

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50081081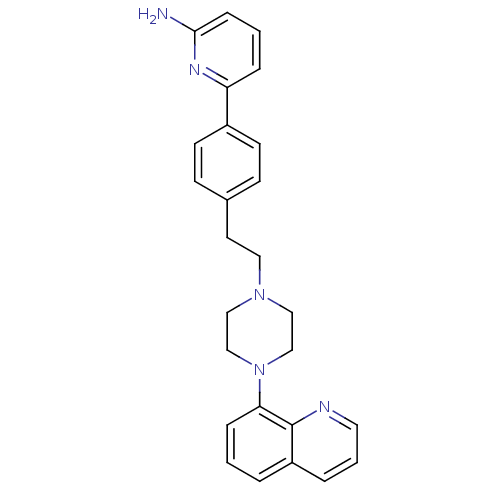 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50081081 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding ability of compound to human Dopamine receptor D2 | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50081081 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to human 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50081082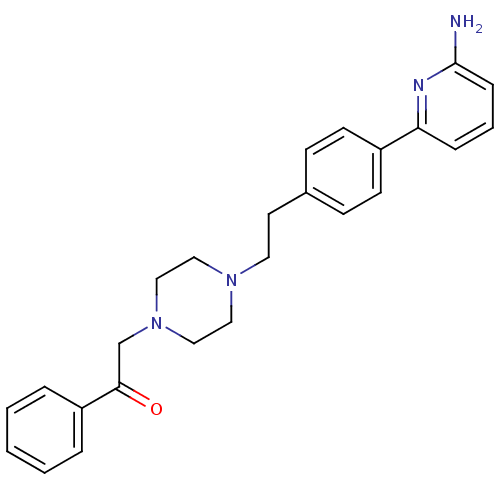 (2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to m2 muscarinic receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281005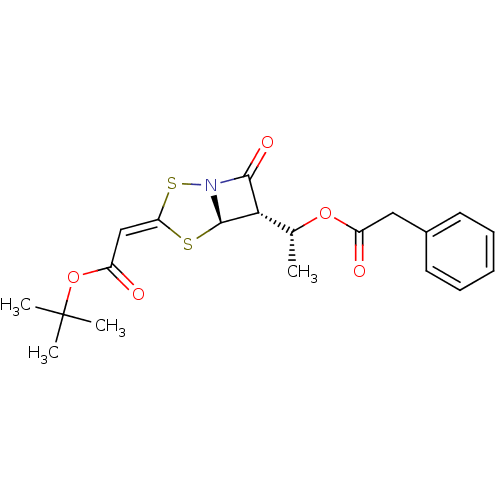 (CHEMBL76445 | [(5R,6S)-7-Oxo-6-((R)-1-phenylacetox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) was determined | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50081082 (2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding ability of compound to m4 muscarinic receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281006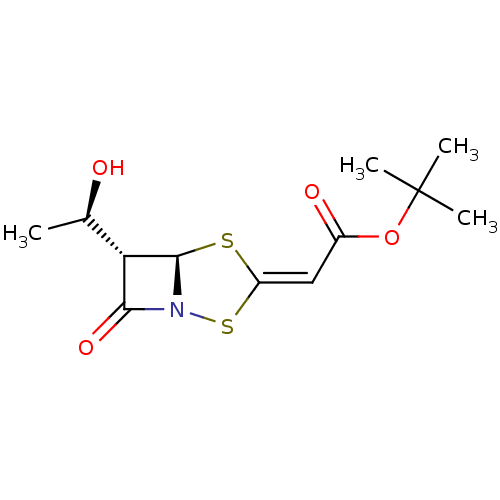 (CHEMBL72889 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281004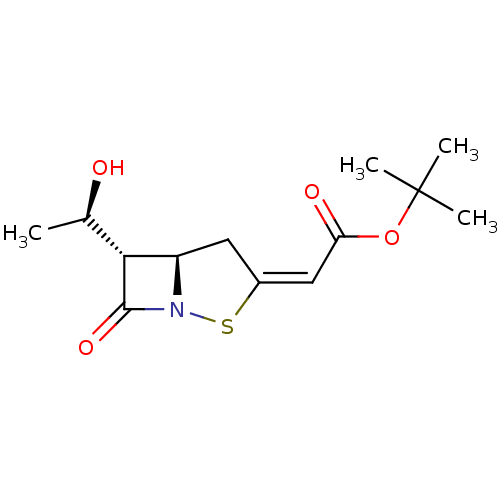 (CHEMBL419820 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281003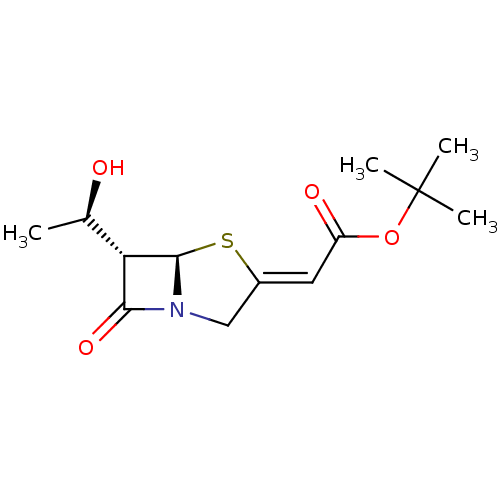 (CHEMBL306859 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 170 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281009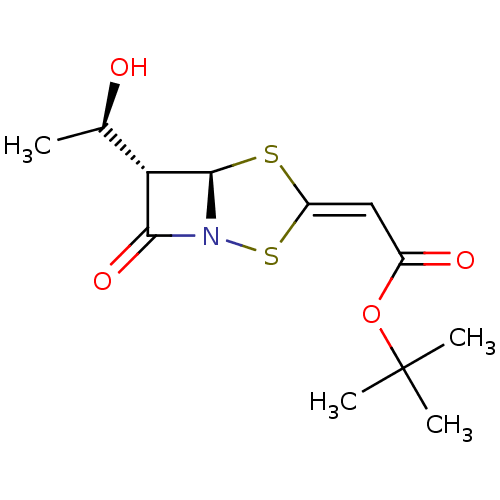 (CHEMBL74813 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) was determined | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281007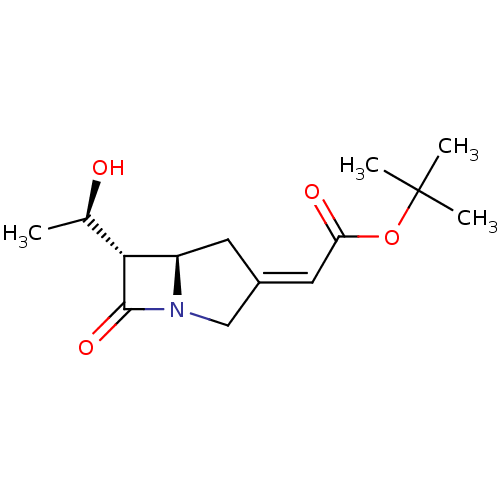 (CHEMBL75220 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281008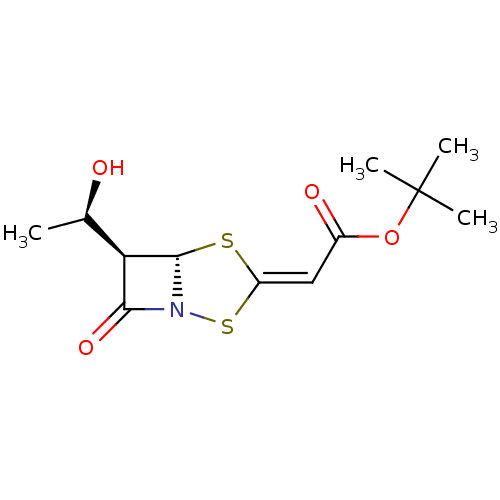 (CHEMBL311390 | [(5S,6R)-6-((R)-1-Hydroxy-ethyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370772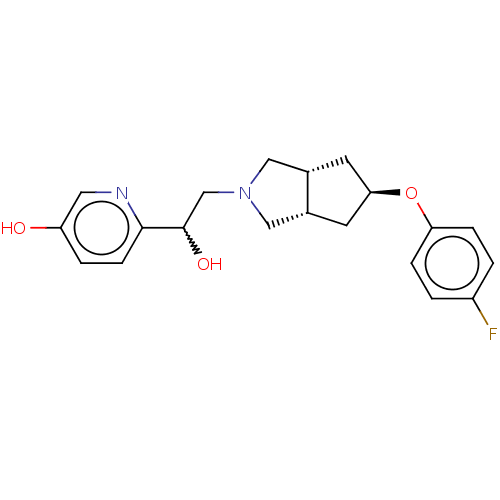 (US10239835, Example 00324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370754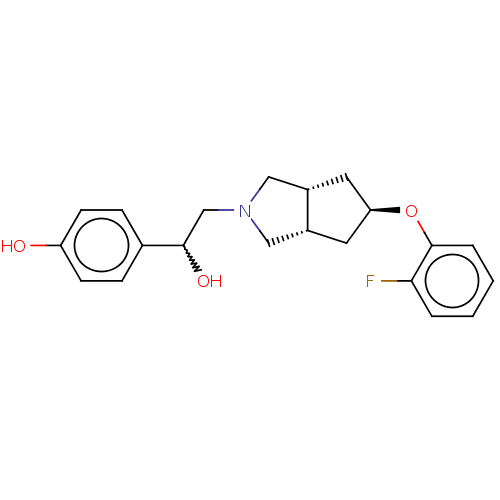 (US10239835, Example 00306) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370814 (US10239835, Example 00365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370813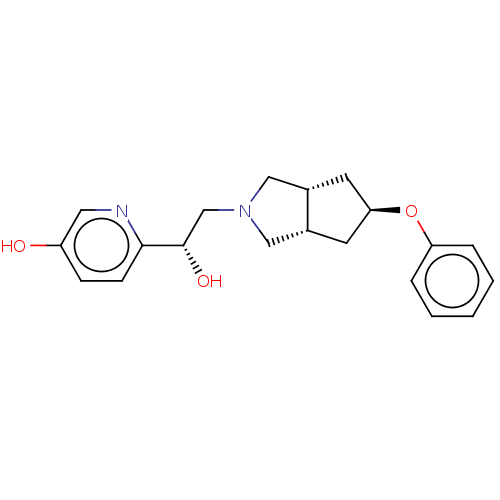 (US10239835, Example 00364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370756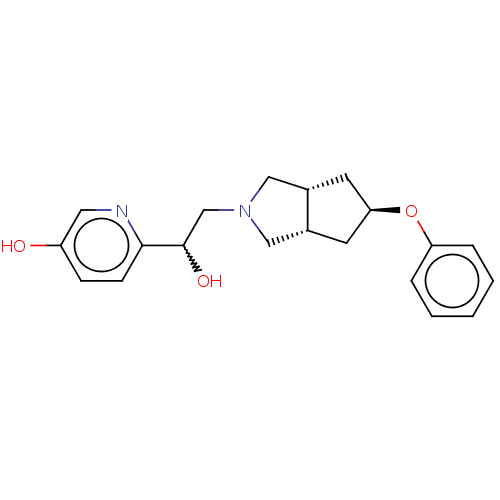 (US10239835, Example 00308) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370745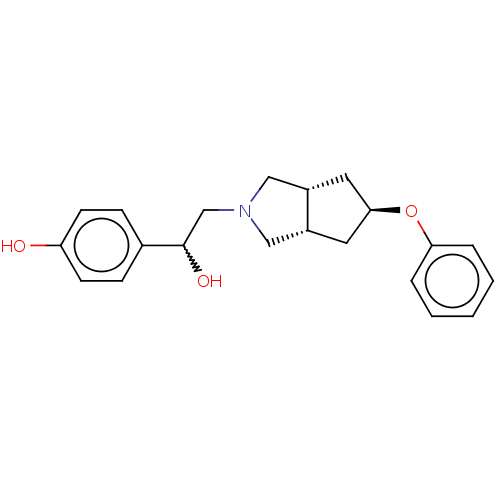 (US10239835, Example 00297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370743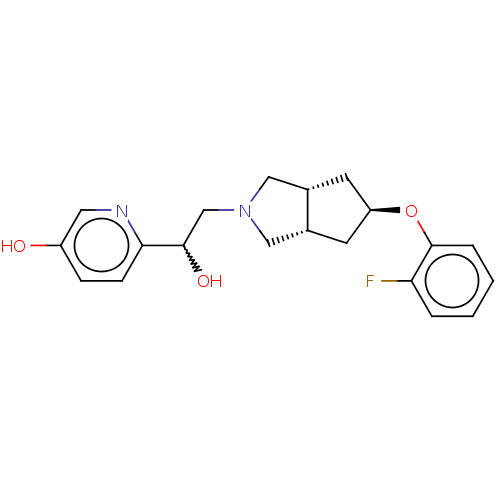 (US10239835, Example 00295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370781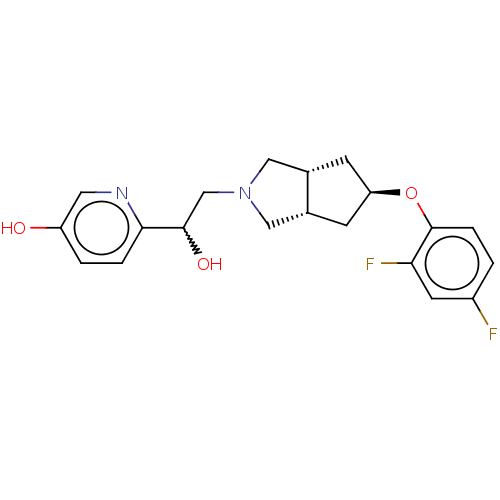 (US10239835, Example 00333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370815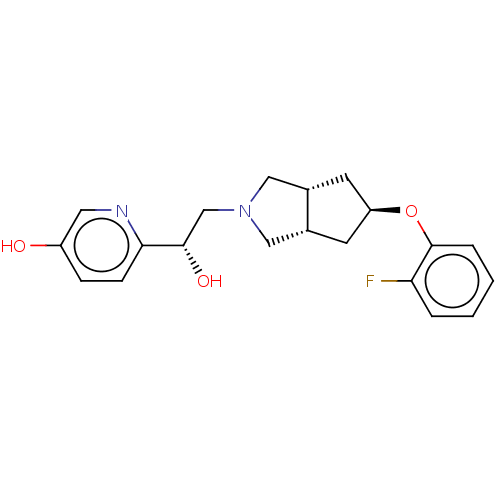 (US10239835, Example 00366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370816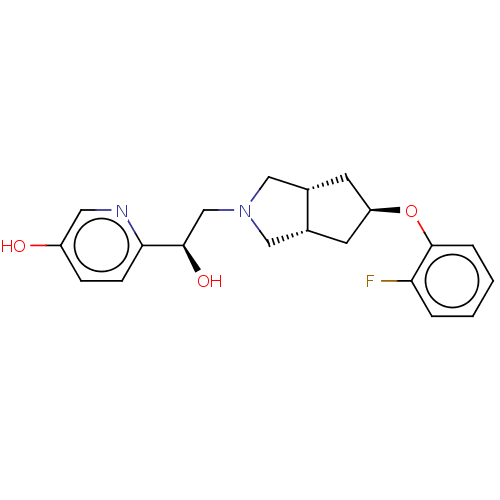 (US10239835, Example 00367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370769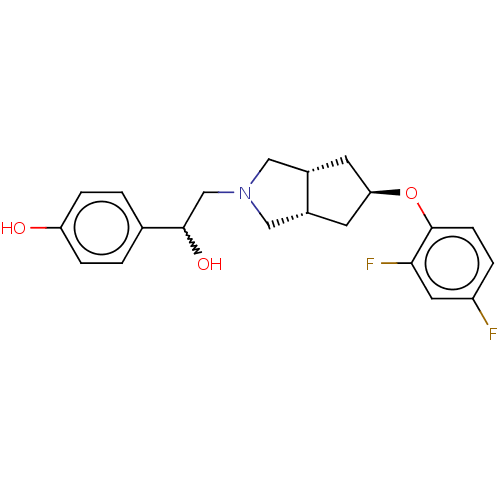 (US10239835, Example 00321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370797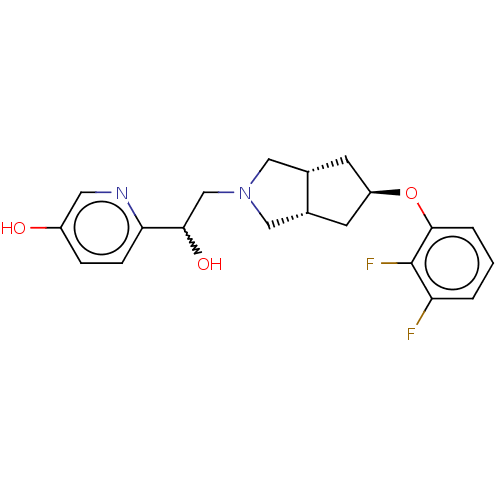 (US10239835, Example 00349) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370802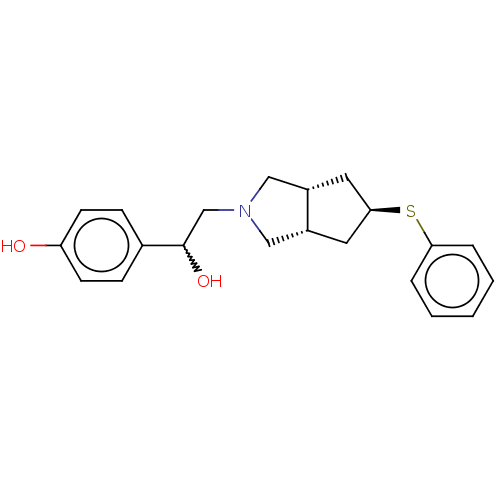 (US10239835, Example 00354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370768 (US10239835, Example 00320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091800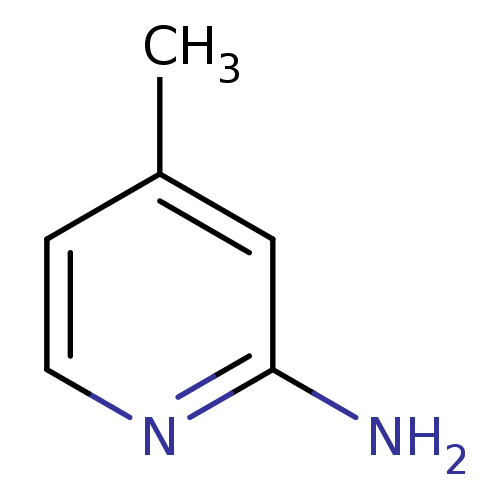 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human inducible nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370744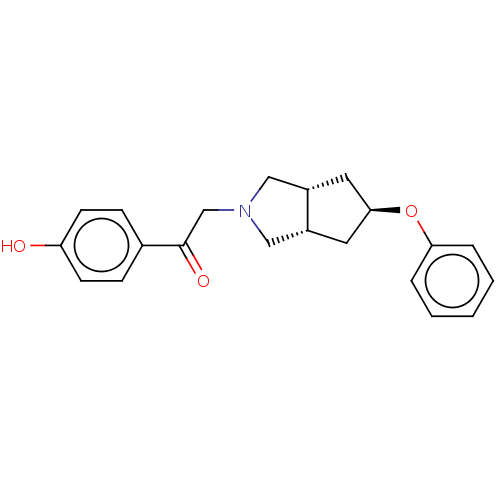 (US10239835, Example 00296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM237552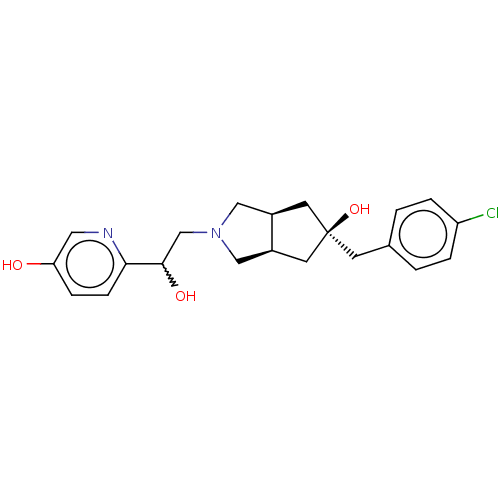 (US10052306, 223 | rac-(3aR,5r,6aS)-5-(4- chloroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM238867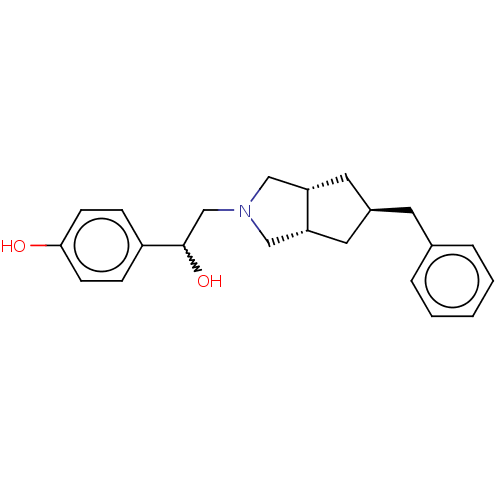 (US10052306, 306 | rac-4-{2-[(3aR,5R,6aS)- 5-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM238862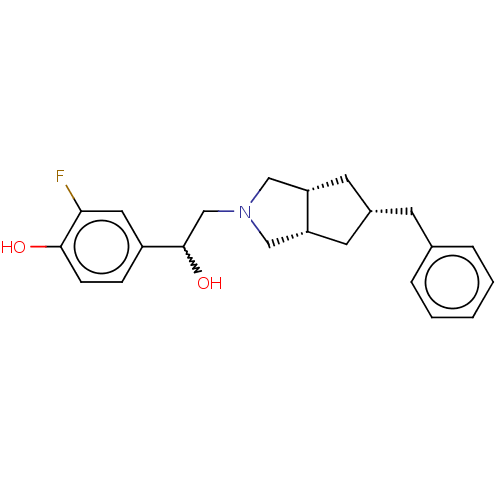 (US10052306, 301 | rac-4-{2-[(3aR,5S,6aS)- 5-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM237388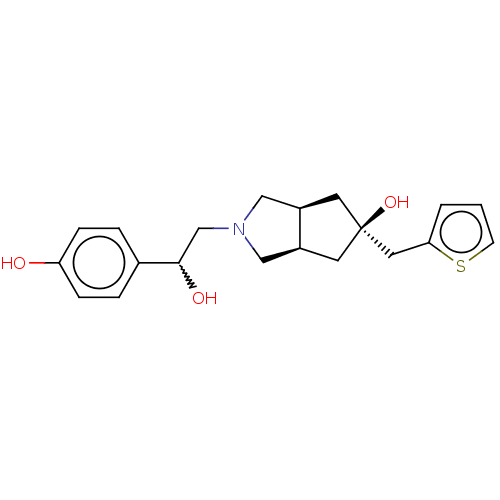 (US10052306, 204 | rac-(3aR,5r,6aS)-2-(2- hydroxy-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370676 (US10239835, Example 00228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM238861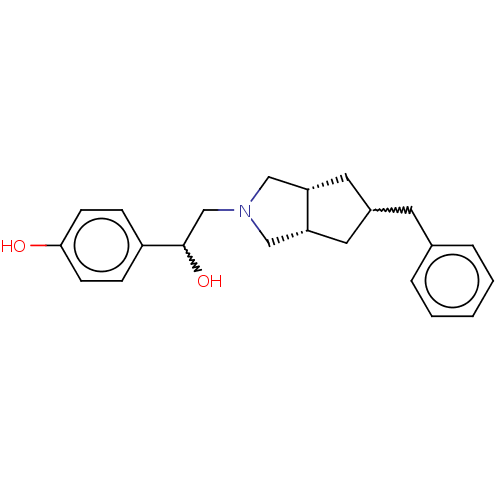 (US10052306, 300 | rac-4-(2-((3aR,6aS)-5- benzylhex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370678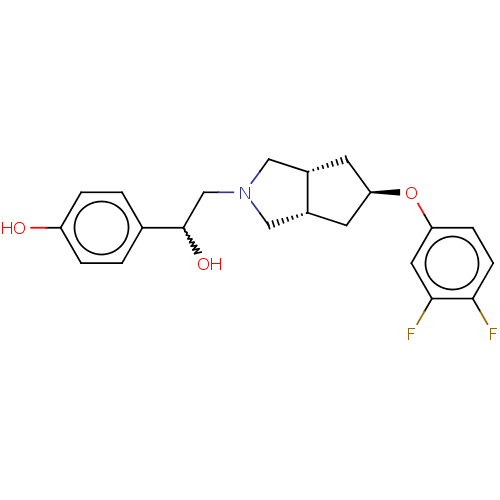 (US10239835, Example 00230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370782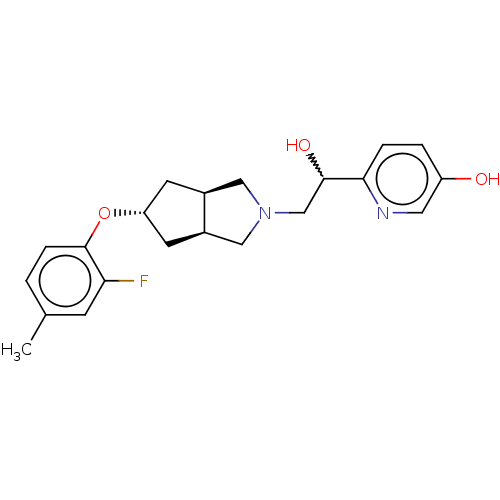 (US10239835, Example 00334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM237683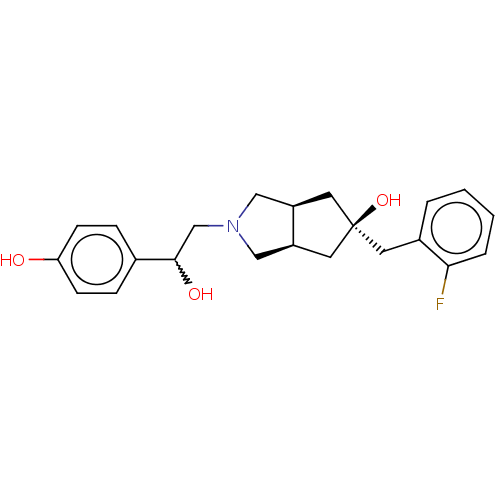 (US10052306, 229 | rac-(3aR,5r,6aS)-5-(2- fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Homo sapiens (Human)) | BDBM393276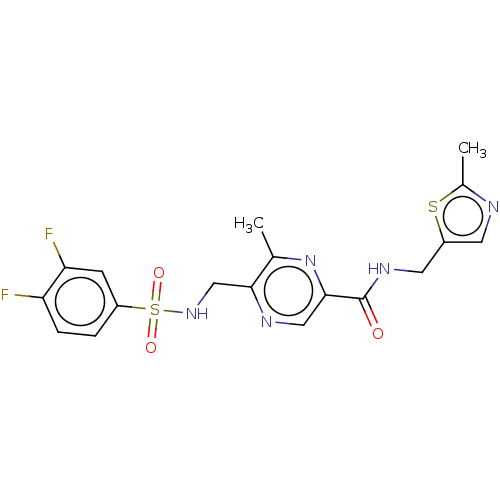 (5-(3,4- difluorobenzenesulfon- amidomethyl)-6- met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2A (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2KD217B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM238865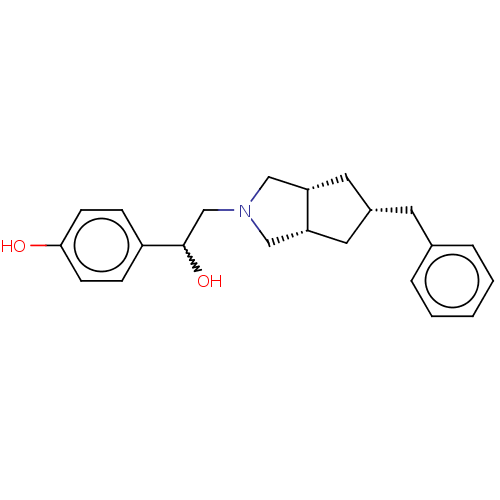 (US10052306, 304 | rac-4-{2-[(3aR,5S,6aS)- 5-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370755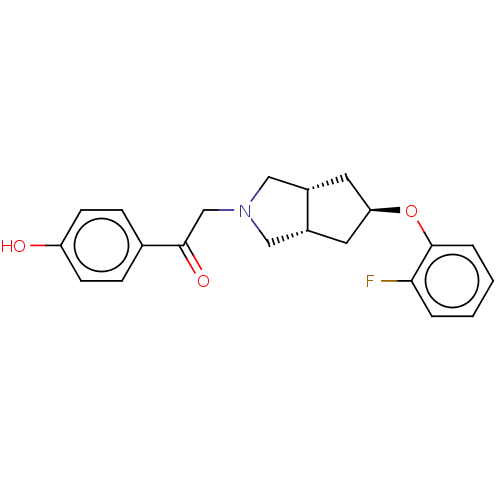 (US10239835, Example 00307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM235335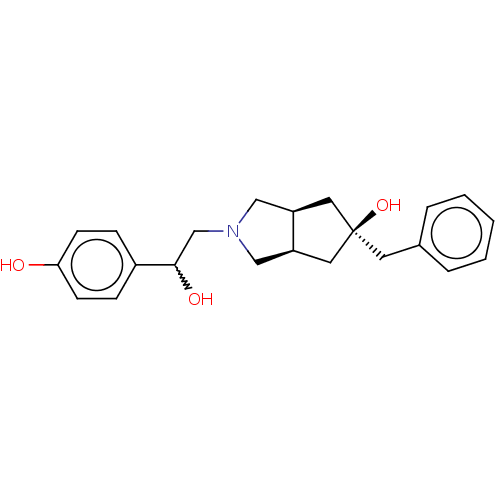 (US10052306, 131 | US10052306, 281 | rac-(3aR,5r,6a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM237322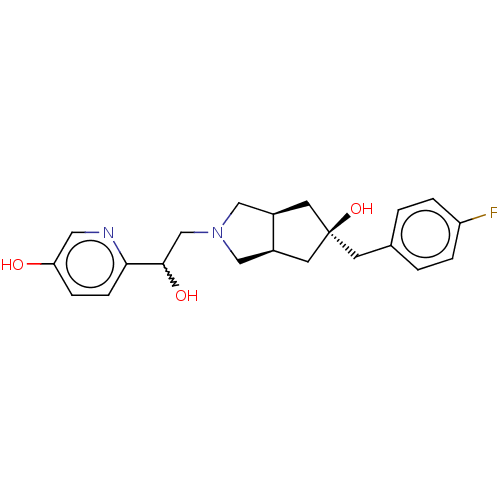 (US10052306, 168 | rac-(3aR,5r,6aS)-5-(4- fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370681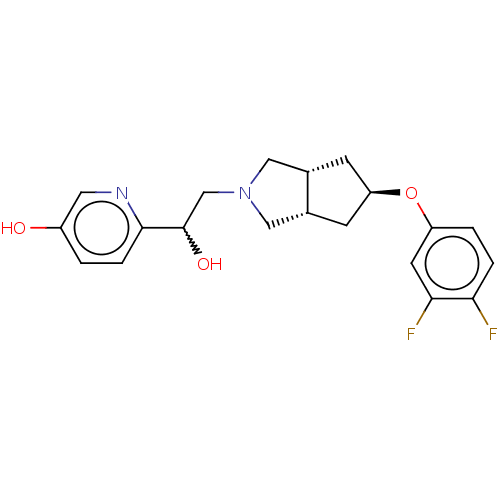 (US10239835, Example 00233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370727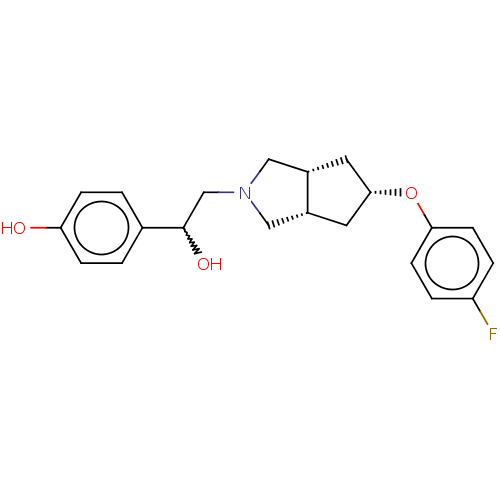 (US10239835, Example 00279) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370694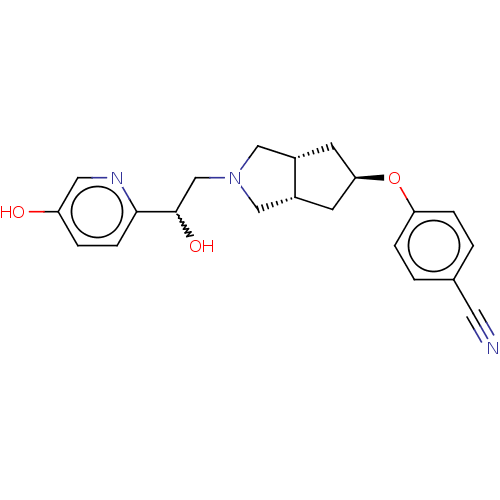 (US10239835, Example 00246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370809 (US10239835, Example 00361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM237551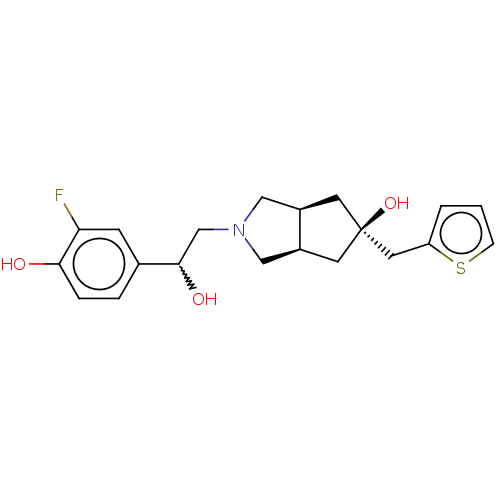 (US10052306, 222 | rac-2-(2-(3-fluoro-4- hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Homo sapiens (Human)) | BDBM393246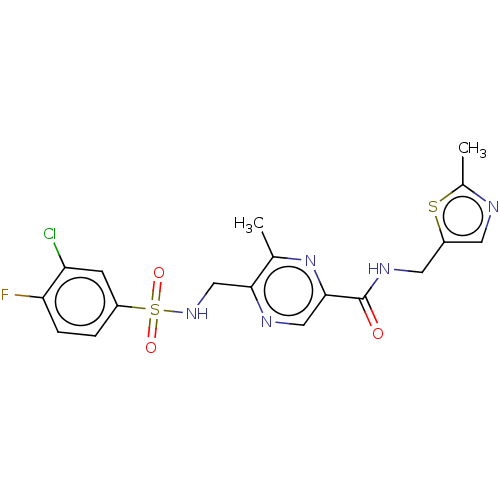 (5-(3-chloro-4- fluorobenzenesulfon- amidomethyl)-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2A (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2KD217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM370817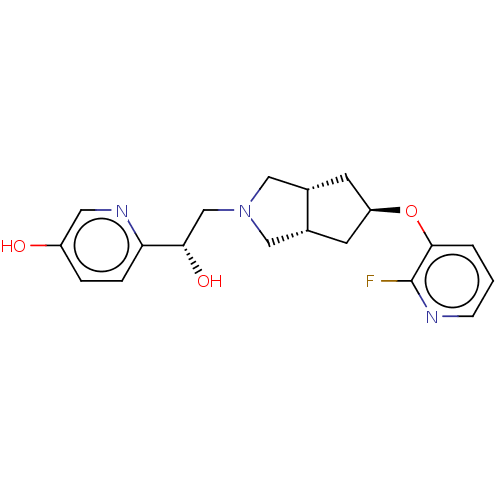 (US10239835, Example 00368) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Cell Culture and plating: HEK293 cells expressing NR1/NR2B (Chantest, Cleveland, Ohio) were grown to 70-80% confluency as an adherent monolayer in st... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2F19207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM238863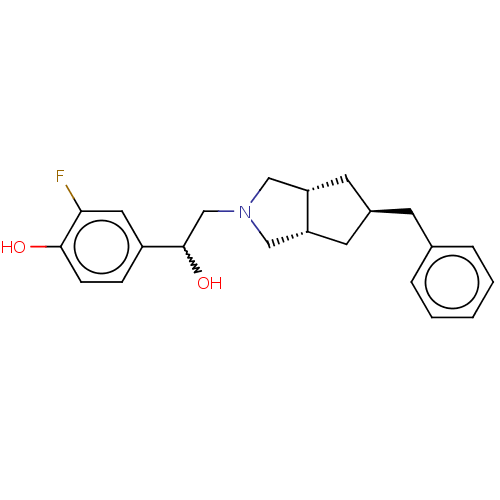 (US10052306, 302 | rac-4-{2-[(3aR,5R,6aS)- 5-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a |
CADENT THERAPEUTICS, INC. US Patent | Assay Description To each well of the plate, 10 μL test compound, control (MK801) or HHnoCa buffer was added to a final concentration of 10 μM with a final c... | US Patent US10052306 (2018) BindingDB Entry DOI: 10.7270/Q20R9RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 830 total ) | Next | Last >> |