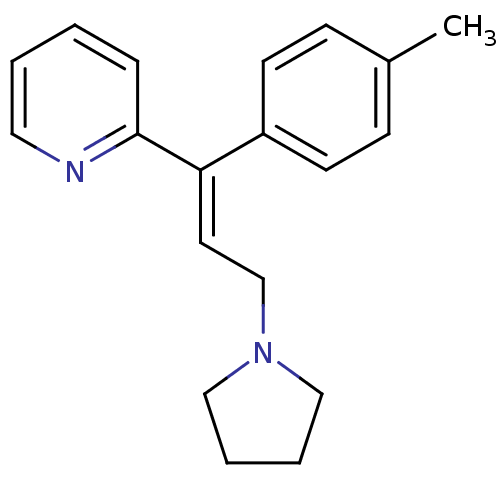 ((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM35938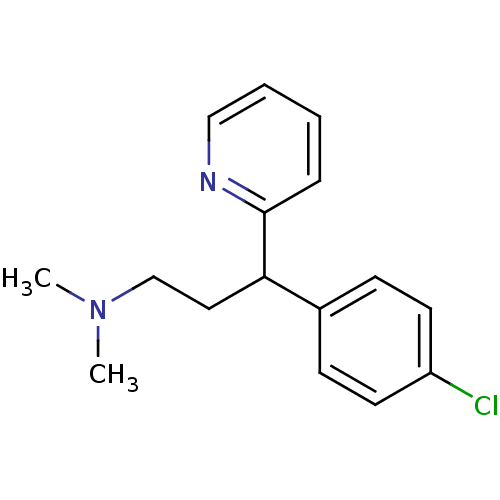 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22910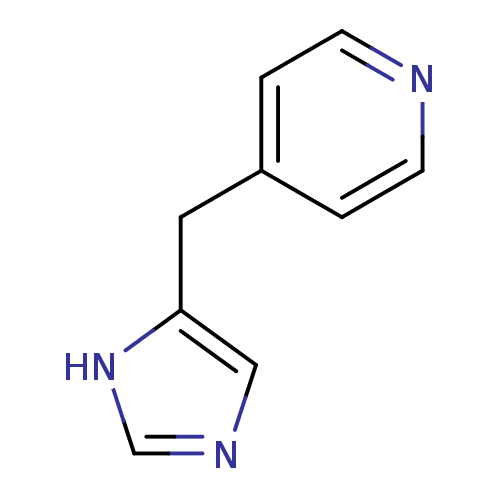 (4-(1H-imidazol-5-ylmethyl)pyridine | Immethridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50292411 ((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22548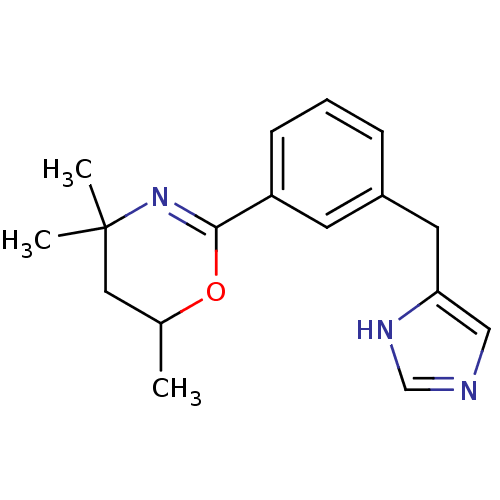 (2-[3-(1H-imidazol-4-ylmethyl)phenyl]-4,4,6-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | 2 | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22909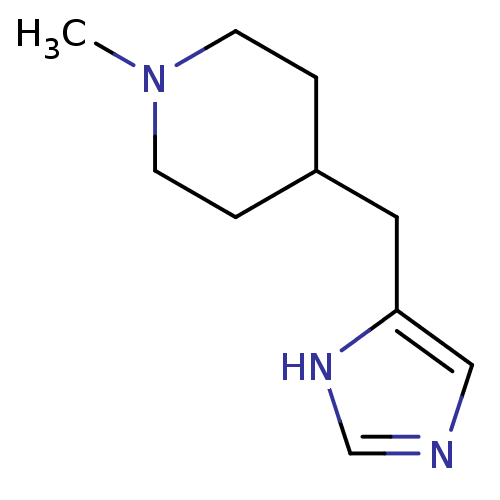 (4-(1H-imidazol-5-ylmethyl)-1-methylpiperidine | Me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50475340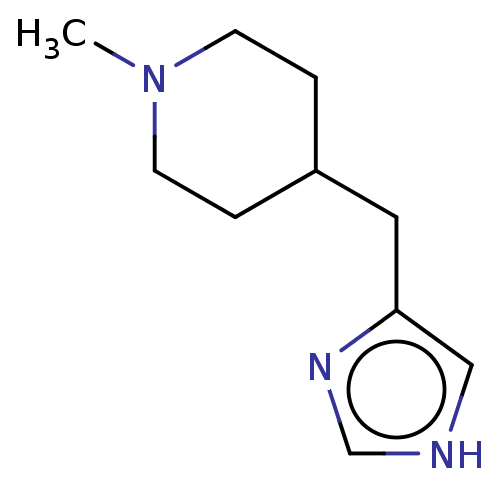 (Methimepip) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-R-methylhistamine binding to SK-N-MC cell membranes expressing human H3 receptor | J Med Chem 48: 2100-7 (2005) Article DOI: 10.1021/jm049475h BindingDB Entry DOI: 10.7270/Q28K7CVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423184 (US10501440, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50292411 ((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86032 (trans-H2-PAT(-) | trans-PAT) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86033 (cis-H2-PAT(+/-) | trans-H2-PAT(+/-)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423181 (US10501440, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50292411 ((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86033 (cis-H2-PAT(+/-) | trans-H2-PAT(+/-)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50414391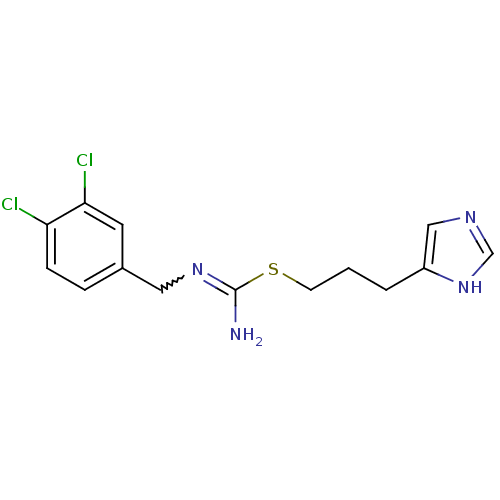 (CHEMBL1202332 | CHEMBL553423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM86032 (trans-H2-PAT(-) | trans-PAT) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423176 (US10501440, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM35938 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 328-36 (2002) Article DOI: 10.1124/jpet.302.1.328 BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22538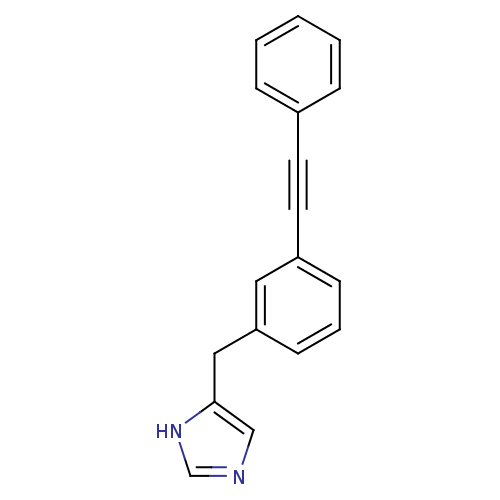 (4-Benzyl-1H-imidazole derivative, 19 | 4-{[3-(2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam | Assay Description Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... | J Med Chem 51: 2944-53 (2008) Article DOI: 10.1021/jm7014149 BindingDB Entry DOI: 10.7270/Q24F1P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 451 total ) | Next | Last >> |