 Found 393 hits with Last Name = 'speth' and Initial = 'rc'
Found 393 hits with Last Name = 'speth' and Initial = 'rc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A
(RAT) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228201

(CHEMBL265084)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,60.62,wD:43.43,24.23,6.5,(-1.11,-12.66,;-1.11,-11.12,;.24,-10.35,;1.55,-11.12,;1.55,-12.66,;2.9,-10.35,;4.24,-11.12,;4.24,-12.66,;5.56,-13.44,;5.56,-14.98,;6.9,-15.75,;6.9,-17.28,;8.25,-18.05,;5.56,-18.05,;5.56,-10.35,;5.56,-8.81,;6.9,-11.12,;8.25,-10.35,;8.25,-8.81,;9.56,-8.02,;6.9,-8.02,;9.56,-11.12,;9.56,-12.66,;10.91,-10.35,;12.25,-11.12,;12.25,-12.66,;13.57,-13.44,;13.57,-14.98,;14.91,-15.75,;16.26,-14.98,;17.57,-15.75,;16.26,-13.44,;14.91,-12.66,;13.57,-10.35,;13.57,-8.81,;14.91,-11.12,;16.26,-10.35,;16.26,-8.81,;17.57,-8.02,;14.91,-8.02,;17.57,-11.12,;17.57,-12.66,;18.92,-10.35,;20.27,-11.12,;20.27,-12.66,;20.81,-14.14,;20.59,-15.7,;22.19,-16.42,;23.31,-15.06,;22.28,-13.86,;21.58,-10.35,;21.58,-8.81,;22.93,-11.12,;23.09,-12.66,;24.59,-12.98,;25.36,-11.64,;24.34,-10.49,;24.66,-8.98,;26.13,-8.5,;23.5,-7.96,;23.85,-6.45,;25.39,-5.8,;26.45,-4.72,;25.97,-3.27,;27,-2.13,;28.5,-2.47,;28.98,-3.94,;27.93,-5.04,;29.56,-1.32,;29.08,.15,;30.14,1.3,;31.66,.98,;32.12,-.51,;31.09,-1.65,;22.7,-5.42,;23.02,-3.91,;21.23,-5.9,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)45(65-47(70)39(61-44(69)29-57-5)13-9-23-59-54(55)56)50(73)62-40(25-34-17-21-38(68)22-18-34)48(71)66-46(32(3)4)51(74)63-41(27-37-28-58-30-60-37)52(75)67-24-10-14-43(67)49(72)64-42(53(76)77)26-33-15-19-36(20-16-33)35-11-7-6-8-12-35/h6-8,11-12,15-22,28,30-32,39-43,45-46,57,68H,9-10,13-14,23-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,72)(H,65,70)(H,66,71)(H,76,77)(H4,55,56,59)/t39-,40-,41-,42+,43+,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228201

(CHEMBL265084)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,60.62,wD:43.43,24.23,6.5,(-1.11,-12.66,;-1.11,-11.12,;.24,-10.35,;1.55,-11.12,;1.55,-12.66,;2.9,-10.35,;4.24,-11.12,;4.24,-12.66,;5.56,-13.44,;5.56,-14.98,;6.9,-15.75,;6.9,-17.28,;8.25,-18.05,;5.56,-18.05,;5.56,-10.35,;5.56,-8.81,;6.9,-11.12,;8.25,-10.35,;8.25,-8.81,;9.56,-8.02,;6.9,-8.02,;9.56,-11.12,;9.56,-12.66,;10.91,-10.35,;12.25,-11.12,;12.25,-12.66,;13.57,-13.44,;13.57,-14.98,;14.91,-15.75,;16.26,-14.98,;17.57,-15.75,;16.26,-13.44,;14.91,-12.66,;13.57,-10.35,;13.57,-8.81,;14.91,-11.12,;16.26,-10.35,;16.26,-8.81,;17.57,-8.02,;14.91,-8.02,;17.57,-11.12,;17.57,-12.66,;18.92,-10.35,;20.27,-11.12,;20.27,-12.66,;20.81,-14.14,;20.59,-15.7,;22.19,-16.42,;23.31,-15.06,;22.28,-13.86,;21.58,-10.35,;21.58,-8.81,;22.93,-11.12,;23.09,-12.66,;24.59,-12.98,;25.36,-11.64,;24.34,-10.49,;24.66,-8.98,;26.13,-8.5,;23.5,-7.96,;23.85,-6.45,;25.39,-5.8,;26.45,-4.72,;25.97,-3.27,;27,-2.13,;28.5,-2.47,;28.98,-3.94,;27.93,-5.04,;29.56,-1.32,;29.08,.15,;30.14,1.3,;31.66,.98,;32.12,-.51,;31.09,-1.65,;22.7,-5.42,;23.02,-3.91,;21.23,-5.9,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)45(65-47(70)39(61-44(69)29-57-5)13-9-23-59-54(55)56)50(73)62-40(25-34-17-21-38(68)22-18-34)48(71)66-46(32(3)4)51(74)63-41(27-37-28-58-30-60-37)52(75)67-24-10-14-43(67)49(72)64-42(53(76)77)26-33-15-19-36(20-16-33)35-11-7-6-8-12-35/h6-8,11-12,15-22,28,30-32,39-43,45-46,57,68H,9-10,13-14,23-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,72)(H,65,70)(H,66,71)(H,76,77)(H4,55,56,59)/t39-,40-,41-,42+,43+,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50266734
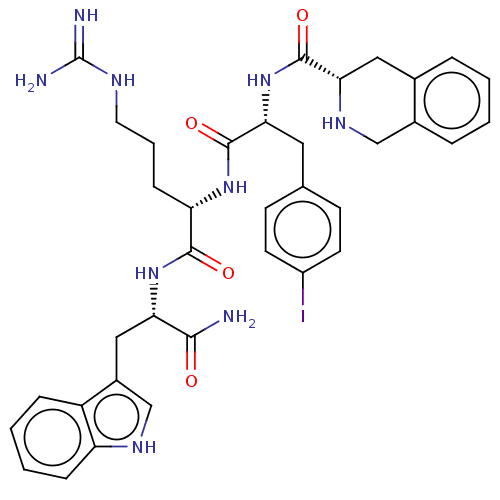
(CHEMBL4096081)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H](Cc1ccc(I)cc1)NC(=O)[C@@H]1Cc2ccccc2CN1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C36H42IN9O4/c37-25-13-11-21(12-14-25)16-31(46-34(49)30-17-22-6-1-2-7-23(22)19-43-30)35(50)44-28(10-5-15-41-36(39)40)33(48)45-29(32(38)47)18-24-20-42-27-9-4-3-8-26(24)27/h1-4,6-9,11-14,20,28-31,42-43H,5,10,15-19H2,(H2,38,47)(H,44,50)(H,45,48)(H,46,49)(H4,39,40,41)/t28-,29-,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of aplha MSH-induced cAMP activation after 45 mins |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228204
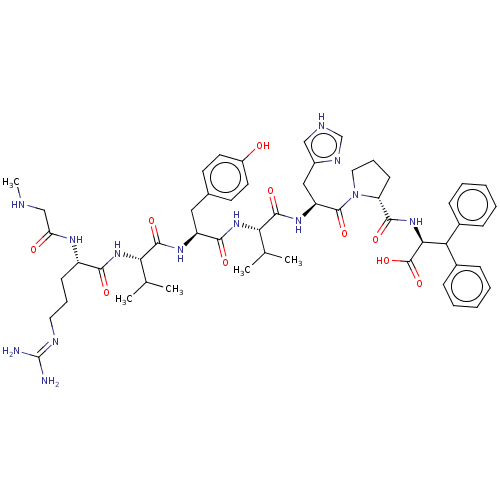
(CHEMBL405389)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,wD:60.62,43.43,24.23,6.5,(2.9,-15.86,;2.9,-14.33,;4.23,-13.56,;5.56,-14.33,;5.56,-15.86,;6.9,-13.56,;8.24,-14.33,;8.24,-15.86,;9.56,-16.64,;9.56,-18.18,;10.91,-18.95,;10.91,-20.49,;12.24,-21.26,;9.56,-21.26,;9.56,-13.56,;9.56,-12.02,;10.91,-14.33,;12.24,-13.56,;12.24,-12.02,;13.57,-11.23,;10.91,-11.23,;13.57,-14.33,;13.57,-15.86,;14.91,-13.56,;16.25,-14.33,;16.25,-15.86,;17.57,-16.64,;17.57,-18.18,;18.92,-18.95,;20.25,-18.18,;21.58,-18.95,;20.25,-16.64,;18.92,-15.86,;17.57,-13.56,;17.57,-12.02,;18.92,-14.33,;20.25,-13.56,;20.25,-12.02,;21.58,-11.23,;18.92,-11.23,;21.58,-14.33,;21.58,-15.86,;22.93,-13.56,;24.26,-14.33,;24.26,-15.86,;24.82,-17.35,;24.98,-18.88,;26.48,-19.2,;27.27,-17.86,;26.23,-16.71,;25.59,-13.56,;25.59,-12.02,;26.93,-14.33,;27.09,-15.86,;28.6,-16.18,;29.37,-14.85,;28.34,-13.7,;28.66,-12.19,;30.13,-11.71,;27.51,-11.16,;27.85,-9.66,;29.3,-9.18,;29.62,-7.67,;31.09,-7.19,;31.41,-5.68,;30.26,-4.65,;28.79,-5.13,;28.47,-6.63,;30.84,-9.18,;31.6,-10.49,;33.14,-10.49,;33.89,-9.17,;33.12,-7.83,;31.58,-7.85,;26.7,-8.62,;27.02,-7.11,;25.23,-9.1,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)44(64-47(70)38(61-42(69)29-57-5)18-12-24-59-54(55)56)50(73)62-39(26-33-20-22-37(68)23-21-33)48(71)65-45(32(3)4)51(74)63-40(27-36-28-58-30-60-36)52(75)67-25-13-19-41(67)49(72)66-46(53(76)77)43(34-14-8-6-9-15-34)35-16-10-7-11-17-35/h6-11,14-17,20-23,28,30-32,38-41,43-46,57,68H,12-13,18-19,24-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,70)(H,65,71)(H,66,72)(H,76,77)(H4,55,56,59)/t38-,39-,40-,41+,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228203

(CHEMBL262025)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,wD:43.43,24.23,60.62,6.5,(-1.11,-12.7,;-1.11,-11.16,;.24,-10.39,;1.55,-11.16,;1.55,-12.7,;2.9,-10.39,;4.24,-11.16,;4.24,-12.7,;5.56,-13.47,;5.56,-15.01,;6.9,-15.78,;6.9,-17.32,;8.25,-18.09,;5.56,-18.09,;5.56,-10.39,;5.56,-8.86,;6.9,-11.16,;8.25,-10.39,;8.25,-8.86,;9.56,-8.05,;6.9,-8.05,;9.56,-11.16,;9.56,-12.7,;10.91,-10.39,;12.25,-11.16,;12.25,-12.7,;13.57,-13.47,;13.57,-15.01,;14.91,-15.78,;16.26,-15.01,;17.57,-15.78,;16.26,-13.47,;14.91,-12.7,;13.57,-10.39,;13.57,-8.86,;14.91,-11.16,;16.26,-10.39,;16.26,-8.86,;17.57,-8.05,;14.91,-8.05,;17.57,-11.16,;17.57,-12.7,;18.92,-10.39,;20.27,-11.16,;20.27,-12.7,;20.81,-14.18,;20.59,-15.75,;22.19,-16.45,;23.31,-15.11,;22.28,-13.91,;21.58,-10.39,;21.58,-8.86,;22.93,-11.16,;23.09,-12.7,;24.59,-13.02,;25.36,-11.68,;24.34,-10.52,;24.66,-9.02,;26.13,-8.54,;23.5,-7.99,;23.85,-6.48,;25.39,-5.84,;26.45,-4.75,;25.97,-3.31,;27,-2.18,;28.5,-2.51,;28.98,-3.98,;27.93,-5.1,;29.56,-1.36,;29.08,.12,;30.14,1.27,;31.66,.93,;32.12,-.56,;31.09,-1.7,;22.7,-5.46,;23.02,-3.95,;21.23,-5.94,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)45(65-47(70)39(61-44(69)29-57-5)13-9-23-59-54(55)56)50(73)62-40(25-34-17-21-38(68)22-18-34)48(71)66-46(32(3)4)51(74)63-41(27-37-28-58-30-60-37)52(75)67-24-10-14-43(67)49(72)64-42(53(76)77)26-33-15-19-36(20-16-33)35-11-7-6-8-12-35/h6-8,11-12,15-22,28,30-32,39-43,45-46,57,68H,9-10,13-14,23-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,72)(H,65,70)(H,66,71)(H,76,77)(H4,55,56,59)/t39-,40-,41-,42-,43+,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228204

(CHEMBL405389)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,wD:60.62,43.43,24.23,6.5,(2.9,-15.86,;2.9,-14.33,;4.23,-13.56,;5.56,-14.33,;5.56,-15.86,;6.9,-13.56,;8.24,-14.33,;8.24,-15.86,;9.56,-16.64,;9.56,-18.18,;10.91,-18.95,;10.91,-20.49,;12.24,-21.26,;9.56,-21.26,;9.56,-13.56,;9.56,-12.02,;10.91,-14.33,;12.24,-13.56,;12.24,-12.02,;13.57,-11.23,;10.91,-11.23,;13.57,-14.33,;13.57,-15.86,;14.91,-13.56,;16.25,-14.33,;16.25,-15.86,;17.57,-16.64,;17.57,-18.18,;18.92,-18.95,;20.25,-18.18,;21.58,-18.95,;20.25,-16.64,;18.92,-15.86,;17.57,-13.56,;17.57,-12.02,;18.92,-14.33,;20.25,-13.56,;20.25,-12.02,;21.58,-11.23,;18.92,-11.23,;21.58,-14.33,;21.58,-15.86,;22.93,-13.56,;24.26,-14.33,;24.26,-15.86,;24.82,-17.35,;24.98,-18.88,;26.48,-19.2,;27.27,-17.86,;26.23,-16.71,;25.59,-13.56,;25.59,-12.02,;26.93,-14.33,;27.09,-15.86,;28.6,-16.18,;29.37,-14.85,;28.34,-13.7,;28.66,-12.19,;30.13,-11.71,;27.51,-11.16,;27.85,-9.66,;29.3,-9.18,;29.62,-7.67,;31.09,-7.19,;31.41,-5.68,;30.26,-4.65,;28.79,-5.13,;28.47,-6.63,;30.84,-9.18,;31.6,-10.49,;33.14,-10.49,;33.89,-9.17,;33.12,-7.83,;31.58,-7.85,;26.7,-8.62,;27.02,-7.11,;25.23,-9.1,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)44(64-47(70)38(61-42(69)29-57-5)18-12-24-59-54(55)56)50(73)62-39(26-33-20-22-37(68)23-21-33)48(71)65-45(32(3)4)51(74)63-40(27-36-28-58-30-60-36)52(75)67-25-13-19-41(67)49(72)66-46(53(76)77)43(34-14-8-6-9-15-34)35-16-10-7-11-17-35/h6-11,14-17,20-23,28,30-32,38-41,43-46,57,68H,12-13,18-19,24-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,70)(H,65,71)(H,66,72)(H,76,77)(H4,55,56,59)/t38-,39-,40-,41+,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228198

(CHEMBL414796)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)NC1(Cc2ccccc2C1)C(O)=O |wU:56.59,36.36,17.16,wD:43.43,24.23,6.5,(2.9,-15.88,;2.9,-14.34,;4.23,-13.57,;5.56,-14.34,;5.56,-15.88,;6.91,-13.57,;8.24,-14.34,;8.24,-15.88,;9.57,-16.66,;9.57,-18.2,;10.92,-18.97,;10.92,-20.51,;12.25,-21.28,;9.57,-21.28,;9.57,-13.57,;9.57,-12.03,;10.92,-14.34,;12.25,-13.57,;12.25,-12.03,;13.58,-11.24,;10.92,-11.24,;13.58,-14.34,;13.58,-15.88,;14.93,-13.57,;16.26,-14.34,;16.26,-15.88,;17.59,-16.66,;17.59,-18.2,;18.94,-18.97,;20.27,-18.2,;21.6,-18.97,;20.27,-16.66,;18.94,-15.88,;17.59,-13.57,;17.59,-12.03,;18.94,-14.34,;20.27,-13.57,;20.27,-12.03,;21.6,-11.24,;18.94,-11.24,;21.6,-14.34,;21.6,-15.88,;22.95,-13.57,;24.28,-14.34,;24.28,-15.88,;24.84,-17.37,;25,-18.9,;26.51,-19.22,;27.3,-17.88,;26.25,-16.72,;25.61,-13.57,;25.61,-12.03,;26.96,-14.34,;27.12,-15.88,;28.63,-16.2,;29.4,-14.86,;28.37,-13.71,;28.69,-12.2,;30.16,-11.72,;27.54,-11.17,;27.88,-9.67,;28.79,-8.42,;30.25,-8.9,;31.58,-8.13,;32.89,-8.9,;32.89,-10.44,;31.57,-11.21,;30.23,-10.44,;28.78,-10.92,;26.72,-8.63,;27.04,-7.12,;25.26,-9.11,)| Show InChI InChI=1S/C49H69N13O10/c1-27(2)39(59-41(65)34(56-38(64)25-52-5)12-8-18-54-48(50)51)44(68)57-35(20-29-14-16-33(63)17-15-29)42(66)60-40(28(3)4)45(69)58-36(21-32-24-53-26-55-32)46(70)62-19-9-13-37(62)43(67)61-49(47(71)72)22-30-10-6-7-11-31(30)23-49/h6-7,10-11,14-17,24,26-28,34-37,39-40,52,63H,8-9,12-13,18-23,25H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,65)(H,60,66)(H,61,67)(H,71,72)(H4,50,51,54)/t34-,35-,36-,37+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228199

(ANGIOTENSIN AMIDE | Angiotensinamide)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:59.62,39.39,46.46,27.26,18.18,7.7,wD:3.3,63.65,(-.29,-6,;-1.35,-5.38,;-2.42,-5.99,;-1.34,-3.84,;-2.68,-3.06,;-4.01,-3.83,;-4.02,-5.06,;-5.35,-3.05,;-5.34,-1.51,;-6.67,-.74,;-6.67,.8,;-8,1.57,;-8,3.11,;-9.06,3.73,;-6.93,3.73,;-6.68,-3.82,;-8.01,-3.05,;-8.01,-1.81,;-9.35,-3.81,;-9.35,-5.04,;-10.68,-3.04,;-12.02,-3.8,;-12.02,-5.03,;-13.08,-3.18,;-.01,-3.07,;-0,-1.84,;1.33,-3.85,;2.66,-3.08,;2.67,-1.54,;4,-.77,;4,.77,;5.34,1.54,;6.67,.76,;7.74,1.37,;6.66,-.78,;5.33,-1.54,;4,-3.85,;5.06,-3.24,;3.99,-5.39,;5.33,-6.17,;6.66,-5.4,;7.73,-6.02,;6.67,-4.17,;5.33,-7.71,;4.26,-8.32,;6.66,-8.48,;6.66,-10.02,;7.99,-10.8,;9.33,-10.03,;10.71,-10.67,;11.74,-9.53,;10.97,-8.19,;9.47,-8.51,;5.32,-10.79,;4.25,-10.18,;5.32,-12.34,;6.57,-13.21,;6.1,-14.68,;4.56,-14.69,;4.08,-13.22,;2.62,-12.73,;1.69,-13.55,;2.31,-11.22,;.85,-10.74,;.53,-9.23,;-.92,-8.74,;-1.24,-7.23,;-2.7,-6.75,;-3.85,-7.77,;-3.54,-9.28,;-2.07,-9.76,;-.31,-11.76,;-.06,-12.96,;-1.48,-11.37,)| Show InChI InChI=1S/C49H70N14O11/c1-26(2)39(61-42(67)33(12-8-18-55-49(52)53)57-41(66)32(50)23-38(51)65)45(70)58-34(20-29-14-16-31(64)17-15-29)43(68)62-40(27(3)4)46(71)59-35(22-30-24-54-25-56-30)47(72)63-19-9-13-37(63)44(69)60-36(48(73)74)21-28-10-6-5-7-11-28/h5-7,10-11,14-17,24-27,32-37,39-40,64H,8-9,12-13,18-23,50H2,1-4H3,(H2,51,65)(H,54,56)(H,57,66)(H,58,70)(H,59,71)(H,60,69)(H,61,67)(H,62,68)(H,73,74)(H4,52,53,55)/t32-,33-,34-,35-,36-,37-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228200
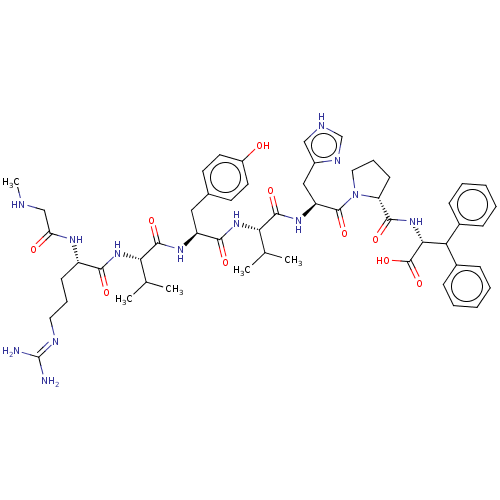
(CHEMBL217516)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(O)=O |wU:60.62,56.59,36.36,17.16,wD:43.43,24.23,6.5,(2.9,-15.86,;2.9,-14.33,;4.23,-13.56,;5.56,-14.33,;5.56,-15.86,;6.9,-13.56,;8.24,-14.33,;8.24,-15.86,;9.56,-16.64,;9.56,-18.18,;10.91,-18.95,;10.91,-20.49,;12.24,-21.26,;9.56,-21.26,;9.56,-13.56,;9.56,-12.02,;10.91,-14.33,;12.24,-13.56,;12.24,-12.02,;13.57,-11.23,;10.91,-11.23,;13.57,-14.33,;13.57,-15.86,;14.91,-13.56,;16.25,-14.33,;16.25,-15.86,;17.57,-16.64,;17.57,-18.18,;18.92,-18.95,;20.25,-18.18,;21.58,-18.95,;20.25,-16.64,;18.92,-15.86,;17.57,-13.56,;17.57,-12.02,;18.92,-14.33,;20.25,-13.56,;20.25,-12.02,;21.58,-11.23,;18.92,-11.23,;21.58,-14.33,;21.58,-15.86,;22.93,-13.56,;24.26,-14.33,;24.26,-15.86,;24.82,-17.35,;24.98,-18.88,;26.48,-19.2,;27.27,-17.86,;26.23,-16.71,;25.59,-13.56,;25.59,-12.02,;26.93,-14.33,;27.09,-15.86,;28.6,-16.18,;29.37,-14.85,;28.34,-13.7,;28.66,-12.19,;30.13,-11.71,;27.51,-11.16,;27.85,-9.66,;29.3,-9.18,;29.62,-7.67,;31.09,-7.19,;31.41,-5.68,;30.26,-4.65,;28.79,-5.13,;28.47,-6.63,;30.84,-9.18,;31.6,-10.49,;33.14,-10.49,;33.89,-9.17,;33.12,-7.83,;31.58,-7.85,;26.7,-8.62,;27.02,-7.11,;25.23,-9.1,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)44(64-47(70)38(61-42(69)29-57-5)18-12-24-59-54(55)56)50(73)62-39(26-33-20-22-37(68)23-21-33)48(71)65-45(32(3)4)51(74)63-40(27-36-28-58-30-60-36)52(75)67-25-13-19-41(67)49(72)66-46(53(76)77)43(34-14-8-6-9-15-34)35-16-10-7-11-17-35/h6-11,14-17,20-23,28,30-32,38-41,43-46,57,68H,12-13,18-19,24-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,70)(H,65,71)(H,66,72)(H,76,77)(H4,55,56,59)/t38-,39-,40-,41+,44-,45-,46+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228203

(CHEMBL262025)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O |wU:56.59,36.36,17.16,wD:43.43,24.23,60.62,6.5,(-1.11,-12.7,;-1.11,-11.16,;.24,-10.39,;1.55,-11.16,;1.55,-12.7,;2.9,-10.39,;4.24,-11.16,;4.24,-12.7,;5.56,-13.47,;5.56,-15.01,;6.9,-15.78,;6.9,-17.32,;8.25,-18.09,;5.56,-18.09,;5.56,-10.39,;5.56,-8.86,;6.9,-11.16,;8.25,-10.39,;8.25,-8.86,;9.56,-8.05,;6.9,-8.05,;9.56,-11.16,;9.56,-12.7,;10.91,-10.39,;12.25,-11.16,;12.25,-12.7,;13.57,-13.47,;13.57,-15.01,;14.91,-15.78,;16.26,-15.01,;17.57,-15.78,;16.26,-13.47,;14.91,-12.7,;13.57,-10.39,;13.57,-8.86,;14.91,-11.16,;16.26,-10.39,;16.26,-8.86,;17.57,-8.05,;14.91,-8.05,;17.57,-11.16,;17.57,-12.7,;18.92,-10.39,;20.27,-11.16,;20.27,-12.7,;20.81,-14.18,;20.59,-15.75,;22.19,-16.45,;23.31,-15.11,;22.28,-13.91,;21.58,-10.39,;21.58,-8.86,;22.93,-11.16,;23.09,-12.7,;24.59,-13.02,;25.36,-11.68,;24.34,-10.52,;24.66,-9.02,;26.13,-8.54,;23.5,-7.99,;23.85,-6.48,;25.39,-5.84,;26.45,-4.75,;25.97,-3.31,;27,-2.18,;28.5,-2.51,;28.98,-3.98,;27.93,-5.1,;29.56,-1.36,;29.08,.12,;30.14,1.27,;31.66,.93,;32.12,-.56,;31.09,-1.7,;22.7,-5.46,;23.02,-3.95,;21.23,-5.94,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)45(65-47(70)39(61-44(69)29-57-5)13-9-23-59-54(55)56)50(73)62-40(25-34-17-21-38(68)22-18-34)48(71)66-46(32(3)4)51(74)63-41(27-37-28-58-30-60-37)52(75)67-24-10-14-43(67)49(72)64-42(53(76)77)26-33-15-19-36(20-16-33)35-11-7-6-8-12-35/h6-8,11-12,15-22,28,30-32,39-43,45-46,57,68H,9-10,13-14,23-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,72)(H,65,70)(H,66,71)(H,76,77)(H4,55,56,59)/t39-,40-,41-,42-,43+,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228198

(CHEMBL414796)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)NC1(Cc2ccccc2C1)C(O)=O |wU:56.59,36.36,17.16,wD:43.43,24.23,6.5,(2.9,-15.88,;2.9,-14.34,;4.23,-13.57,;5.56,-14.34,;5.56,-15.88,;6.91,-13.57,;8.24,-14.34,;8.24,-15.88,;9.57,-16.66,;9.57,-18.2,;10.92,-18.97,;10.92,-20.51,;12.25,-21.28,;9.57,-21.28,;9.57,-13.57,;9.57,-12.03,;10.92,-14.34,;12.25,-13.57,;12.25,-12.03,;13.58,-11.24,;10.92,-11.24,;13.58,-14.34,;13.58,-15.88,;14.93,-13.57,;16.26,-14.34,;16.26,-15.88,;17.59,-16.66,;17.59,-18.2,;18.94,-18.97,;20.27,-18.2,;21.6,-18.97,;20.27,-16.66,;18.94,-15.88,;17.59,-13.57,;17.59,-12.03,;18.94,-14.34,;20.27,-13.57,;20.27,-12.03,;21.6,-11.24,;18.94,-11.24,;21.6,-14.34,;21.6,-15.88,;22.95,-13.57,;24.28,-14.34,;24.28,-15.88,;24.84,-17.37,;25,-18.9,;26.51,-19.22,;27.3,-17.88,;26.25,-16.72,;25.61,-13.57,;25.61,-12.03,;26.96,-14.34,;27.12,-15.88,;28.63,-16.2,;29.4,-14.86,;28.37,-13.71,;28.69,-12.2,;30.16,-11.72,;27.54,-11.17,;27.88,-9.67,;28.79,-8.42,;30.25,-8.9,;31.58,-8.13,;32.89,-8.9,;32.89,-10.44,;31.57,-11.21,;30.23,-10.44,;28.78,-10.92,;26.72,-8.63,;27.04,-7.12,;25.26,-9.11,)| Show InChI InChI=1S/C49H69N13O10/c1-27(2)39(59-41(65)34(56-38(64)25-52-5)12-8-18-54-48(50)51)44(68)57-35(20-29-14-16-33(63)17-15-29)42(66)60-40(28(3)4)45(69)58-36(21-32-24-53-26-55-32)46(70)62-19-9-13-37(62)43(67)61-49(47(71)72)22-30-10-6-7-11-31(30)23-49/h6-7,10-11,14-17,24,26-28,34-37,39-40,52,63H,8-9,12-13,18-23,25H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,65)(H,60,66)(H,61,67)(H,71,72)(H4,50,51,54)/t34-,35-,36-,37+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228199

(ANGIOTENSIN AMIDE | Angiotensinamide)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:59.62,39.39,46.46,27.26,18.18,7.7,wD:3.3,63.65,(-.29,-6,;-1.35,-5.38,;-2.42,-5.99,;-1.34,-3.84,;-2.68,-3.06,;-4.01,-3.83,;-4.02,-5.06,;-5.35,-3.05,;-5.34,-1.51,;-6.67,-.74,;-6.67,.8,;-8,1.57,;-8,3.11,;-9.06,3.73,;-6.93,3.73,;-6.68,-3.82,;-8.01,-3.05,;-8.01,-1.81,;-9.35,-3.81,;-9.35,-5.04,;-10.68,-3.04,;-12.02,-3.8,;-12.02,-5.03,;-13.08,-3.18,;-.01,-3.07,;-0,-1.84,;1.33,-3.85,;2.66,-3.08,;2.67,-1.54,;4,-.77,;4,.77,;5.34,1.54,;6.67,.76,;7.74,1.37,;6.66,-.78,;5.33,-1.54,;4,-3.85,;5.06,-3.24,;3.99,-5.39,;5.33,-6.17,;6.66,-5.4,;7.73,-6.02,;6.67,-4.17,;5.33,-7.71,;4.26,-8.32,;6.66,-8.48,;6.66,-10.02,;7.99,-10.8,;9.33,-10.03,;10.71,-10.67,;11.74,-9.53,;10.97,-8.19,;9.47,-8.51,;5.32,-10.79,;4.25,-10.18,;5.32,-12.34,;6.57,-13.21,;6.1,-14.68,;4.56,-14.69,;4.08,-13.22,;2.62,-12.73,;1.69,-13.55,;2.31,-11.22,;.85,-10.74,;.53,-9.23,;-.92,-8.74,;-1.24,-7.23,;-2.7,-6.75,;-3.85,-7.77,;-3.54,-9.28,;-2.07,-9.76,;-.31,-11.76,;-.06,-12.96,;-1.48,-11.37,)| Show InChI InChI=1S/C49H70N14O11/c1-26(2)39(61-42(67)33(12-8-18-55-49(52)53)57-41(66)32(50)23-38(51)65)45(70)58-34(20-29-14-16-31(64)17-15-29)43(68)62-40(27(3)4)46(71)59-35(22-30-24-54-25-56-30)47(72)63-19-9-13-37(63)44(69)60-36(48(73)74)21-28-10-6-5-7-11-28/h5-7,10-11,14-17,24-27,32-37,39-40,64H,8-9,12-13,18-23,50H2,1-4H3,(H2,51,65)(H,54,56)(H,57,66)(H,58,70)(H,59,71)(H,60,69)(H,61,67)(H,62,68)(H,73,74)(H4,52,53,55)/t32-,33-,34-,35-,36-,37-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228200

(CHEMBL217516)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(O)=O |wU:60.62,56.59,36.36,17.16,wD:43.43,24.23,6.5,(2.9,-15.86,;2.9,-14.33,;4.23,-13.56,;5.56,-14.33,;5.56,-15.86,;6.9,-13.56,;8.24,-14.33,;8.24,-15.86,;9.56,-16.64,;9.56,-18.18,;10.91,-18.95,;10.91,-20.49,;12.24,-21.26,;9.56,-21.26,;9.56,-13.56,;9.56,-12.02,;10.91,-14.33,;12.24,-13.56,;12.24,-12.02,;13.57,-11.23,;10.91,-11.23,;13.57,-14.33,;13.57,-15.86,;14.91,-13.56,;16.25,-14.33,;16.25,-15.86,;17.57,-16.64,;17.57,-18.18,;18.92,-18.95,;20.25,-18.18,;21.58,-18.95,;20.25,-16.64,;18.92,-15.86,;17.57,-13.56,;17.57,-12.02,;18.92,-14.33,;20.25,-13.56,;20.25,-12.02,;21.58,-11.23,;18.92,-11.23,;21.58,-14.33,;21.58,-15.86,;22.93,-13.56,;24.26,-14.33,;24.26,-15.86,;24.82,-17.35,;24.98,-18.88,;26.48,-19.2,;27.27,-17.86,;26.23,-16.71,;25.59,-13.56,;25.59,-12.02,;26.93,-14.33,;27.09,-15.86,;28.6,-16.18,;29.37,-14.85,;28.34,-13.7,;28.66,-12.19,;30.13,-11.71,;27.51,-11.16,;27.85,-9.66,;29.3,-9.18,;29.62,-7.67,;31.09,-7.19,;31.41,-5.68,;30.26,-4.65,;28.79,-5.13,;28.47,-6.63,;30.84,-9.18,;31.6,-10.49,;33.14,-10.49,;33.89,-9.17,;33.12,-7.83,;31.58,-7.85,;26.7,-8.62,;27.02,-7.11,;25.23,-9.1,)| Show InChI InChI=1S/C54H73N13O10/c1-31(2)44(64-47(70)38(61-42(69)29-57-5)18-12-24-59-54(55)56)50(73)62-39(26-33-20-22-37(68)23-21-33)48(71)65-45(32(3)4)51(74)63-40(27-36-28-58-30-60-36)52(75)67-25-13-19-41(67)49(72)66-46(53(76)77)43(34-14-8-6-9-15-34)35-16-10-7-11-17-35/h6-11,14-17,20-23,28,30-32,38-41,43-46,57,68H,12-13,18-19,24-27,29H2,1-5H3,(H,58,60)(H,61,69)(H,62,73)(H,63,74)(H,64,70)(H,65,71)(H,66,72)(H,76,77)(H4,55,56,59)/t38-,39-,40-,41+,44-,45-,46+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303975
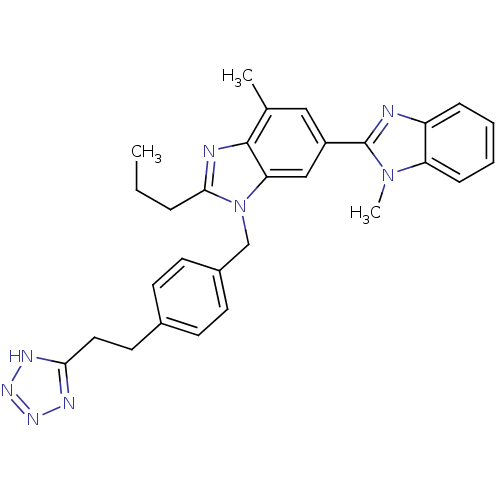
(3'-(4-(2-(1H-Tetrazol-5-yl)ethyl)benzyl)-1,7'-dime...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(CCc2nnn[nH]2)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C29H30N8/c1-4-7-27-31-28-19(2)16-22(29-30-23-8-5-6-9-24(23)36(29)3)17-25(28)37(27)18-21-12-10-20(11-13-21)14-15-26-32-34-35-33-26/h5-6,8-13,16-17H,4,7,14-15,18H2,1-3H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50266704
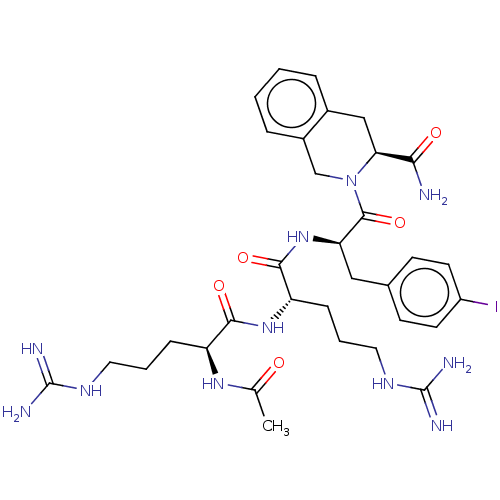
(CHEMBL4083717)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(I)cc1)C(=O)N1Cc2ccccc2C[C@H]1C(N)=O |r| Show InChI InChI=1S/C33H46IN11O5/c1-19(46)42-24(8-4-14-40-32(36)37)29(48)43-25(9-5-15-41-33(38)39)30(49)44-26(16-20-10-12-23(34)13-11-20)31(50)45-18-22-7-3-2-6-21(22)17-27(45)28(35)47/h2-3,6-7,10-13,24-27H,4-5,8-9,14-18H2,1H3,(H2,35,47)(H,42,46)(H,43,48)(H,44,49)(H4,36,37,40)(H4,38,39,41)/t24-,25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228202
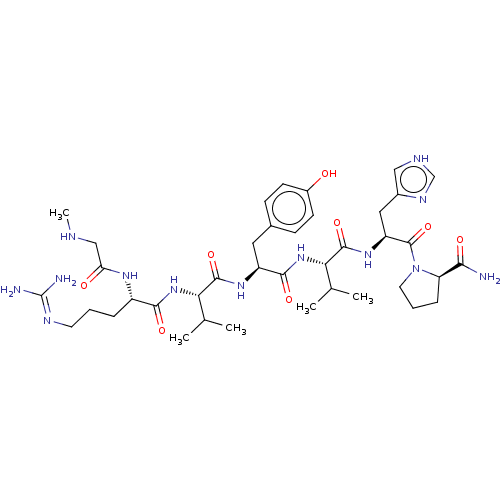
(CHEMBL368638)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(N)=O |wU:36.36,17.16,56.59,wD:43.43,24.23,6.5,(1.06,-8.48,;1.83,-7.17,;3.16,-6.41,;4.5,-7.17,;4.5,-8.7,;5.8,-6.41,;7.14,-7.17,;7.14,-8.7,;8.45,-9.47,;8.45,-10.99,;9.78,-11.78,;9.78,-13.31,;11.12,-14.07,;8.45,-14.07,;8.45,-6.41,;8.45,-4.88,;9.78,-7.17,;11.12,-6.41,;11.12,-4.88,;12.46,-4.11,;9.78,-4.11,;12.46,-7.17,;12.46,-8.7,;13.76,-6.41,;15.1,-7.17,;15.1,-8.7,;16.44,-9.47,;17.74,-8.7,;19.08,-9.47,;19.08,-10.99,;20.42,-11.78,;17.74,-11.78,;16.44,-10.99,;16.44,-6.41,;16.44,-4.88,;17.74,-7.17,;19.08,-6.41,;19.08,-4.88,;20.42,-4.11,;17.74,-4.11,;20.42,-7.17,;20.42,-8.7,;21.72,-6.41,;23.06,-7.17,;23.06,-8.7,;23.63,-10.17,;23.19,-11.76,;24.62,-12.62,;25.89,-11.52,;25.18,-10.17,;24.4,-6.41,;24.4,-4.88,;25.7,-7.17,;25.86,-8.7,;27.36,-9.02,;28.15,-7.68,;27.1,-6.54,;27.42,-5.04,;26.31,-4.02,;28.89,-4.58,)| Show InChI InChI=1S/C39H61N13O8/c1-21(2)31(50-34(56)26(47-30(54)19-43-5)8-6-14-45-39(41)42)36(58)48-27(16-23-10-12-25(53)13-11-23)35(57)51-32(22(3)4)37(59)49-28(17-24-18-44-20-46-24)38(60)52-15-7-9-29(52)33(40)55/h10-13,18,20-22,26-29,31-32,43,53H,6-9,14-17,19H2,1-5H3,(H2,40,55)(H,44,46)(H,47,54)(H,48,58)(H,49,59)(H,50,56)(H,51,57)(H4,41,42,45)/t26-,27-,28-,29+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat uterus |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50266713
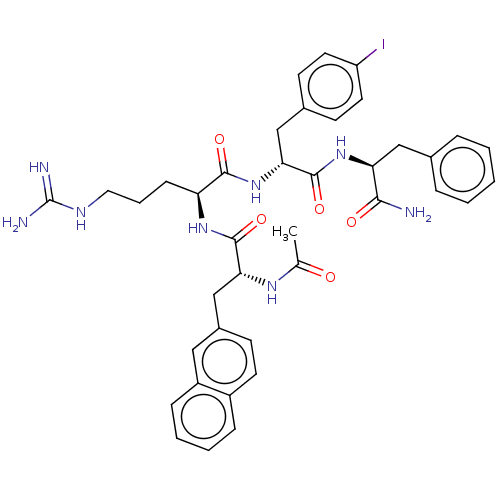
(CHEMBL4089759)Show SMILES CC(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(I)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C39H45IN8O5/c1-24(49)45-33(23-27-13-16-28-10-5-6-11-29(28)20-27)37(52)46-31(12-7-19-44-39(42)43)36(51)48-34(22-26-14-17-30(40)18-15-26)38(53)47-32(35(41)50)21-25-8-3-2-4-9-25/h2-6,8-11,13-18,20,31-34H,7,12,19,21-23H2,1H3,(H2,41,50)(H,45,49)(H,46,52)(H,47,53)(H,48,51)(H4,42,43,44)/t31-,32-,33+,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303976

(3'-(2-(4-(2-(1H-Tetrazol-5-yl)ethyl)phenoxy)ethyl)...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CCc2nnn[nH]2)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C30H32N8O/c1-4-7-28-32-29-20(2)18-22(30-31-24-8-5-6-9-25(24)37(30)3)19-26(29)38(28)16-17-39-23-13-10-21(11-14-23)12-15-27-33-35-36-34-27/h5-6,8-11,13-14,18-19H,4,7,12,15-17H2,1-3H3,(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303977

(3-(4-(2-(1,7'-Dimethyl-2'-propyl-1H,3'H-2,5'-biben...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C37H38N4O4/c1-5-11-33-39-34-25(2)22-27(35-38-30-14-9-10-15-31(30)40(35)4)23-32(34)41(33)20-21-44-28-18-16-26(17-19-28)24-37(3,36(42)43)45-29-12-7-6-8-13-29/h6-10,12-19,22-23H,5,11,20-21,24H2,1-4H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303979

(CHEMBL571763 | Ethyl 3-(4-(2-(1,7'-Dimethyl-2'-pro...)Show SMILES CCCc1nc2c(C)cc(cc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(=O)OCC)cc1)-c1nc2ccccc2n1C Show InChI InChI=1S/C39H42N4O4/c1-6-13-35-41-36-27(3)24-29(37-40-32-16-11-12-17-33(32)42(37)5)25-34(36)43(35)22-23-46-30-20-18-28(19-21-30)26-39(4,38(44)45-7-2)47-31-14-9-8-10-15-31/h8-12,14-21,24-25H,6-7,13,22-23,26H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303978
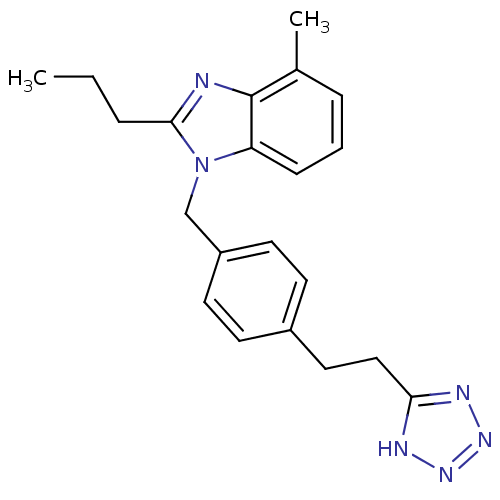
(1-(4-(2-(1H-tetrazol-5-yl)ethyl)benzyl)-4-methyl-2...)Show InChI InChI=1S/C21H24N6/c1-3-5-20-22-21-15(2)6-4-7-18(21)27(20)14-17-10-8-16(9-11-17)12-13-19-23-25-26-24-19/h4,6-11H,3,5,12-14H2,1-2H3,(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303980
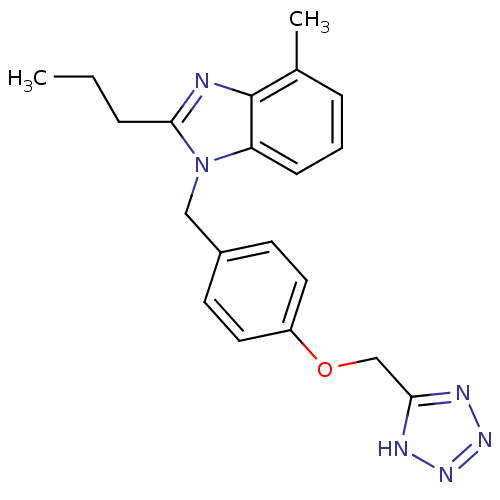
(1-(4-((1H-Tetrazol-5-yl)methoxy)benzyl)-4-methyl-2...)Show InChI InChI=1S/C20H22N6O/c1-3-5-19-21-20-14(2)6-4-7-17(20)26(19)12-15-8-10-16(11-9-15)27-13-18-22-24-25-23-18/h4,6-11H,3,5,12-13H2,1-2H3,(H,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228202

(CHEMBL368638)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@@H]1C(N)=O |wU:36.36,17.16,56.59,wD:43.43,24.23,6.5,(1.06,-8.48,;1.83,-7.17,;3.16,-6.41,;4.5,-7.17,;4.5,-8.7,;5.8,-6.41,;7.14,-7.17,;7.14,-8.7,;8.45,-9.47,;8.45,-10.99,;9.78,-11.78,;9.78,-13.31,;11.12,-14.07,;8.45,-14.07,;8.45,-6.41,;8.45,-4.88,;9.78,-7.17,;11.12,-6.41,;11.12,-4.88,;12.46,-4.11,;9.78,-4.11,;12.46,-7.17,;12.46,-8.7,;13.76,-6.41,;15.1,-7.17,;15.1,-8.7,;16.44,-9.47,;17.74,-8.7,;19.08,-9.47,;19.08,-10.99,;20.42,-11.78,;17.74,-11.78,;16.44,-10.99,;16.44,-6.41,;16.44,-4.88,;17.74,-7.17,;19.08,-6.41,;19.08,-4.88,;20.42,-4.11,;17.74,-4.11,;20.42,-7.17,;20.42,-8.7,;21.72,-6.41,;23.06,-7.17,;23.06,-8.7,;23.63,-10.17,;23.19,-11.76,;24.62,-12.62,;25.89,-11.52,;25.18,-10.17,;24.4,-6.41,;24.4,-4.88,;25.7,-7.17,;25.86,-8.7,;27.36,-9.02,;28.15,-7.68,;27.1,-6.54,;27.42,-5.04,;26.31,-4.02,;28.89,-4.58,)| Show InChI InChI=1S/C39H61N13O8/c1-21(2)31(50-34(56)26(47-30(54)19-43-5)8-6-14-45-39(41)42)36(58)48-27(16-23-10-12-25(53)13-11-23)35(57)51-32(22(3)4)37(59)49-28(17-24-18-44-20-46-24)38(60)52-15-7-9-29(52)33(40)55/h10-13,18,20-22,26-29,31-32,43,53H,6-9,14-17,19H2,1-5H3,(H2,40,55)(H,44,46)(H,47,54)(H,48,58)(H,49,59)(H,50,56)(H,51,57)(H4,41,42,45)/t26-,27-,28-,29+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand [Sar1,Ile5,8]AII binding to angiotensin II receptor in rat brain |
J Med Chem 32: 898-903 (1989)
BindingDB Entry DOI: 10.7270/Q2FJ2K0Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303981

(CHEMBL567063 | Ethyl 3-(4-((4-Methyl-2-propyl-1H-b...)Show SMILES CCCc1nc2c(C)cccc2n1Cc1ccc(\C=C\C(=O)OCC)cc1 Show InChI InChI=1S/C23H26N2O2/c1-4-7-21-24-23-17(3)8-6-9-20(23)25(21)16-19-12-10-18(11-13-19)14-15-22(26)27-5-2/h6,8-15H,4-5,7,16H2,1-3H3/b15-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303986

(CHEMBL584704 | Ethyl 2-Methyl-3-(4-(2-(4-methyl-2-...)Show SMILES CCCc1nc2c(C)cccc2n1CCOc1ccc(CC(C)(Oc2ccccc2)C(=O)OCC)cc1 Show InChI InChI=1S/C31H36N2O4/c1-5-11-28-32-29-23(3)12-10-15-27(29)33(28)20-21-36-25-18-16-24(17-19-25)22-31(4,30(34)35-6-2)37-26-13-8-7-9-14-26/h7-10,12-19H,5-6,11,20-22H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303982

(CHEMBL567689 | Methyl 3-(4-((4-Methyl-2-propyl-1H-...)Show InChI InChI=1S/C23H28N2O2/c1-4-7-21-24-23-17(3)8-6-9-20(23)25(21)16-19-12-10-18(11-13-19)14-15-22(26)27-5-2/h6,8-13H,4-5,7,14-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303983
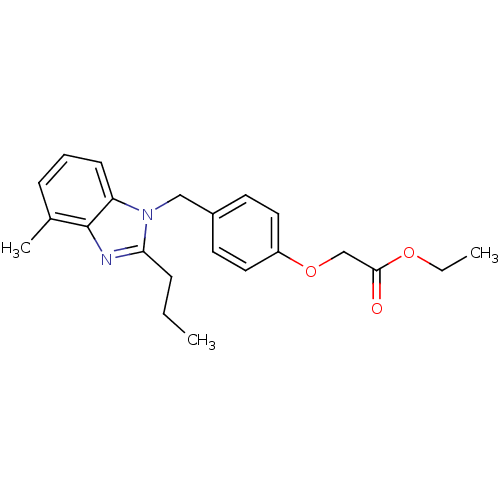
(CHEMBL565793 | ethyl 2-(4-((4-methyl-2-propyl-1H-b...)Show InChI InChI=1S/C22H26N2O3/c1-4-7-20-23-22-16(3)8-6-9-19(22)24(20)14-17-10-12-18(13-11-17)27-15-21(25)26-5-2/h6,8-13H,4-5,7,14-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303984
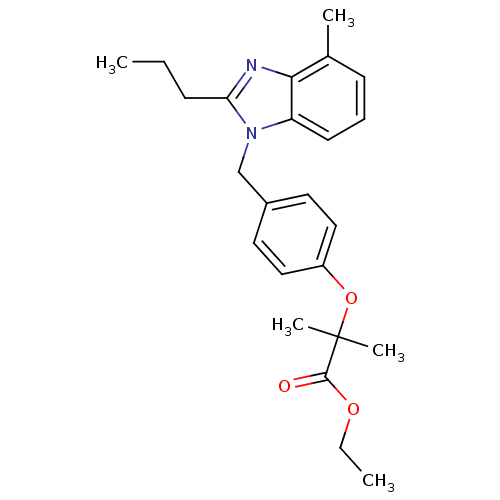
(CHEMBL565379 | Ethyl 2-Methyl-2-(4-((4-methyl-2-pr...)Show SMILES CCCc1nc2c(C)cccc2n1Cc1ccc(OC(C)(C)C(=O)OCC)cc1 Show InChI InChI=1S/C24H30N2O3/c1-6-9-21-25-22-17(3)10-8-11-20(22)26(21)16-18-12-14-19(15-13-18)29-24(4,5)23(27)28-7-2/h8,10-15H,6-7,9,16H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50303985

(CHEMBL567269 | Ethyl 2,2-Dimethyl-3-(4-(2-(4-methy...)Show SMILES CCCc1nc2c(C)cccc2n1CCOc1ccc(CC(C)(C)C(=O)OCC)cc1 Show InChI InChI=1S/C26H34N2O3/c1-6-9-23-27-24-19(3)10-8-11-22(24)28(23)16-17-31-21-14-12-20(13-15-21)18-26(4,5)25(29)30-7-2/h8,10-15H,6-7,9,16-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane |
J Med Chem 53: 1076-85 (2010)
Article DOI: 10.1021/jm901272d
BindingDB Entry DOI: 10.7270/Q22V2H2Q |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse melanocortin receptor 1 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC1R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC4R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse melanocortin receptor 4 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50258965

(CHEMBL4102048)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C49H64N12O8/c1-30-41(62)58-37(28-33-18-9-4-10-19-33)47(68)61-25-13-22-40(61)48(69)60-24-12-21-39(60)46(67)55-34(20-11-23-53-49(51)52)42(63)56-35(26-31-14-5-2-6-15-31)43(64)57-36(27-32-16-7-3-8-17-32)44(65)59-38(29-50)45(66)54-30/h2-10,14-19,30,34-40H,11-13,20-29,50H2,1H3,(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,62)(H,59,65)(H4,51,52,53)/t30-,34-,35-,36-,37-,38-,39-,40+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse melanocortin receptor 4 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC3R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration of compound for 50% displacement of [3H]QNB from Muscarinic acetylcholine receptor in rat brain |
J Med Chem 23: 865-73 (1980)
BindingDB Entry DOI: 10.7270/Q2542QTR |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM31046
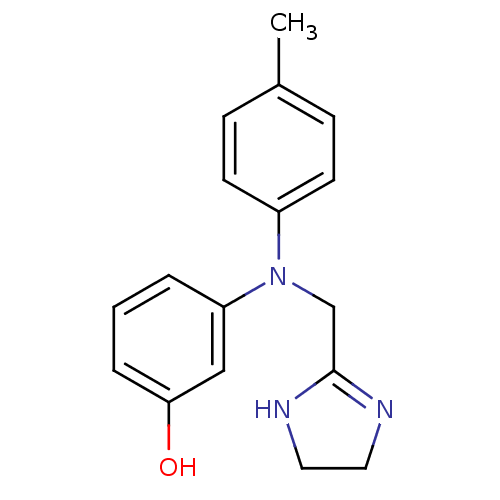
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration of compound for 50% displacement of [3H]WB-4101 from Alpha-1 adrenergic receptor of rat brain |
J Med Chem 23: 865-73 (1980)
BindingDB Entry DOI: 10.7270/Q2542QTR |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50258965

(CHEMBL4102048)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C49H64N12O8/c1-30-41(62)58-37(28-33-18-9-4-10-19-33)47(68)61-25-13-22-40(61)48(69)60-24-12-21-39(60)46(67)55-34(20-11-23-53-49(51)52)42(63)56-35(26-31-14-5-2-6-15-31)43(64)57-36(27-32-16-7-3-8-17-32)44(65)59-38(29-50)45(66)54-30/h2-10,14-19,30,34-40H,11-13,20-29,50H2,1H3,(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,62)(H,59,65)(H4,51,52,53)/t30-,34-,35-,36-,37-,38-,39-,40+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from human melanocortin receptor 4 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50514457
(CHEMBL4565367)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(C)C |r| Show InChI InChI=1S/C51H68N12O8/c1-31(2)42-48(69)59-38(29-34-19-10-5-11-20-34)49(70)63-26-14-23-41(63)50(71)62-25-13-22-40(62)47(68)56-35(21-12-24-55-51(53)54)43(64)57-36(27-32-15-6-3-7-16-32)44(65)58-37(28-33-17-8-4-9-18-33)45(66)60-39(30-52)46(67)61-42/h3-11,15-20,31,35-42H,12-14,21-30,52H2,1-2H3,(H,56,68)(H,57,64)(H,58,65)(H,59,69)(H,60,66)(H,61,67)(H4,53,54,55)/t35-,36-,37-,38-,39-,40-,41+,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse melanocortin receptor 4 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse melanocortin receptor 3 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50164804

(CHEMBL3798421)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C80H107N23O15/c1-50(104)96-67(40-55-44-86-48-94-55)77(113)101-64(36-51-16-4-2-5-17-51)75(111)99-62(25-13-27-91-80(84)85)74(110)103-66(39-54-43-93-60-23-11-9-21-58(54)60)72(108)89-29-15-31-116-33-35-117-34-32-115-30-14-28-88-69(105)46-118-47-70(106)97-68(41-56-45-87-49-95-56)78(114)102-65(37-52-18-6-3-7-19-52)76(112)98-61(24-12-26-90-79(82)83)73(109)100-63(71(81)107)38-53-42-92-59-22-10-8-20-57(53)59/h2-11,16-23,42-45,48-49,61-68,92-93H,12-15,24-41,46-47H2,1H3,(H2,81,107)(H,86,94)(H,87,95)(H,88,105)(H,89,108)(H,96,104)(H,97,106)(H,98,112)(H,99,111)(H,100,109)(H,101,113)(H,102,114)(H,103,110)(H4,82,83,90)(H4,84,85,91)/t61-,62-,63-,64+,65+,66-,67-,68-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC4R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50164800
(CHEMBL3798768)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C88H111N23O15/c1-54(112)104-75(44-63-48-94-52-102-63)85(121)109-72(40-55-24-26-57-14-2-4-16-59(57)38-55)83(119)107-70(23-11-29-99-88(92)93)82(118)111-74(43-62-47-101-68-21-9-7-19-66(62)68)80(116)97-31-13-33-124-35-37-125-36-34-123-32-12-30-96-77(113)50-126-51-78(114)105-76(45-64-49-95-53-103-64)86(122)110-73(41-56-25-27-58-15-3-5-17-60(58)39-56)84(120)106-69(22-10-28-98-87(90)91)81(117)108-71(79(89)115)42-61-46-100-67-20-8-6-18-65(61)67/h2-9,14-21,24-27,38-39,46-49,52-53,69-76,100-101H,10-13,22-23,28-37,40-45,50-51H2,1H3,(H2,89,115)(H,94,102)(H,95,103)(H,96,113)(H,97,116)(H,104,112)(H,105,114)(H,106,120)(H,107,119)(H,108,117)(H,109,121)(H,110,122)(H,111,118)(H4,90,91,98)(H4,92,93,99)/t69-,70-,71-,72+,73+,74-,75-,76-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC4R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from human melanocortin receptor 4 expressed in HEK293 cell mebranes incubated for 1 hr by automatic gamma counting me... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50164802

(CHEMBL3799408)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O |r| Show InChI InChI=1S/C52H71N13O11/c1-34(66)62-45(28-39-30-56-33-61-39)51(72)64-43(26-35-14-15-36-9-2-3-10-37(36)25-35)50(71)63-42(13-6-16-59-52(54)55)49(70)65-44(27-38-29-60-41-12-5-4-11-40(38)41)48(69)58-18-8-20-74-22-24-75-23-21-73-19-7-17-57-47(68)32-76-31-46(53)67/h2-5,9-12,14-15,25,29-30,33,42-45,60H,6-8,13,16-24,26-28,31-32H2,1H3,(H2,53,67)(H,56,61)(H,57,68)(H,58,69)(H,62,66)(H,63,71)(H,64,72)(H,65,70)(H4,54,55,59)/t42-,43+,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of 125I-NDP-MSH from mouse MC4R expressed in HEK293 cells incubated for 1 hr by gamma counting analysis |
J Med Chem 59: 3112-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01894
BindingDB Entry DOI: 10.7270/Q2R2139S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50266704

(CHEMBL4083717)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(I)cc1)C(=O)N1Cc2ccccc2C[C@H]1C(N)=O |r| Show InChI InChI=1S/C33H46IN11O5/c1-19(46)42-24(8-4-14-40-32(36)37)29(48)43-25(9-5-15-41-33(38)39)30(49)44-26(16-20-10-12-23(34)13-11-20)31(50)45-18-22-7-3-2-6-21(22)17-27(45)28(35)47/h2-3,6-7,10-13,24-27H,4-5,8-9,14-18H2,1H3,(H2,35,47)(H,42,46)(H,43,48)(H,44,49)(H4,36,37,40)(H4,38,39,41)/t24-,25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting |
J Med Chem 60: 4342-4357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00301
BindingDB Entry DOI: 10.7270/Q24170J1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data