 Found 1130 hits with Last Name = 'massa' and Initial = 's'
Found 1130 hits with Last Name = 'massa' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253154

(CHEMBL492265 | N-Cyclopropyl-4-methyl-3-(1-(2-meth...)Show SMILES Cc1ccccc1-c1nncc2cc(ccc12)-c1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C26H23N3O/c1-16-5-3-4-6-22(16)25-23-12-9-18(13-20(23)15-27-29-25)24-14-19(8-7-17(24)2)26(30)28-21-10-11-21/h3-9,12-15,21H,10-11H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50313107
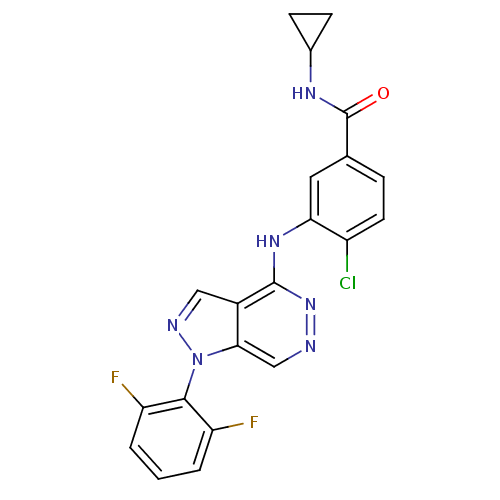
(4-chloro-N-cyclopropyl-3-(1-(2,6-difluorophenyl)-1...)Show SMILES Fc1cccc(F)c1-n1ncc2c(Nc3cc(ccc3Cl)C(=O)NC3CC3)nncc12 |(-6.01,-8.16,;-5.23,-9.48,;-5.98,-10.82,;-5.21,-12.15,;-3.66,-12.13,;-2.9,-10.79,;-1.37,-10.77,;-3.69,-9.47,;-2.93,-8.14,;-3.84,-6.9,;-2.95,-5.64,;-1.48,-6.1,;-.16,-5.33,;-.1,-3.79,;1.27,-3.07,;2.56,-3.9,;3.92,-3.18,;3.98,-1.64,;2.68,-.83,;1.32,-1.54,;.02,-.73,;5.22,-4.01,;5.16,-5.55,;6.59,-3.29,;7.89,-4.12,;8.6,-5.48,;9.43,-4.18,;1.18,-6.08,;1.19,-7.63,;-.14,-8.4,;-1.47,-7.64,)| Show InChI InChI=1S/C21H15ClF2N6O/c22-14-7-4-11(21(31)27-12-5-6-12)8-17(14)28-20-13-9-26-30(18(13)10-25-29-20)19-15(23)2-1-3-16(19)24/h1-4,7-10,12H,5-6H2,(H,27,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314780
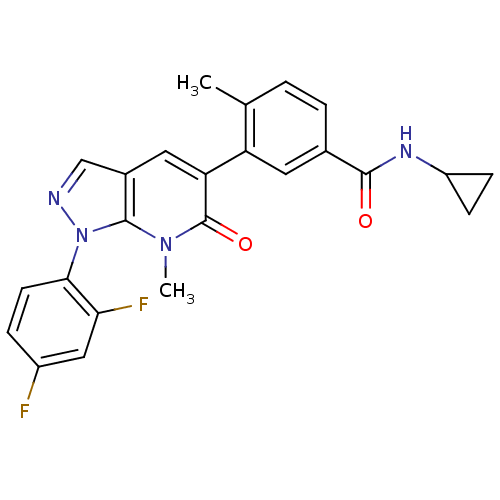
(CHEMBL1091199 | N-Cyclopropyl-3-(1-(2,4-difluoroph...)Show SMILES Cc1ccc(cc1-c1cc2cnn(-c3ccc(F)cc3F)c2n(C)c1=O)C(=O)NC1CC1 Show InChI InChI=1S/C24H20F2N4O2/c1-13-3-4-14(22(31)28-17-6-7-17)9-18(13)19-10-15-12-27-30(23(15)29(2)24(19)32)21-8-5-16(25)11-20(21)26/h3-5,8-12,17H,6-7H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110715
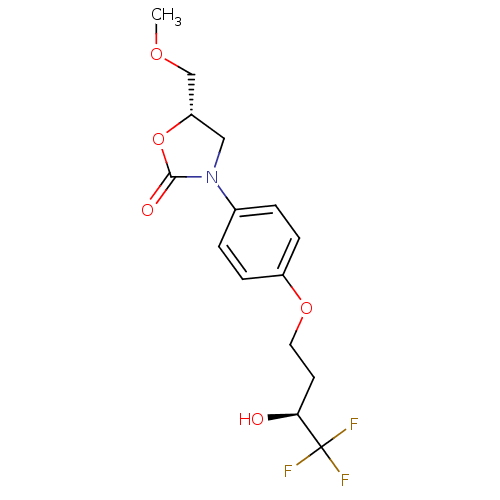
(5-Methoxymethyl-3-[4-(4,4,4-trifluoro-3-hydroxy-bu...)Show SMILES COC[C@@H]1CN(C(=O)O1)c1ccc(OCC[C@H](O)C(F)(F)F)cc1 Show InChI InChI=1S/C15H18F3NO5/c1-22-9-12-8-19(14(21)24-12)10-2-4-11(5-3-10)23-7-6-13(20)15(16,17)18/h2-5,12-13,20H,6-9H2,1H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110719
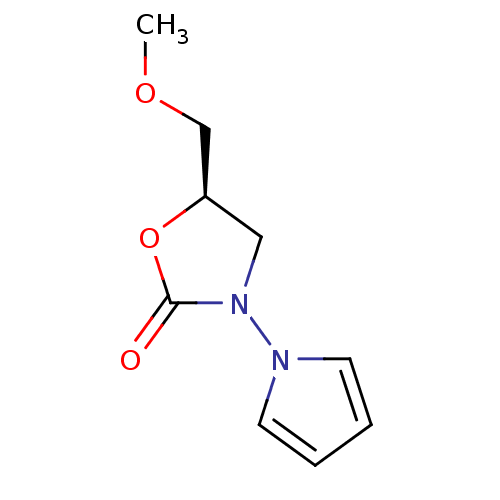
(5-Methoxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C9H12N2O3/c1-13-7-8-6-11(9(12)14-8)10-4-2-3-5-10/h2-5,8H,6-7H2,1H3/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50002338
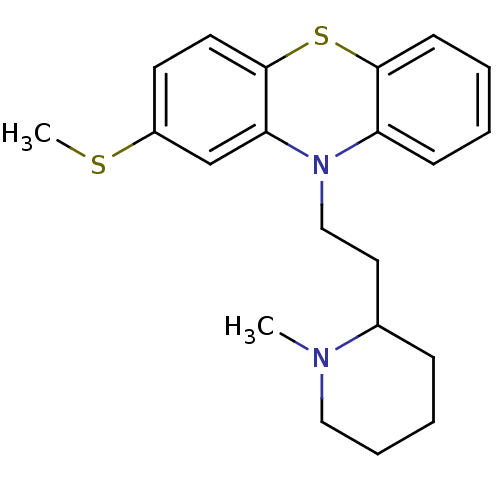
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Raclopride from human D2 receptor (unknown origin) expressed in HEK293 cell membranes |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50418613
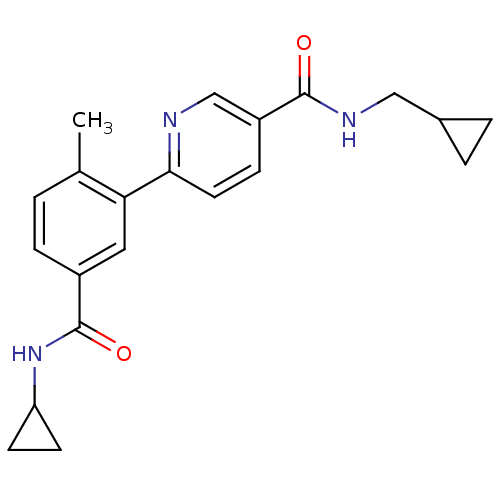
(CHEMBL1232887)Show SMILES Cc1ccc(cc1-c1ccc(cn1)C(=O)NCC1CC1)C(=O)NC1CC1 Show InChI InChI=1S/C21H23N3O2/c1-13-2-5-15(21(26)24-17-7-8-17)10-18(13)19-9-6-16(12-22-19)20(25)23-11-14-3-4-14/h2,5-6,9-10,12,14,17H,3-4,7-8,11H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265033

(CHEMBL522579 | N-cyclopropyl-2',6-dimethyl-4'-(5-m...)Show SMILES Cc1nnc(o1)-c1ccc(c(C)c1)-c1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C21H21N3O2/c1-12-4-5-15(20(25)22-17-7-8-17)11-19(12)18-9-6-16(10-13(18)2)21-24-23-14(3)26-21/h4-6,9-11,17H,7-8H2,1-3H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263093
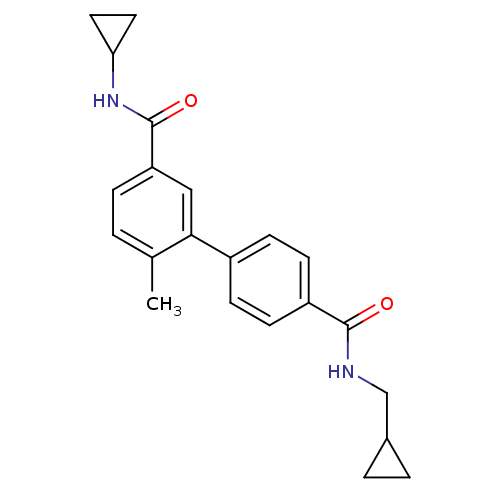
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...)Show SMILES Cc1ccc(cc1-c1ccc(cc1)C(=O)NCC1CC1)C(=O)NC1CC1 Show InChI InChI=1S/C22H24N2O2/c1-14-2-5-18(22(26)24-19-10-11-19)12-20(14)16-6-8-17(9-7-16)21(25)23-13-15-3-4-15/h2,5-9,12,15,19H,3-4,10-11,13H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Ketanserin from human 5HT2A receptor expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110714
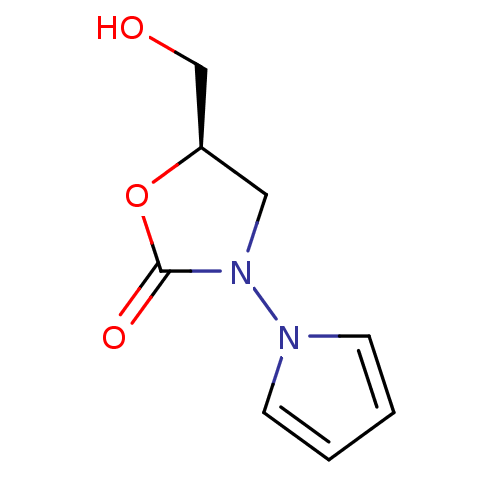
(5-Hydroxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110717
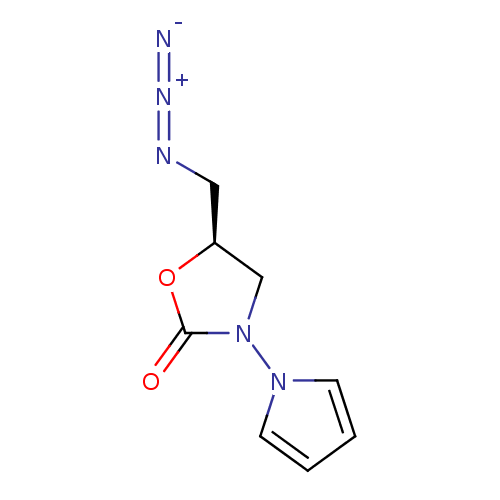
(5-Azidomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H9N5O2/c9-11-10-5-7-6-13(8(14)15-7)12-3-1-2-4-12/h1-4,7H,5-6H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110715

(5-Methoxymethyl-3-[4-(4,4,4-trifluoro-3-hydroxy-bu...)Show SMILES COC[C@@H]1CN(C(=O)O1)c1ccc(OCC[C@H](O)C(F)(F)F)cc1 Show InChI InChI=1S/C15H18F3NO5/c1-22-9-12-8-19(14(21)24-12)10-2-4-11(5-3-10)23-7-6-13(20)15(16,17)18/h2-5,12-13,20H,6-9H2,1H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50565002

(CHEMBL4778666)Show SMILES Cl.[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1-[#6]-[#6]-[#7]-1-c2ccc(Br)cc2[Se;v2]c2cc(Br)ccc-12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM25980
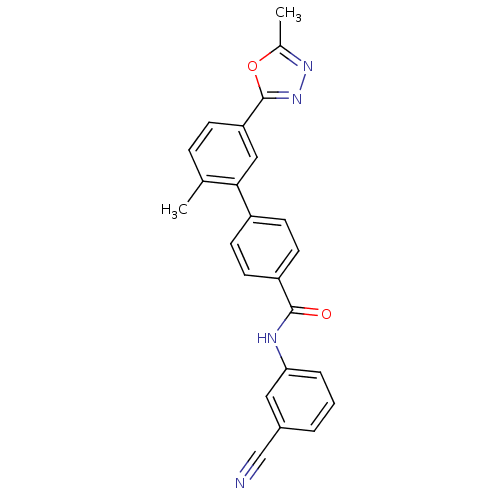
(BMCL18324 Compound 3 | CHEMBL270164 | N-(3-cyanoph...)Show SMILES Cc1nnc(o1)-c1ccc(C)c(c1)-c1ccc(cc1)C(=O)Nc1cccc(c1)C#N Show InChI InChI=1S/C24H18N4O2/c1-15-6-7-20(24-28-27-16(2)30-24)13-22(15)18-8-10-19(11-9-18)23(29)26-21-5-3-4-17(12-21)14-25/h3-13H,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Dengue virus) | BDBM50464499
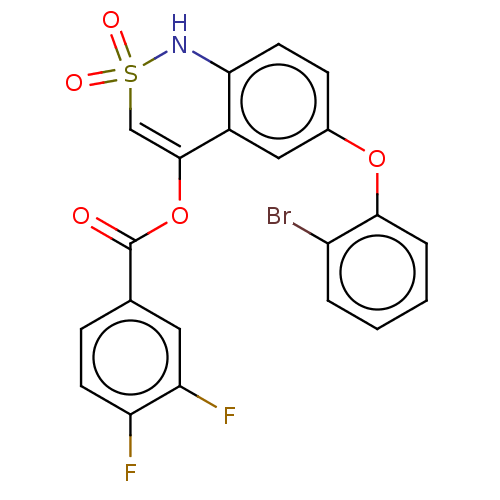
(CHEMBL4288319)Show SMILES Fc1ccc(cc1F)C(=O)OC1=CS(=O)(=O)Nc2ccc(Oc3ccccc3Br)cc12 |t:12| Show InChI InChI=1S/C21H12BrF2NO5S/c22-15-3-1-2-4-19(15)29-13-6-8-18-14(10-13)20(11-31(27,28)25-18)30-21(26)12-5-7-16(23)17(24)9-12/h1-11,25H | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Mixed type inhibition of DENV3 NS5 RdRp using increasing concentration of GTP as substrate assessed as free enzyme after 1 hr in presence of ss-RNA P... |
Eur J Med Chem 143: 1667-1676 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.064
BindingDB Entry DOI: 10.7270/Q26M39GT |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50370193
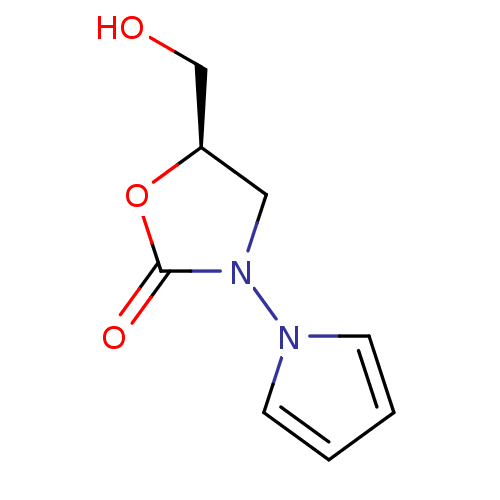
(CHEMBL1794849)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50565002

(CHEMBL4778666)Show SMILES Cl.[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1-[#6]-[#6]-[#7]-1-c2ccc(Br)cc2[Se;v2]c2cc(Br)ccc-12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Raclopride from human D2 receptor (unknown origin) expressed in HEK293 cell membranes |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110725
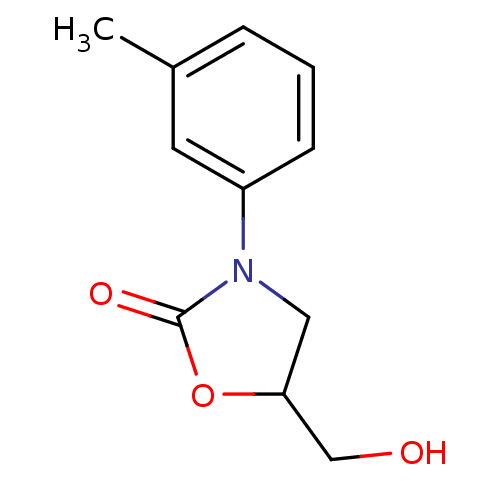
(5-(hydroxymethyl)-3-(3-methylphenyl)-oxazolidin-2-...)Show InChI InChI=1S/C11H13NO3/c1-8-3-2-4-9(5-8)12-6-10(7-13)15-11(12)14/h2-5,10,13H,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50565001
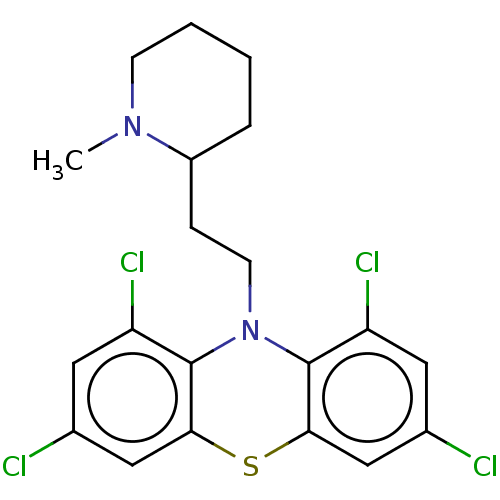
(CHEMBL4797066)Show SMILES Cl.CN1CCCCC1CCN1c2c(Cl)cc(Cl)cc2Sc2cc(Cl)cc(Cl)c12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM25981

(BMCL18324 Compound 17 | CHEMBL273158 | D3RKN_78 | ...)Show SMILES Cc1nnc(o1)-c1ccc(C)c(c1)-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C21H21N3O2/c1-13-3-6-18(21-24-23-14(2)26-21)11-19(13)16-7-9-17(10-8-16)20(25)22-12-15-4-5-15/h3,6-11,15H,4-5,12H2,1-2H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibition of P38alpha MAPK (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111624
BindingDB Entry DOI: 10.7270/Q2J38WW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50565001

(CHEMBL4797066)Show SMILES Cl.CN1CCCCC1CCN1c2c(Cl)cc(Cl)cc2Sc2cc(Cl)cc(Cl)c12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 639 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Raclopride from human D2 receptor (unknown origin) expressed in HEK293 cell membranes |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50565000

(CHEMBL4791959) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Raclopride from human D2 receptor (unknown origin) expressed in HEK293 cell membranes |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50565000

(CHEMBL4791959) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 758 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110723
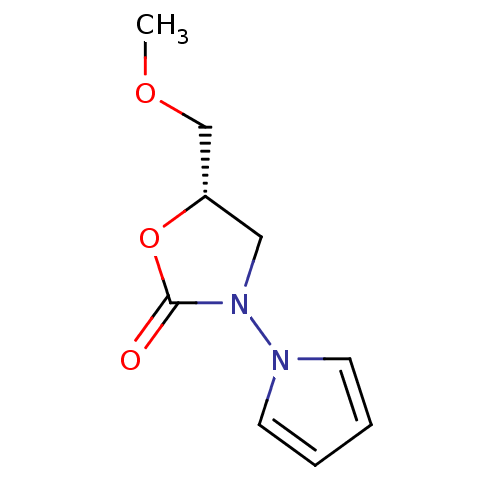
(5-Methoxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C9H12N2O3/c1-13-7-8-6-11(9(12)14-8)10-4-2-3-5-10/h2-5,8H,6-7H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50565001

(CHEMBL4797066)Show SMILES Cl.CN1CCCCC1CCN1c2c(Cl)cc(Cl)cc2Sc2cc(Cl)cc(Cl)c12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110716
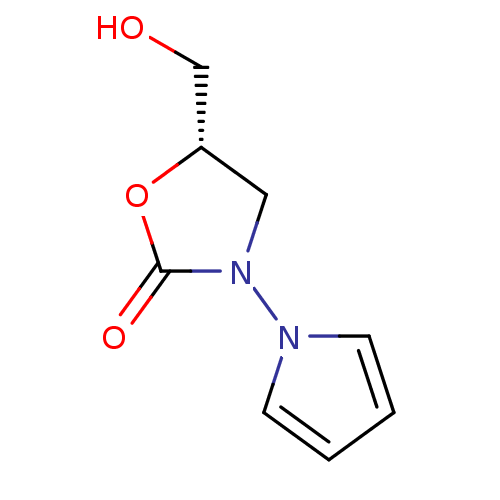
(5-Hydroxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110724
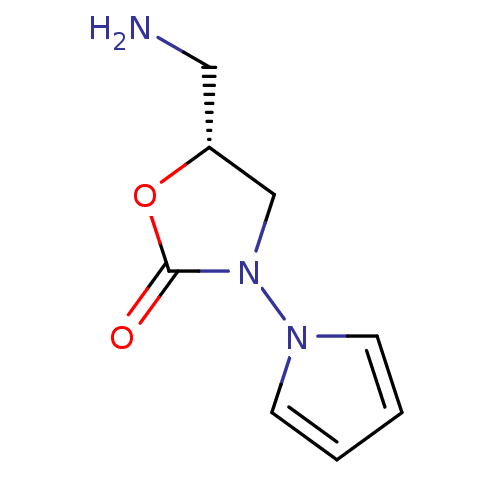
(5-Aminomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H11N3O2/c9-5-7-6-11(8(12)13-7)10-3-1-2-4-10/h1-4,7H,5-6,9H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50565001

(CHEMBL4797066)Show SMILES Cl.CN1CCCCC1CCN1c2c(Cl)cc(Cl)cc2Sc2cc(Cl)cc(Cl)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Ketanserin from human 5HT2A receptor expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50464499

(CHEMBL4288319)Show SMILES Fc1ccc(cc1F)C(=O)OC1=CS(=O)(=O)Nc2ccc(Oc3ccccc3Br)cc12 |t:12| Show InChI InChI=1S/C21H12BrF2NO5S/c22-15-3-1-2-4-19(15)29-13-6-8-18-14(10-13)20(11-31(27,28)25-18)30-21(26)12-5-7-16(23)17(24)9-12/h1-11,25H | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Mixed type inhibition of DENV3 NS5 RdRp using increasing concentration of ss-RNA PolyC as template assessed as free enzyme after 1 hr in presence of ... |
Eur J Med Chem 143: 1667-1676 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.064
BindingDB Entry DOI: 10.7270/Q26M39GT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50464499

(CHEMBL4288319)Show SMILES Fc1ccc(cc1F)C(=O)OC1=CS(=O)(=O)Nc2ccc(Oc3ccccc3Br)cc12 |t:12| Show InChI InChI=1S/C21H12BrF2NO5S/c22-15-3-1-2-4-19(15)29-13-6-8-18-14(10-13)20(11-31(27,28)25-18)30-21(26)12-5-7-16(23)17(24)9-12/h1-11,25H | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Mixed type inhibition of DENV3 NS5 RdRp using increasing concentration of GTP as substrate assessed as enzyme-substrate complex after 1 hr in presenc... |
Eur J Med Chem 143: 1667-1676 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.064
BindingDB Entry DOI: 10.7270/Q26M39GT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50565000

(CHEMBL4791959) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Ketanserin from human 5HT2A receptor expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50565002

(CHEMBL4778666)Show SMILES Cl.[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1-[#6]-[#6]-[#7]-1-c2ccc(Br)cc2[Se;v2]c2cc(Br)ccc-12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Ketanserin from human 5HT2A receptor expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50370193

(CHEMBL1794849)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110716

(5-Hydroxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50565000

(CHEMBL4791959) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50565002

(CHEMBL4778666)Show SMILES Cl.[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1-[#6]-[#6]-[#7]-1-c2ccc(Br)cc2[Se;v2]c2cc(Br)ccc-12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112420
BindingDB Entry DOI: 10.7270/Q2G73JGD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110720
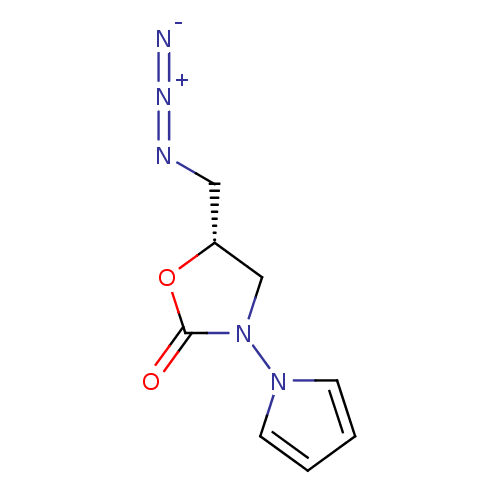
(5-Azidomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H9N5O2/c9-11-10-5-7-6-13(8(14)15-7)12-3-1-2-4-12/h1-4,7H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50464499

(CHEMBL4288319)Show SMILES Fc1ccc(cc1F)C(=O)OC1=CS(=O)(=O)Nc2ccc(Oc3ccccc3Br)cc12 |t:12| Show InChI InChI=1S/C21H12BrF2NO5S/c22-15-3-1-2-4-19(15)29-13-6-8-18-14(10-13)20(11-31(27,28)25-18)30-21(26)12-5-7-16(23)17(24)9-12/h1-11,25H | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia
Curated by ChEMBL
| Assay Description
Mixed type inhibition of DENV3 NS5 RdRp using increasing concentration of ss-RNA PolyC as template assessed as enzyme-substrate complex after 1 hr in... |
Eur J Med Chem 143: 1667-1676 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.064
BindingDB Entry DOI: 10.7270/Q26M39GT |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110721

(5-Aminomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H11N3O2/c9-5-7-6-11(8(12)13-7)10-3-1-2-4-10/h1-4,7H,5-6,9H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110714

(5-Hydroxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C8H10N2O3/c11-6-7-5-10(8(12)13-7)9-3-1-2-4-9/h1-4,7,11H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110725

(5-(hydroxymethyl)-3-(3-methylphenyl)-oxazolidin-2-...)Show InChI InChI=1S/C11H13NO3/c1-8-3-2-4-9(5-8)12-6-10(7-13)15-11(12)14/h2-5,10,13H,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110723

(5-Methoxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C9H12N2O3/c1-13-7-8-6-11(9(12)14-8)10-4-2-3-5-10/h2-5,8H,6-7H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110720

(5-Azidomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H9N5O2/c9-11-10-5-7-6-13(8(14)15-7)12-3-1-2-4-12/h1-4,7H,5-6H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110724

(5-Aminomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H11N3O2/c9-5-7-6-11(8(12)13-7)10-3-1-2-4-10/h1-4,7H,5-6,9H2/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110718
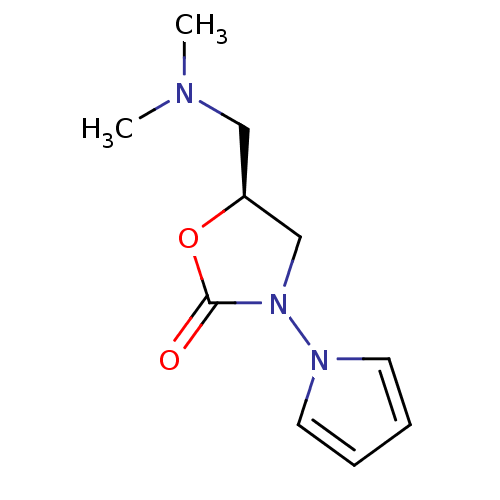
(5-Dimethylaminomethyl-3-pyrrol-1-yl-oxazolidin-2-o...)Show InChI InChI=1S/C10H15N3O2/c1-11(2)7-9-8-13(10(14)15-9)12-5-3-4-6-12/h3-6,9H,7-8H2,1-2H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50110719

(5-Methoxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | C...)Show InChI InChI=1S/C9H12N2O3/c1-13-7-8-6-11(9(12)14-8)10-4-2-3-5-10/h2-5,8H,6-7H2,1H3/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase B (MAO-B) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110721

(5-Aminomethyl-3-pyrrol-1-yl-oxazolidin-2-one | CHE...)Show InChI InChI=1S/C8H11N3O2/c9-5-7-6-11(8(12)13-7)10-3-1-2-4-10/h1-4,7H,5-6,9H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial Monoamine oxidase A (MAO-A) compared to toloxatone |
J Med Chem 45: 1180-3 (2002)
BindingDB Entry DOI: 10.7270/Q28W3F11 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data