 Found 368 hits with Last Name = 'hussain' and Initial = 's'
Found 368 hits with Last Name = 'hussain' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433602
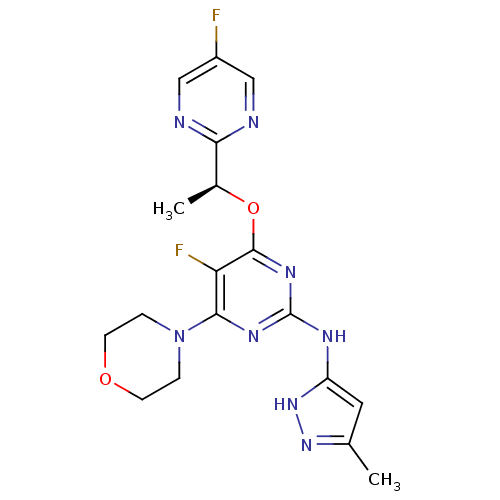
(CHEMBL2381980)Show SMILES C[C@H](Oc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H20F2N8O2/c1-10-7-13(27-26-10)23-18-24-16(28-3-5-29-6-4-28)14(20)17(25-18)30-11(2)15-21-8-12(19)9-22-15/h7-9,11H,3-6H2,1-2H3,(H2,23,24,25,26,27)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433601
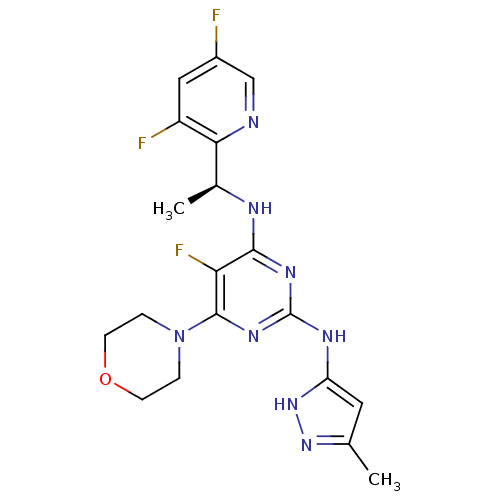
(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052
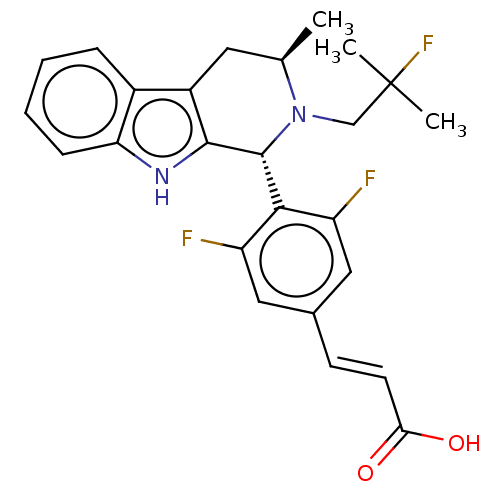
(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
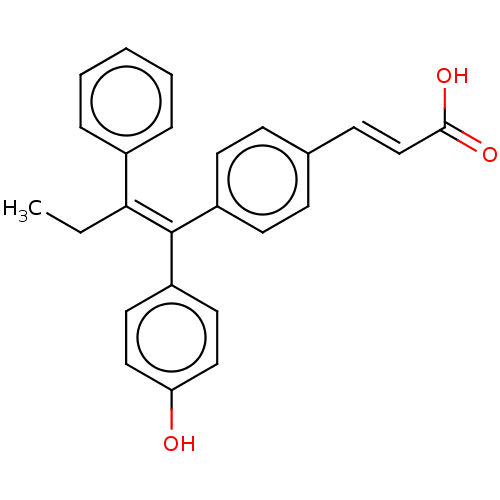
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
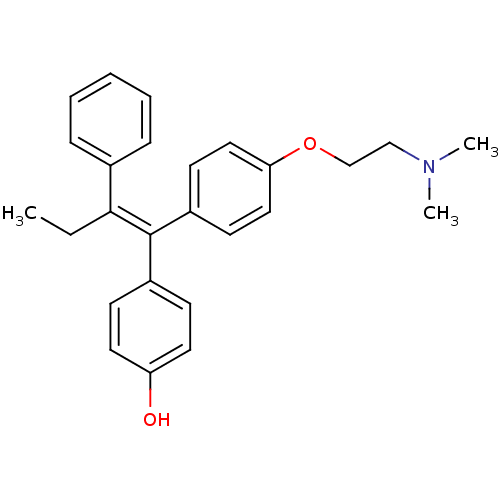
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8349
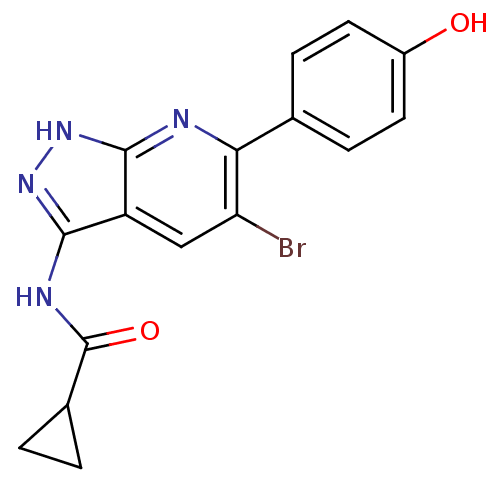
(6-aryl-pyrazolo[3,4-b]pyridine analogue 13 | CHEMB...)Show SMILES Oc1ccc(cc1)-c1nc2[nH]nc(NC(=O)C3CC3)c2cc1Br Show InChI InChI=1S/C16H13BrN4O2/c17-12-7-11-14(18-13(12)8-3-5-10(22)6-4-8)20-21-15(11)19-16(23)9-1-2-9/h3-7,9,22H,1-2H2,(H2,18,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8350

(6-aryl-pyrazolo[3,4-b]pyridine analogue 14 | N-[5-...)Show SMILES Oc1ccc(cc1)-c1nc2[nH]nc(NC(=O)C3CC3)c2cc1Cl Show InChI InChI=1S/C16H13ClN4O2/c17-12-7-11-14(18-13(12)8-3-5-10(22)6-4-8)20-21-15(11)19-16(23)9-1-2-9/h3-7,9,22H,1-2H2,(H2,18,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291344

(CHEMBL4165510)Show SMILES Fc1cc2CN(C=O)C(Cn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)N1CCCCC1 |t:13| Show InChI InChI=1S/C28H25FN6O3/c29-18-10-17-13-34(16-36)23(32-7-3-1-4-8-32)15-33-14-20(19(11-18)26(17)33)24-25(28(38)31-27(24)37)21-12-30-22-6-2-5-9-35(21)22/h2,5-6,9-12,14,16,23H,1,3-4,7-8,13,15H2,(H,31,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291346
(CHEMBL4162850)Show SMILES O=CN1Cc2cccc3c(cn(CC1N1CCOCC1)c23)C1=C(C(=O)NC1=O)c1cnc2ccccn12 |t:25| Show InChI InChI=1S/C27H24N6O4/c34-16-32-13-17-4-3-5-18-19(14-31(25(17)18)15-22(32)30-8-10-37-11-9-30)23-24(27(36)29-26(23)35)20-12-28-21-6-1-2-7-33(20)21/h1-7,12,14,16,22H,8-11,13,15H2,(H,29,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291367
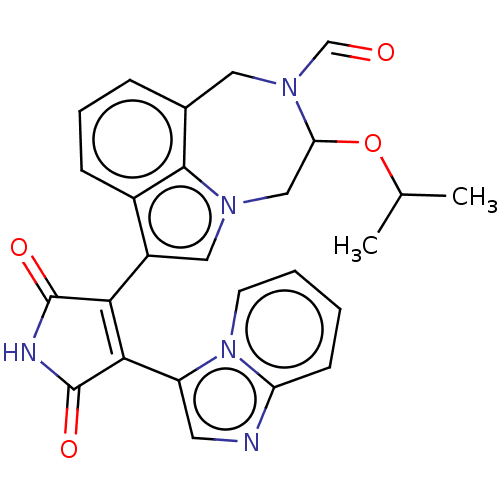
(CHEMBL4164416)Show SMILES CC(C)OC1Cn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(CN1C=O)c23 |t:9| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-21-13-29-12-18(17-7-5-6-16(24(17)29)11-30(21)14-32)22-23(26(34)28-25(22)33)19-10-27-20-8-3-4-9-31(19)20/h3-10,12,14-15,21H,11,13H2,1-2H3,(H,28,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291345
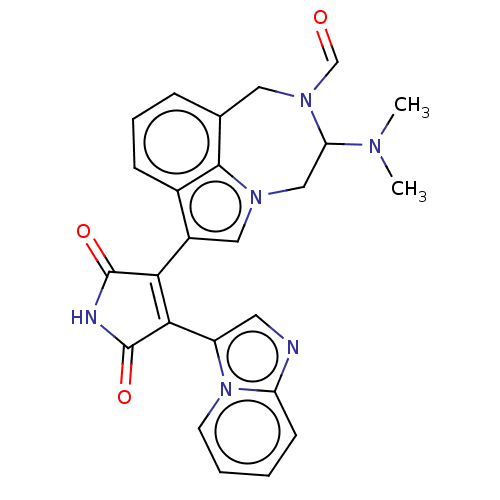
(CHEMBL4169204)Show SMILES CN(C)C1Cn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(CN1C=O)c23 |t:8| Show InChI InChI=1S/C25H22N6O3/c1-28(2)20-13-29-12-17(16-7-5-6-15(23(16)29)11-30(20)14-32)21-22(25(34)27-24(21)33)18-10-26-19-8-3-4-9-31(18)19/h3-10,12,14,20H,11,13H2,1-2H3,(H,27,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291366
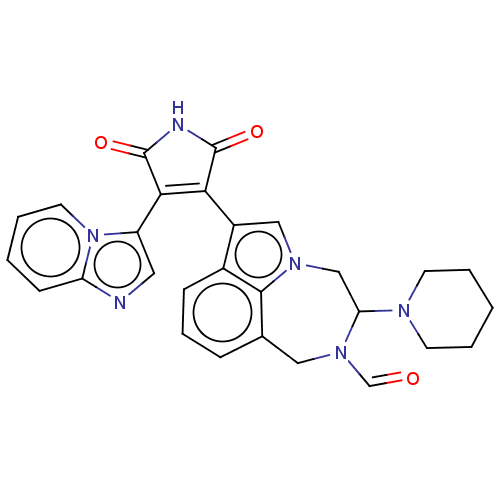
(CHEMBL4172295)Show SMILES O=CN1Cc2cccc3c(cn(CC1N1CCCCC1)c23)C1=C(C(=O)NC1=O)c1cnc2ccccn12 |t:25| Show InChI InChI=1S/C28H26N6O3/c35-17-33-14-18-7-6-8-19-20(15-32(26(18)19)16-23(33)31-10-3-1-4-11-31)24-25(28(37)30-27(24)36)21-13-29-22-9-2-5-12-34(21)22/h2,5-9,12-13,15,17,23H,1,3-4,10-11,14,16H2,(H,30,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433605
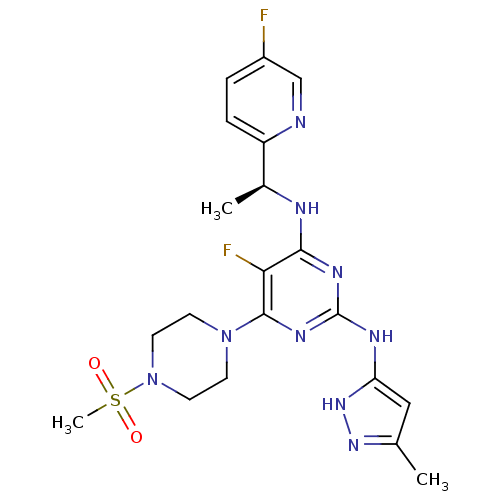
(CHEMBL2381975)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCN(CC2)S(C)(=O)=O)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N9O2S/c1-12-10-16(29-28-12)25-20-26-18(24-13(2)15-5-4-14(21)11-23-15)17(22)19(27-20)30-6-8-31(9-7-30)34(3,32)33/h4-5,10-11,13H,6-9H2,1-3H3,(H3,24,25,26,27,28,29)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
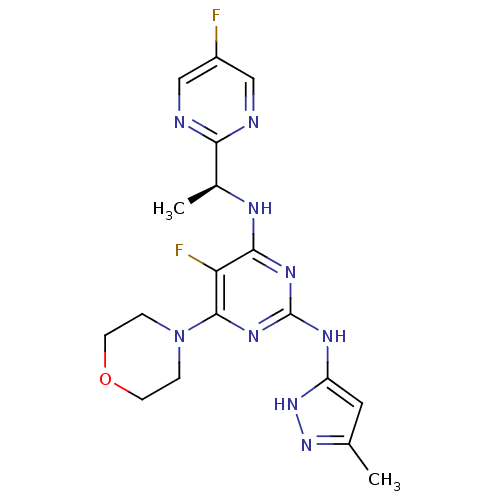
(Homo sapiens (Human)) | BDBM50433604
(CHEMBL2381986)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCS(=O)(=O)CC2)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H22F2N8O2S/c1-11-9-15(28-27-11)24-19-25-17(23-12(2)14-4-3-13(20)10-22-14)16(21)18(26-19)29-5-7-32(30,31)8-6-29/h3-4,9-10,12H,5-8H2,1-2H3,(H3,23,24,25,26,27,28)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433601

(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
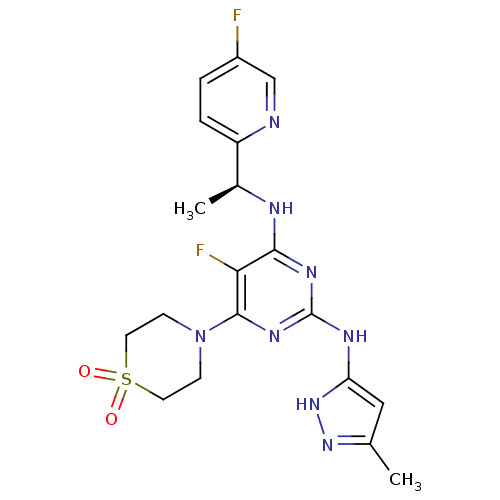
(Homo sapiens (Human)) | BDBM50433611
(CHEMBL2381979)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H21F2N9O/c1-10-7-13(28-27-10)24-18-25-16(23-11(2)15-21-8-12(19)9-22-15)14(20)17(26-18)29-3-5-30-6-4-29/h7-9,11H,3-6H2,1-2H3,(H3,23,24,25,26,27,28)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50041611
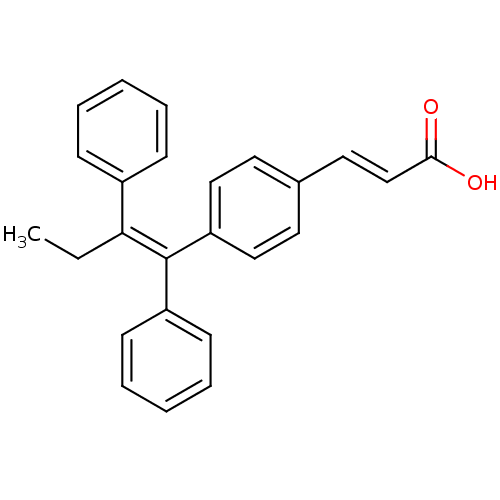
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433608

(CHEMBL2381984)Show SMILES C[C@H](Oc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H20F3N7O2/c1-10-7-14(28-27-10)24-19-25-17(29-3-5-30-6-4-29)15(22)18(26-19)31-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H2,24,25,26,27,28)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433615

(CHEMBL2381976)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCNCC2)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H23F2N9/c1-11-9-15(29-28-11)25-19-26-17(24-12(2)14-4-3-13(20)10-23-14)16(21)18(27-19)30-7-5-22-6-8-30/h3-4,9-10,12,22H,5-8H2,1-2H3,(H3,24,25,26,27,28,29)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50291370

(CHEMBL4172418)Show InChI InChI=1S/C16H17N5O/c1-2-3-9-14(22)17-15-12-10-13(11-7-5-4-6-8-11)18-20-16(12)21-19-15/h4-8,10H,2-3,9H2,1H3,(H2,17,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433609
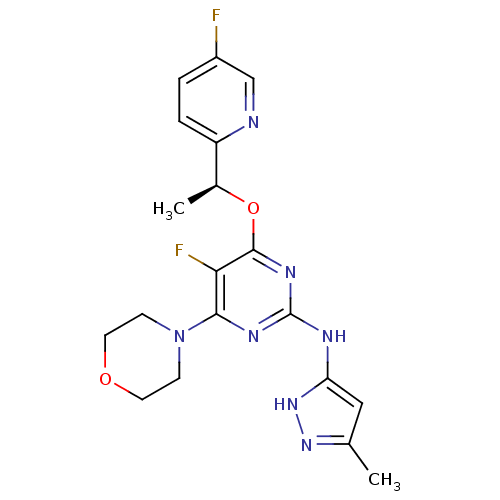
(CHEMBL2381982)Show SMILES C[C@H](Oc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H21F2N7O2/c1-11-9-15(27-26-11)23-19-24-17(28-5-7-29-8-6-28)16(21)18(25-19)30-12(2)14-4-3-13(20)10-22-14/h3-4,9-10,12H,5-8H2,1-2H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433604

(CHEMBL2381986)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCS(=O)(=O)CC2)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H22F2N8O2S/c1-11-9-15(28-27-11)24-19-25-17(23-12(2)14-4-3-13(20)10-22-14)16(21)18(26-19)29-5-7-32(30,31)8-6-29/h3-4,9-10,12H,5-8H2,1-2H3,(H3,23,24,25,26,27,28)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using biotinylated poly-EY peptide as substrate after 60 mins by HTRF assay |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433607
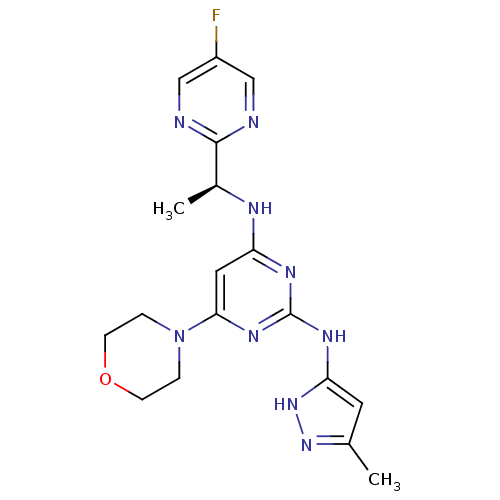
(CHEMBL2381985)Show SMILES C[C@H](Nc1cc(nc(Nc2cc(C)n[nH]2)n1)N1CCOCC1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H22FN9O/c1-11-7-15(27-26-11)24-18-23-14(8-16(25-18)28-3-5-29-6-4-28)22-12(2)17-20-9-13(19)10-21-17/h7-10,12H,3-6H2,1-2H3,(H3,22,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579
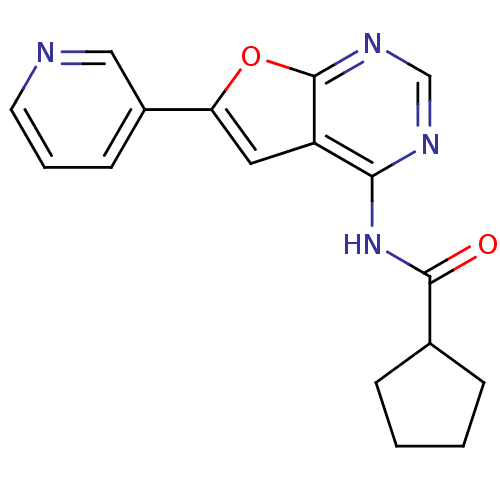
(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta by scintillation proximity assay |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8310
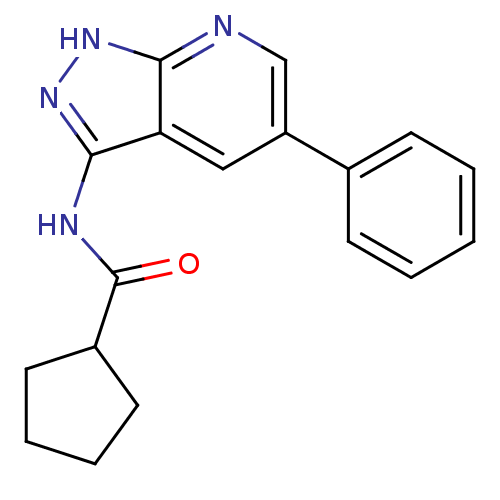
(N-{5-phenyl-1H-pyrazolo[3,4-b]pyridin-3-yl}cyclope...)Show InChI InChI=1S/C18H18N4O/c23-18(13-8-4-5-9-13)20-17-15-10-14(11-19-16(15)21-22-17)12-6-2-1-3-7-12/h1-3,6-7,10-11,13H,4-5,8-9H2,(H2,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50291365
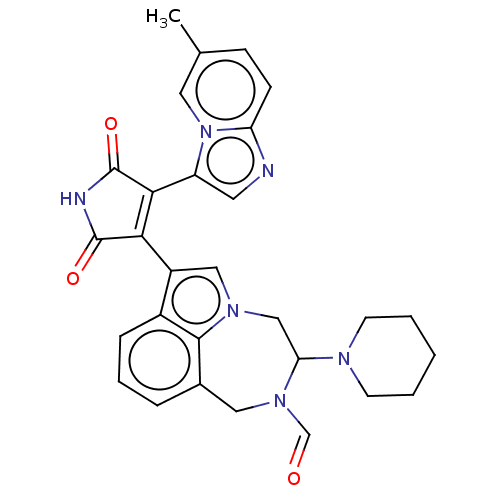
(CHEMBL4161007)Show SMILES Cc1ccc2ncc(C3=C(C(=O)NC3=O)c3cn4CC(N5CCCCC5)N(Cc5cccc3c45)C=O)n2c1 |t:8| Show InChI InChI=1S/C29H28N6O3/c1-18-8-9-23-30-12-22(35(23)13-18)26-25(28(37)31-29(26)38)21-15-33-16-24(32-10-3-2-4-11-32)34(17-36)14-19-6-5-7-20(21)27(19)33/h5-9,12-13,15,17,24H,2-4,10-11,14,16H2,1H3,(H,31,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta (unknown origin) assessed as decrease in phosphorylation of CREB at serine-129 in presence of ATP[gamma-33P] by f... |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433606

(CHEMBL2380329)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1Cl)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H22ClFN8O/c1-11-9-15(28-27-11)24-19-25-17(23-12(2)14-4-3-13(21)10-22-14)16(20)18(26-19)29-5-7-30-8-6-29/h3-4,9-10,12H,5-8H2,1-2H3,(H3,23,24,25,26,27,28)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433610
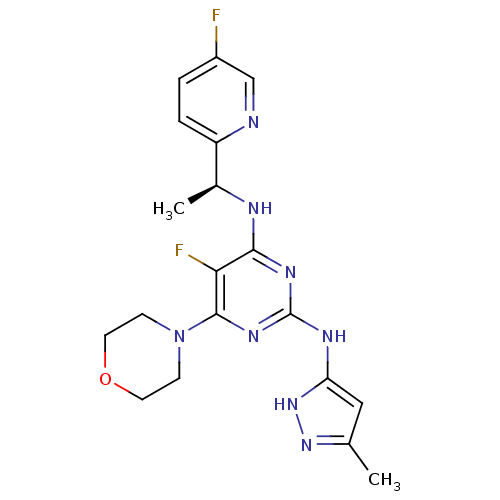
(CHEMBL2381981)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H22F2N8O/c1-11-9-15(28-27-11)24-19-25-17(23-12(2)14-4-3-13(20)10-22-14)16(21)18(26-19)29-5-7-30-8-6-29/h3-4,9-10,12H,5-8H2,1-2H3,(H3,23,24,25,26,27,28)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using biotinylated TYK2 peptide as substrate after 60 mins |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50219330
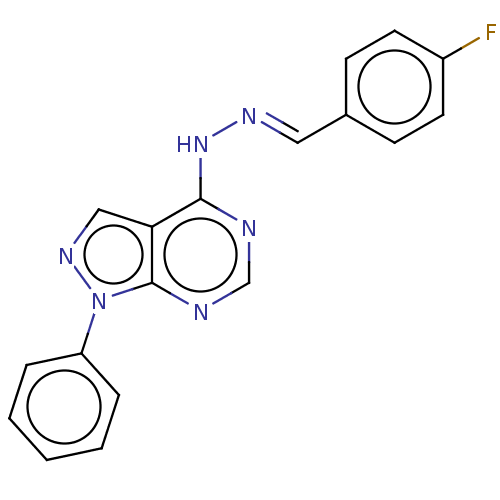
(CHEMBL303926)Show InChI InChI=1S/C18H13FN6/c19-14-8-6-13(7-9-14)10-22-24-17-16-11-23-25(18(16)21-12-20-17)15-4-2-1-3-5-15/h1-12H,(H,20,21,24)/b22-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-Ahx-AAAKRREILSRRP-S(PO3)YR-amide as substrate after 40 mins in presence of [33gammaP]-ATP by SPA |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433601

(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 TEL domain (unknown origin) transfected in mouse Ba/F3 cells |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147458
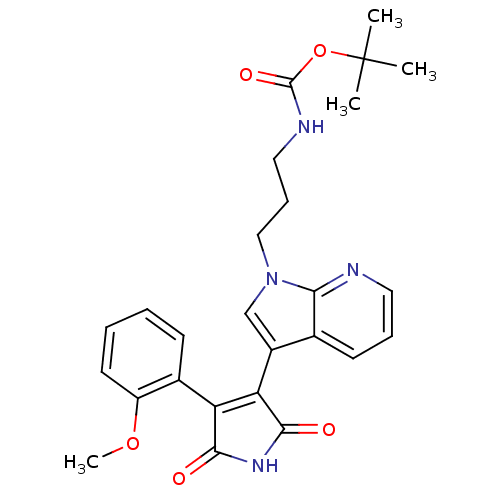
((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433601

(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using biotinylated poly-EY peptide as substrate after 60 mins by HTRF assay |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data