 Found 645 hits with Last Name = 'omura' and Initial = 's'
Found 645 hits with Last Name = 'omura' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(RAT) | BDBM82260
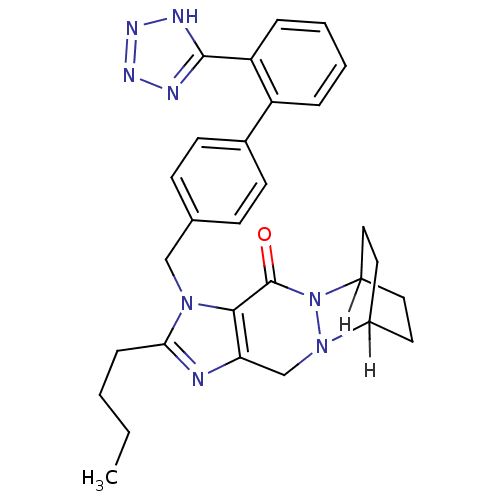
(U-97018)Show SMILES [H]C12CCC([H])(CC1)N1N2Cc2nc(CCCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2C1=O |TLB:38:8:2.3:7.6,THB:10:9:2.3:7.6,(17.17,4.69,;16.48,6.31,;19.3,5.14,;18.9,3.65,;16.08,4.82,;17.54,5.9,;14.57,7.62,;15.91,8.39,;13.41,3.28,;12.64,4.62,;11.04,4.64,;10.22,3.26,;8.67,2.92,;8.5,1.34,;7.17,.57,;5.83,1.34,;4.5,.57,;3.17,1.34,;9.96,.69,;10.27,-.82,;9.13,-1.85,;7.66,-1.37,;6.52,-2.4,;6.84,-3.91,;8.3,-4.38,;9.45,-3.36,;5.69,-4.93,;4.23,-4.46,;3.08,-5.48,;3.4,-6.99,;4.86,-7.47,;6.01,-6.44,;7.47,-6.92,;7.94,-8.38,;9.48,-8.39,;9.96,-6.92,;8.72,-6.02,;11.03,1.87,;12.63,1.88,;13.42,.56,)| Show InChI InChI=1S/C29H32N8O/c1-2-3-8-26-30-25-18-36-21-13-15-22(16-14-21)37(36)29(38)27(25)35(26)17-19-9-11-20(12-10-19)23-6-4-5-7-24(23)28-31-33-34-32-28/h4-7,9-12,21-22H,2-3,8,13-18H2,1H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82260

(U-97018)Show SMILES [H]C12CCC([H])(CC1)N1N2Cc2nc(CCCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2C1=O |TLB:38:8:2.3:7.6,THB:10:9:2.3:7.6,(17.17,4.69,;16.48,6.31,;19.3,5.14,;18.9,3.65,;16.08,4.82,;17.54,5.9,;14.57,7.62,;15.91,8.39,;13.41,3.28,;12.64,4.62,;11.04,4.64,;10.22,3.26,;8.67,2.92,;8.5,1.34,;7.17,.57,;5.83,1.34,;4.5,.57,;3.17,1.34,;9.96,.69,;10.27,-.82,;9.13,-1.85,;7.66,-1.37,;6.52,-2.4,;6.84,-3.91,;8.3,-4.38,;9.45,-3.36,;5.69,-4.93,;4.23,-4.46,;3.08,-5.48,;3.4,-6.99,;4.86,-7.47,;6.01,-6.44,;7.47,-6.92,;7.94,-8.38,;9.48,-8.39,;9.96,-6.92,;8.72,-6.02,;11.03,1.87,;12.63,1.88,;13.42,.56,)| Show InChI InChI=1S/C29H32N8O/c1-2-3-8-26-30-25-18-36-21-13-15-22(16-14-21)37(36)29(38)27(25)35(26)17-19-9-11-20(12-10-19)23-6-4-5-7-24(23)28-31-33-34-32-28/h4-7,9-12,21-22H,2-3,8,13-18H2,1H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50010361
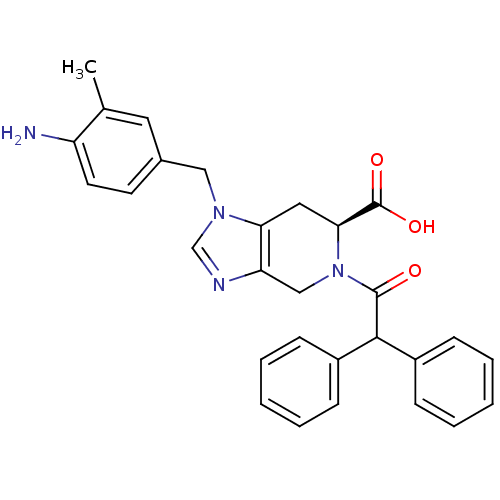
((S)-1-(4-Amino-3-methyl-benzyl)-5-diphenylacetyl-4...)Show SMILES Cc1cc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)ccc1N Show InChI InChI=1S/C29H28N4O3/c1-19-14-20(12-13-23(19)30)16-32-18-31-24-17-33(26(29(35)36)15-25(24)32)28(34)27(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-14,18,26-27H,15-17,30H2,1H3,(H,35,36)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10854
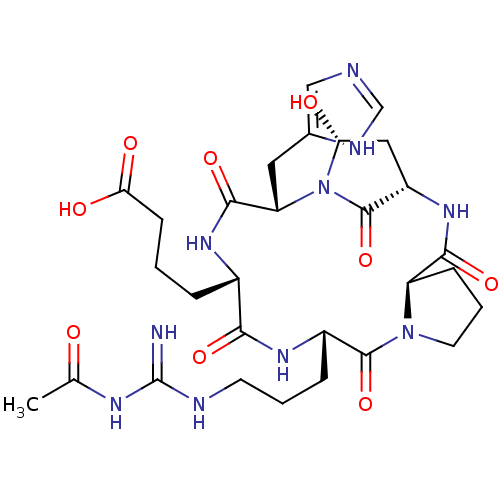
(4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...)Show SMILES CC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCC(O)=O)NC(=O)[C@H](Cc2cnc[nH]2)N2[C@H](O)C[C@H](NC(=O)[C@H]3CCCN3C1=O)C2=O |r| Show InChI InChI=1S/C29H42N10O9/c1-15(40)34-29(30)32-9-3-6-18-27(47)38-10-4-7-20(38)25(45)37-19-12-22(41)39(28(19)48)21(11-16-13-31-14-33-16)26(46)35-17(24(44)36-18)5-2-8-23(42)43/h13-14,17-22,41H,2-12H2,1H3,(H,31,33)(H,35,46)(H,36,44)(H,37,45)(H,42,43)(H3,30,32,34,40)/t17-,18-,19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 33.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82260

(U-97018)Show SMILES [H]C12CCC([H])(CC1)N1N2Cc2nc(CCCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2C1=O |TLB:38:8:2.3:7.6,THB:10:9:2.3:7.6,(17.17,4.69,;16.48,6.31,;19.3,5.14,;18.9,3.65,;16.08,4.82,;17.54,5.9,;14.57,7.62,;15.91,8.39,;13.41,3.28,;12.64,4.62,;11.04,4.64,;10.22,3.26,;8.67,2.92,;8.5,1.34,;7.17,.57,;5.83,1.34,;4.5,.57,;3.17,1.34,;9.96,.69,;10.27,-.82,;9.13,-1.85,;7.66,-1.37,;6.52,-2.4,;6.84,-3.91,;8.3,-4.38,;9.45,-3.36,;5.69,-4.93,;4.23,-4.46,;3.08,-5.48,;3.4,-6.99,;4.86,-7.47,;6.01,-6.44,;7.47,-6.92,;7.94,-8.38,;9.48,-8.39,;9.96,-6.92,;8.72,-6.02,;11.03,1.87,;12.63,1.88,;13.42,.56,)| Show InChI InChI=1S/C29H32N8O/c1-2-3-8-26-30-25-18-36-21-13-15-22(16-14-21)37(36)29(38)27(25)35(26)17-19-9-11-20(12-10-19)23-6-4-5-7-24(23)28-31-33-34-32-28/h4-7,9-12,21-22H,2-3,8,13-18H2,1H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50010361

((S)-1-(4-Amino-3-methyl-benzyl)-5-diphenylacetyl-4...)Show SMILES Cc1cc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)ccc1N Show InChI InChI=1S/C29H28N4O3/c1-19-14-20(12-13-23(19)30)16-32-18-31-24-17-33(26(29(35)36)15-25(24)32)28(34)27(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-14,18,26-27H,15-17,30H2,1H3,(H,35,36)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Pharmaceuticals Limited
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 274: 1042-53 (1995)
BindingDB Entry DOI: 10.7270/Q20G3HN9 |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10853
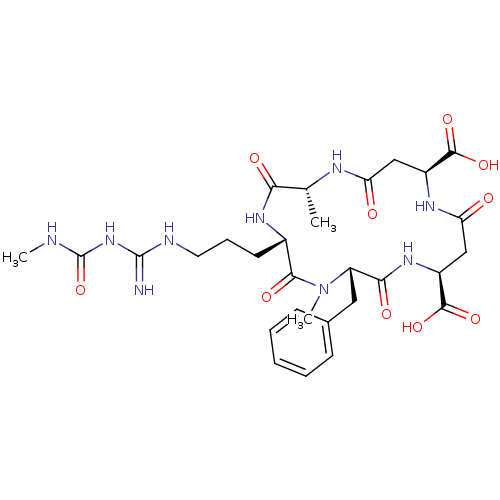
((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...)Show SMILES CNC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@@H](C)NC(=O)C[C@H](NC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H41N9O10/c1-15-23(41)35-17(10-7-11-32-28(30)37-29(48)31-2)25(43)38(3)20(12-16-8-5-4-6-9-16)24(42)36-19(27(46)47)14-22(40)34-18(26(44)45)13-21(39)33-15/h4-6,8-9,15,17-20H,7,10-14H2,1-3H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)(H,44,45)(H,46,47)(H4,30,31,32,37,48)/t15-,17+,18+,19+,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.30E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438462
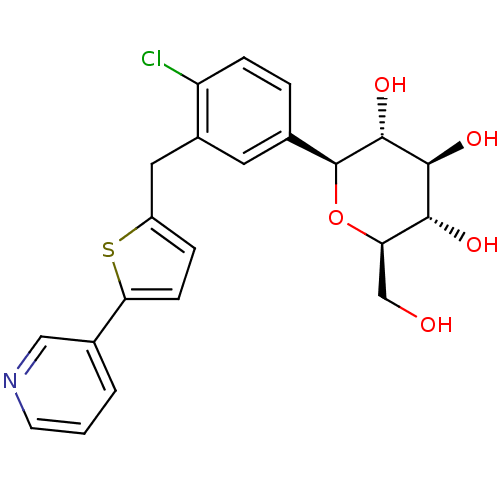
(CHEMBL2414623)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(s2)-c2cccnc2)c1 |r| Show InChI InChI=1S/C22H22ClNO5S/c23-16-5-3-12(22-21(28)20(27)19(26)17(11-25)29-22)8-14(16)9-15-4-6-18(30-15)13-2-1-7-24-10-13/h1-8,10,17,19-22,25-28H,9,11H2/t17-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429605

(CHEMBL2334538)Show SMILES COc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO11/c1-20(39)45-19-36(4)27-17-29(48-33(42)22-9-11-24(44-6)12-10-22)37(5)32(35(27,3)14-13-28(36)46-21(2)40)31(41)30-26(49-37)16-25(47-34(30)43)23-8-7-15-38-18-23/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429604
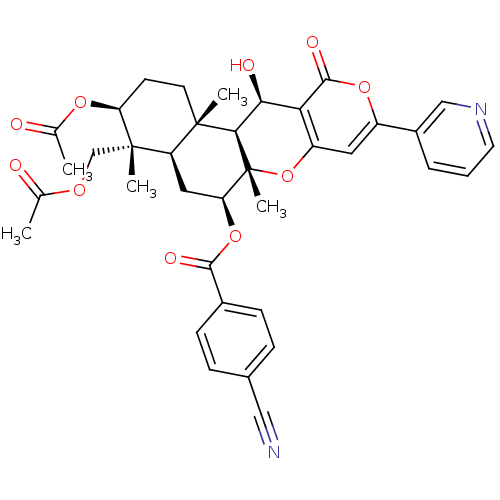
(CHEMBL2334539)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H38N2O10/c1-20(40)45-19-36(4)27-16-29(48-33(43)23-10-8-22(17-38)9-11-23)37(5)32(35(27,3)13-12-28(36)46-21(2)41)31(42)30-26(49-37)15-25(47-34(30)44)24-7-6-14-39-18-24/h6-11,14-15,18,27-29,31-32,42H,12-13,16,19H2,1-5H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429604

(CHEMBL2334539)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)C#N)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H38N2O10/c1-20(40)45-19-36(4)27-16-29(48-33(43)23-10-8-22(17-38)9-11-23)37(5)32(35(27,3)13-12-28(36)46-21(2)41)31(42)30-26(49-37)15-25(47-34(30)44)24-7-6-14-39-18-24/h6-11,14-15,18,27-29,31-32,42H,12-13,16,19H2,1-5H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) |
Bioorg Med Chem Lett 23: 3798-801 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.075
BindingDB Entry DOI: 10.7270/Q2WD41Z9 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429610

(CHEMBL2334222)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10/c1-20-9-11-23(12-10-20)33(42)47-29-17-27-35(4,14-13-28(45-22(3)40)36(27,5)19-44-21(2)39)32-31(41)30-26(48-37(29,32)6)16-25(46-34(30)43)24-8-7-15-38-18-24/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429603

(CHEMBL2334540)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38FNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429602

(CHEMBL2334541)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Cl)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38ClNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50089619
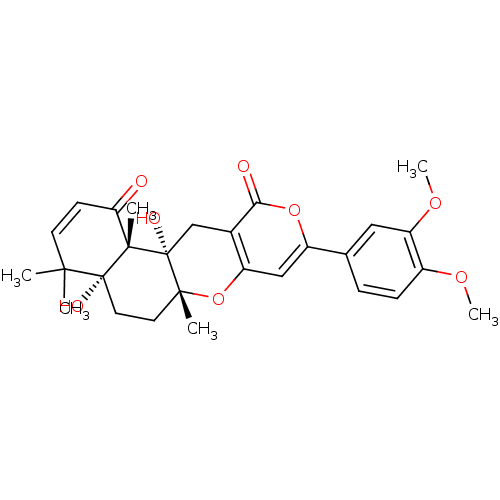
(9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...)Show SMILES COc1ccc(cc1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)C(C)(C)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C28H32O8/c1-24(2)10-9-22(29)26(4)27(24,31)12-11-25(3)28(26,32)15-17-20(36-25)14-19(35-23(17)30)16-7-8-18(33-5)21(13-16)34-6/h7-10,13-14,31-32H,11-12,15H2,1-6H3/t25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 10: 1315-6 (2000)
BindingDB Entry DOI: 10.7270/Q23B5ZCD |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429601

(CHEMBL2333808)Show SMILES CCc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C38H43NO10/c1-7-23-10-12-24(13-11-23)34(43)48-30-18-28-36(4,15-14-29(46-22(3)41)37(28,5)20-45-21(2)40)33-32(42)31-27(49-38(30,33)6)17-26(47-35(31)44)25-9-8-16-39-19-25/h8-13,16-17,19,28-30,32-33,42H,7,14-15,18,20H2,1-6H3/t28-,29+,30+,32+,33-,36+,37+,38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438467
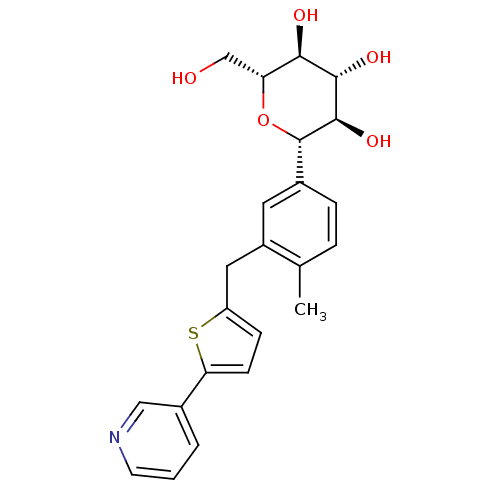
(CHEMBL2414618)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1cccnc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H25NO5S/c1-13-4-5-14(23-22(28)21(27)20(26)18(12-25)29-23)9-16(13)10-17-6-7-19(30-17)15-3-2-8-24-11-15/h2-9,11,18,20-23,25-28H,10,12H2,1H3/t18-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50446188
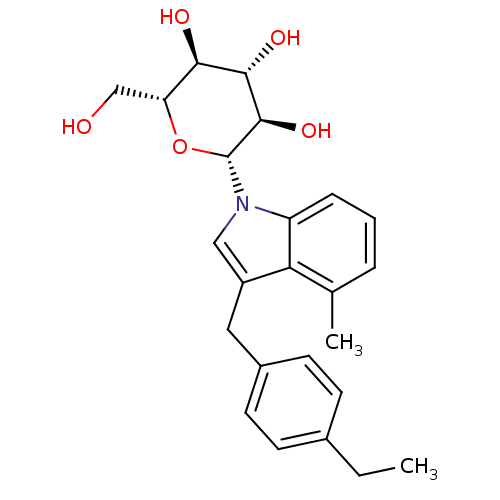
(CHEMBL3109017)Show SMILES CCc1ccc(Cc2cn([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3cccc(C)c23)cc1 |r| Show InChI InChI=1S/C24H29NO5/c1-3-15-7-9-16(10-8-15)11-17-12-25(18-6-4-5-14(2)20(17)18)24-23(29)22(28)21(27)19(13-26)30-24/h4-10,12,19,21-24,26-29H,3,11,13H2,1-2H3/t19-,21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2-mediated [14C]-AMG uptake expressed in CHOK cells preincubated for 10 mins followed by [14C]-AMG addition measured after 12... |
ACS Med Chem Lett 5: 51-5 (2014)
Article DOI: 10.1021/ml400339b
BindingDB Entry DOI: 10.7270/Q24J0GM7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429600

(CHEMBL2333809)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(Br)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38BrNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50346265
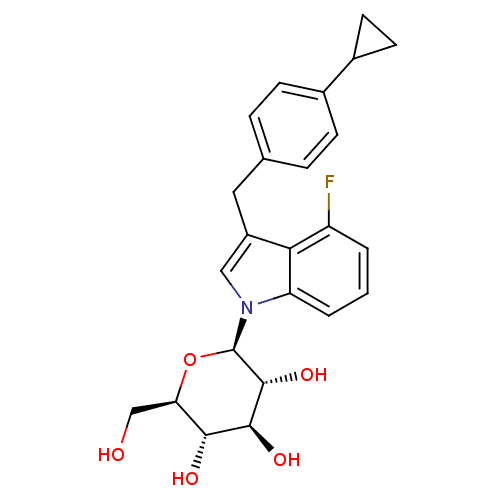
((2R,3R,4S,5S,6R)-2-(3-(4-cyclopropylbenzyl)-4-fluo...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cc2ccc(cc2)C2CC2)c2c(F)cccc12 |r| Show InChI InChI=1S/C24H26FNO5/c25-17-2-1-3-18-20(17)16(10-13-4-6-14(7-5-13)15-8-9-15)11-26(18)24-23(30)22(29)21(28)19(12-27)31-24/h1-7,11,15,19,21-24,27-30H,8-10,12H2/t19-,21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2-mediated [14C]-AMG uptake expressed in CHOK cells preincubated for 10 mins followed by [14C]-AMG addition measured after 12... |
ACS Med Chem Lett 5: 51-5 (2014)
Article DOI: 10.1021/ml400339b
BindingDB Entry DOI: 10.7270/Q24J0GM7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438463
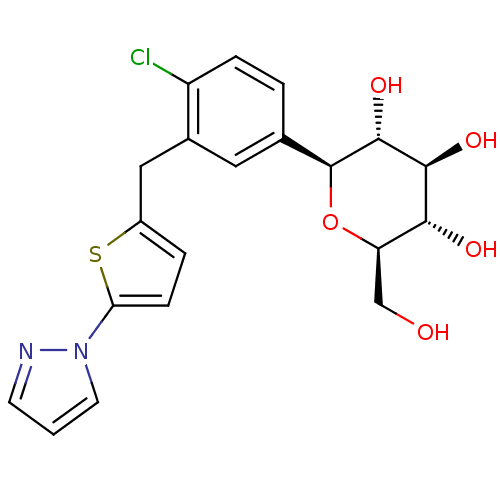
(CHEMBL2414622)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(s2)-n2cccn2)c1 |r| Show InChI InChI=1S/C20H21ClN2O5S/c21-14-4-2-11(20-19(27)18(26)17(25)15(10-24)28-20)8-12(14)9-13-3-5-16(29-13)23-7-1-6-22-23/h1-8,15,17-20,24-27H,9-10H2/t15-,17-,18+,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50446186
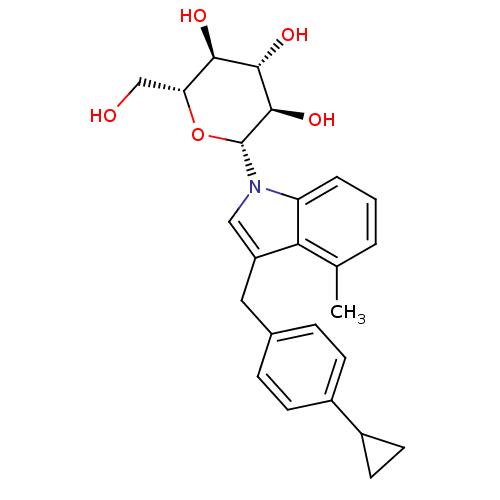
(CHEMBL3109016)Show SMILES Cc1cccc2n(cc(Cc3ccc(cc3)C3CC3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H29NO5/c1-14-3-2-4-19-21(14)18(11-15-5-7-16(8-6-15)17-9-10-17)12-26(19)25-24(30)23(29)22(28)20(13-27)31-25/h2-8,12,17,20,22-25,27-30H,9-11,13H2,1H3/t20-,22-,23+,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2-mediated [14C]-AMG uptake expressed in CHOK cells preincubated for 10 mins followed by [14C]-AMG addition measured after 12... |
ACS Med Chem Lett 5: 51-5 (2014)
Article DOI: 10.1021/ml400339b
BindingDB Entry DOI: 10.7270/Q24J0GM7 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438465
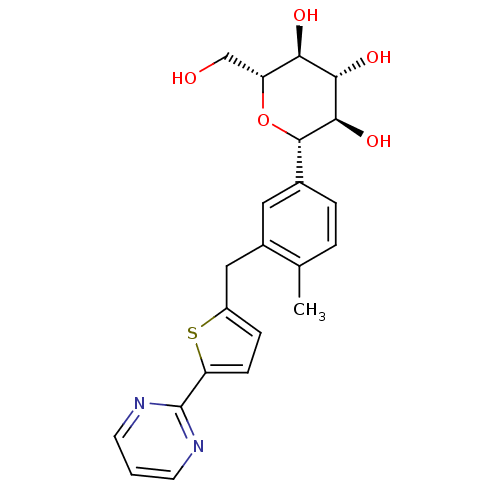
(CHEMBL2414620)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ncccn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H24N2O5S/c1-12-3-4-13(21-20(28)19(27)18(26)16(11-25)29-21)9-14(12)10-15-5-6-17(30-15)22-23-7-2-8-24-22/h2-9,16,18-21,25-28H,10-11H2,1H3/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429599
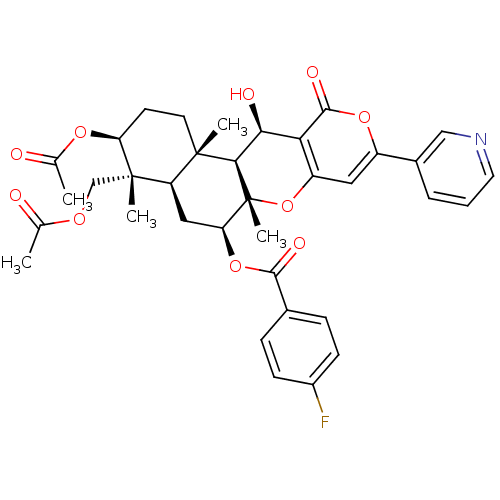
(CHEMBL2333810)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(F)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38FNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438460
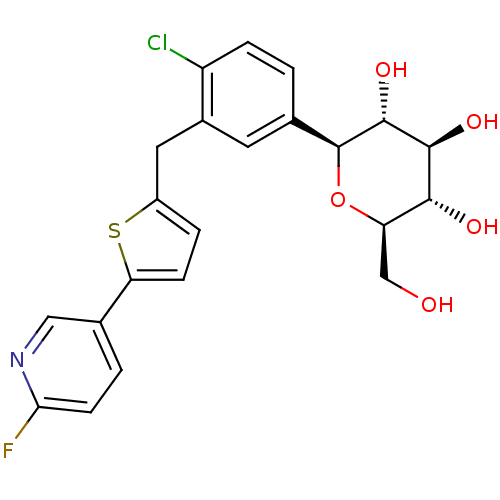
(CHEMBL2414617)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(s2)-c2ccc(F)nc2)c1 |r| Show InChI InChI=1S/C22H21ClFNO5S/c23-15-4-1-11(22-21(29)20(28)19(27)16(10-26)30-22)7-13(15)8-14-3-5-17(31-14)12-2-6-18(24)25-9-12/h1-7,9,16,19-22,26-29H,8,10H2/t16-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429598

(CHEMBL2333811)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Br)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38BrNO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50386885
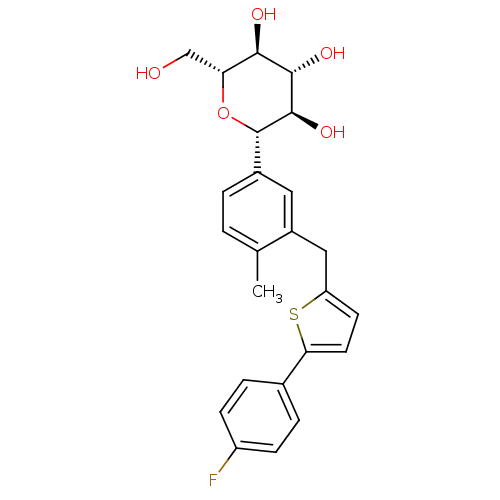
(CANAGLIFLOZIN | CANAGLIFLOZIN HYDRATE | US10752604...)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ccc(F)cc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50446185
(CHEMBL3109015)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(Cc2ccc(cc2)C2CC2)c2ccccc12 |r| Show InChI InChI=1S/C24H27NO5/c26-13-20-21(27)22(28)23(29)24(30-20)25-12-17(18-3-1-2-4-19(18)25)11-14-5-7-15(8-6-14)16-9-10-16/h1-8,12,16,20-24,26-29H,9-11,13H2/t20-,21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2-mediated [14C]-AMG uptake expressed in CHOK cells preincubated for 10 mins followed by [14C]-AMG addition measured after 12... |
ACS Med Chem Lett 5: 51-5 (2014)
Article DOI: 10.1021/ml400339b
BindingDB Entry DOI: 10.7270/Q24J0GM7 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50326284
(1-(beta-D-Glucopyranosyl)-4-methyl-3-(5-(4-fluorop...)Show SMILES Cc1cccc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1Cc1ccc(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H25FO6S/c1-13-3-2-4-18(30-24-23(29)22(28)21(27)19(12-26)31-24)17(13)11-16-9-10-20(32-16)14-5-7-15(25)8-6-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scint... |
J Med Chem 53: 6355-60 (2010)
Article DOI: 10.1021/jm100332n
BindingDB Entry DOI: 10.7270/Q2V69JSS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438464
(CHEMBL2414621)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1cncs1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H23NO5S2/c1-11-2-3-12(21-20(26)19(25)18(24)15(9-23)27-21)6-13(11)7-14-4-5-16(29-14)17-8-22-10-28-17/h2-6,8,10,15,18-21,23-26H,7,9H2,1H3/t15-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... |
Bioorg Med Chem 21: 5561-72 (2013)
Article DOI: 10.1016/j.bmc.2013.05.048
BindingDB Entry DOI: 10.7270/Q2RX9DHV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50326280

(1-(beta-D-Glucopyranosyl)-4-chloro-3-(5-chloro-2-t...)Show SMILES OC[C@H]1O[C@@H](Oc2cccc(Cl)c2Cc2ccc(Cl)s2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C17H18Cl2O6S/c18-10-2-1-3-11(9(10)6-8-4-5-13(19)26-8)24-17-16(23)15(22)14(21)12(7-20)25-17/h1-5,12,14-17,20-23H,6-7H2/t12-,14-,15+,16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scint... |
J Med Chem 53: 6355-60 (2010)
Article DOI: 10.1021/jm100332n
BindingDB Entry DOI: 10.7270/Q2V69JSS |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429597
(CHEMBL2333812)Show SMILES CSc1ccc(cc1)C(=O)O[C@H]1C[C@H]2[C@](C)(COC(C)=O)[C@H](CC[C@]2(C)[C@H]2[C@@H](O)c3c(O[C@]12C)cc(oc3=O)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10S/c1-20(39)44-19-36(4)27-17-29(47-33(42)22-9-11-24(49-6)12-10-22)37(5)32(35(27,3)14-13-28(36)45-21(2)40)31(41)30-26(48-37)16-25(46-34(30)43)23-8-7-15-38-18-23/h7-12,15-16,18,27-29,31-32,41H,13-14,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10854

(4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...)Show SMILES CC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCC(O)=O)NC(=O)[C@H](Cc2cnc[nH]2)N2[C@H](O)C[C@H](NC(=O)[C@H]3CCCN3C1=O)C2=O |r| Show InChI InChI=1S/C29H42N10O9/c1-15(40)34-29(30)32-9-3-6-18-27(47)38-10-4-7-20(38)25(45)37-19-12-22(41)39(28(19)48)21(11-16-13-31-14-33-16)26(46)35-17(24(44)36-18)5-2-8-23(42)43/h13-14,17-22,41H,2-12H2,1H3,(H,31,33)(H,35,46)(H,36,44)(H,37,45)(H,42,43)(H3,30,32,34,40)/t17-,18-,19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to Serratia marcescens chitinase B |
Bioorg Med Chem 16: 3565-79 (2008)
Article DOI: 10.1016/j.bmc.2008.02.017
BindingDB Entry DOI: 10.7270/Q2NG4QCG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50441088
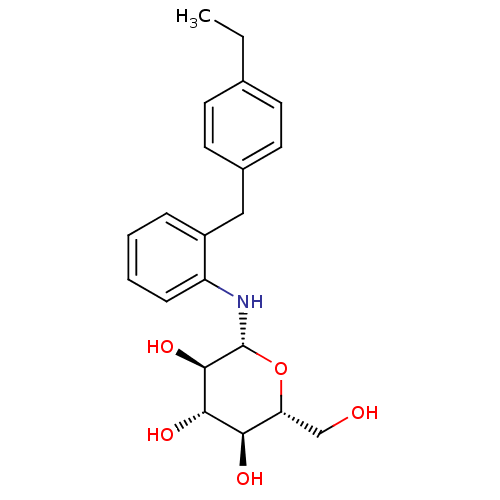
(CHEMBL2430315)Show SMILES CCc1ccc(Cc2ccccc2N[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H27NO5/c1-2-13-7-9-14(10-8-13)11-15-5-3-4-6-16(15)22-21-20(26)19(25)18(24)17(12-23)27-21/h3-10,17-26H,2,11-12H2,1H3/t17-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as [14-C]AMG uptake preincubated for 10 mins followed by [14-C]AMG addition measured after... |
Bioorg Med Chem Lett 23: 5641-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.042
BindingDB Entry DOI: 10.7270/Q2TX3GSM |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429596
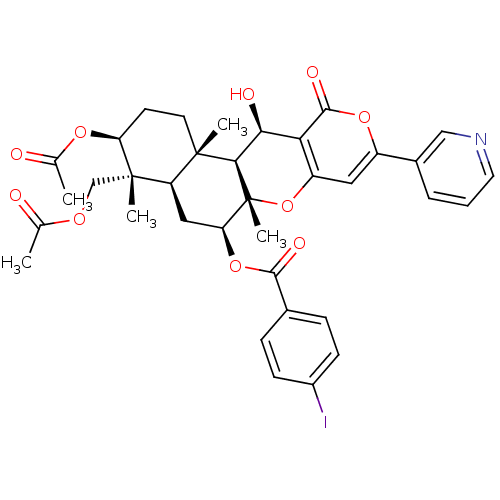
(CHEMBL2333813)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(I)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-10-23(37)11-9-21)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50446190
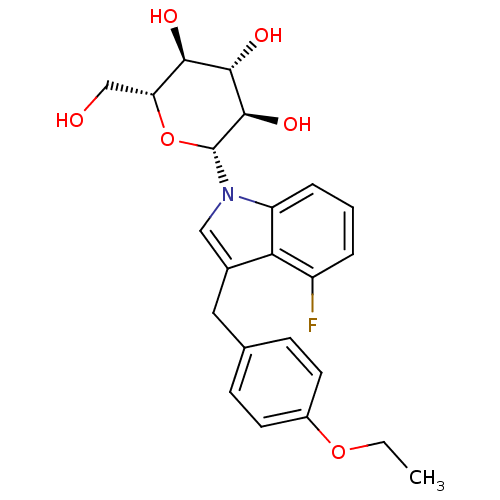
(CHEMBL3109019)Show SMILES CCOc1ccc(Cc2cn([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3cccc(F)c23)cc1 |r| Show InChI InChI=1S/C23H26FNO6/c1-2-30-15-8-6-13(7-9-15)10-14-11-25(17-5-3-4-16(24)19(14)17)23-22(29)21(28)20(27)18(12-26)31-23/h3-9,11,18,20-23,26-29H,2,10,12H2,1H3/t18-,20-,21+,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2-mediated [14C]-AMG uptake expressed in CHOK cells preincubated for 10 mins followed by [14C]-AMG addition measured after 12... |
ACS Med Chem Lett 5: 51-5 (2014)
Article DOI: 10.1021/ml400339b
BindingDB Entry DOI: 10.7270/Q24J0GM7 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429593
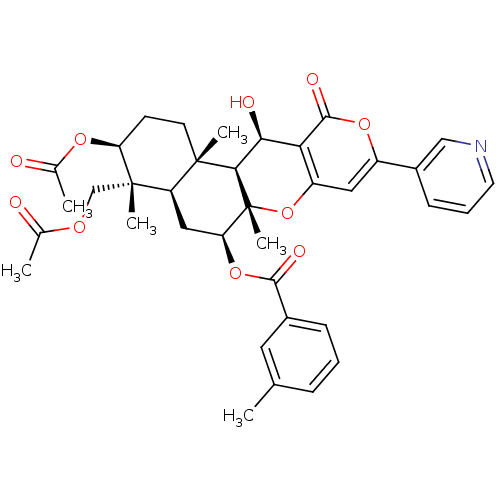
(CHEMBL2334196)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(C)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C37H41NO10/c1-20-9-7-10-23(15-20)33(42)47-29-17-27-35(4,13-12-28(45-22(3)40)36(27,5)19-44-21(2)39)32-31(41)30-26(48-37(29,32)6)16-25(46-34(30)43)24-11-8-14-38-18-24/h7-11,14-16,18,27-29,31-32,41H,12-13,17,19H2,1-6H3/t27-,28+,29+,31+,32-,35+,36+,37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429592
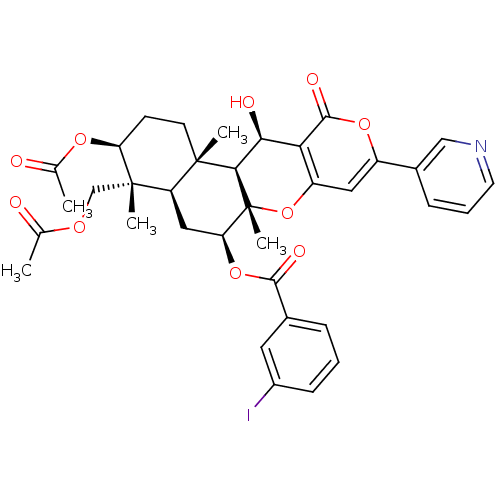
(CHEMBL2334197)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1cccc(I)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)21-8-6-10-23(37)14-21)36(5)31(34(26,3)12-11-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)22-9-7-13-38-17-22/h6-10,13-15,17,26-28,30-31,41H,11-12,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429590

(CHEMBL2334199)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)N=[N+]=[N-])[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38N4O10/c1-19(41)46-18-35(4)26-16-28(49-32(44)21-8-10-23(11-9-21)39-40-37)36(5)31(34(26,3)13-12-27(35)47-20(2)42)30(43)29-25(50-36)15-24(48-33(29)45)22-7-6-14-38-17-22/h6-11,14-15,17,26-28,30-31,43H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429594
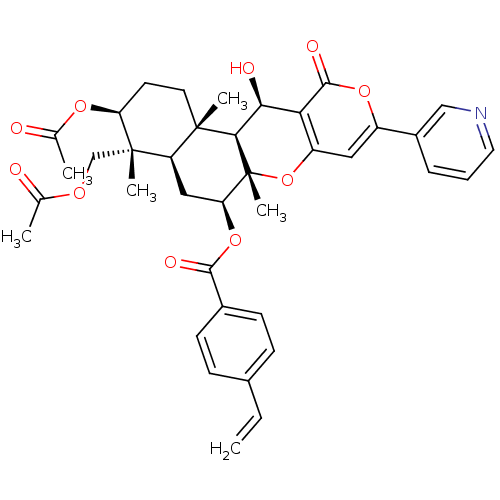
(CHEMBL2334195)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(C=C)cc1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C38H41NO10/c1-7-23-10-12-24(13-11-23)34(43)48-30-18-28-36(4,15-14-29(46-22(3)41)37(28,5)20-45-21(2)40)33-32(42)31-27(49-38(30,33)6)17-26(47-35(31)44)25-9-8-16-39-19-25/h7-13,16-17,19,28-30,32-33,42H,1,14-15,18,20H2,2-6H3/t28-,29+,30+,32+,33-,36+,37+,38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429595
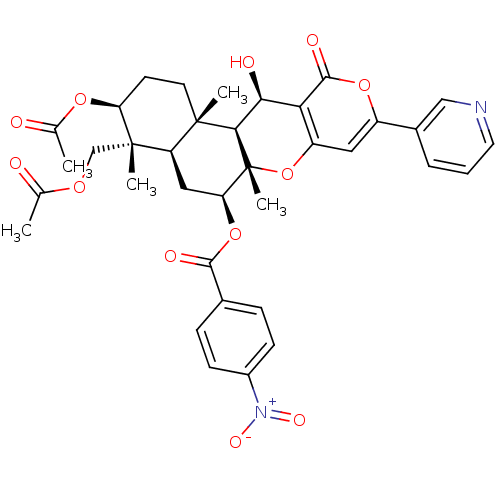
(CHEMBL2334194)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(cc1)[N+]([O-])=O)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38N2O12/c1-19(39)46-18-35(4)26-16-28(49-32(42)21-8-10-23(11-9-21)38(44)45)36(5)31(34(26,3)13-12-27(35)47-20(2)40)30(41)29-25(50-36)15-24(48-33(29)43)22-7-6-14-37-17-22/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429591

(CHEMBL2334198)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccccc1I)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H38INO10/c1-19(39)44-18-35(4)26-16-28(47-32(42)22-10-6-7-11-23(22)37)36(5)31(34(26,3)13-12-27(35)45-20(2)40)30(41)29-25(48-36)15-24(46-33(29)43)21-9-8-14-38-17-21/h6-11,14-15,17,26-28,30-31,41H,12-13,16,18H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50429577

(CHEMBL2334212)Show SMILES CC(=O)OC[C@]1(C)[C@H](CC[C@@]2(C)[C@H]1C[C@H](OC(=O)c1ccc(Br)c(F)c1)[C@@]1(C)Oc3cc(oc(=O)c3[C@H](O)[C@H]21)-c1cccnc1)OC(C)=O |r| Show InChI InChI=1S/C36H37BrFNO10/c1-18(40)45-17-35(4)26-15-28(48-32(43)20-8-9-22(37)23(38)13-20)36(5)31(34(26,3)11-10-27(35)46-19(2)41)30(42)29-25(49-36)14-24(47-33(29)44)21-7-6-12-39-16-21/h6-9,12-14,16,26-28,30-31,42H,10-11,15,17H2,1-5H3/t26-,27+,28+,30+,31-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 23: 1285-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.099
BindingDB Entry DOI: 10.7270/Q2P270HW |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381537

(CHEMBL2017954)Show SMILES CCc1ccc(Cc2cccc(c2)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H26O5/c1-2-13-6-8-14(9-7-13)10-15-4-3-5-16(11-15)21-20(25)19(24)18(23)17(12-22)26-21/h3-9,11,17-25H,2,10,12H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHOK cells assessed as [14-C]AMG uptake preincubated for 10 mins followed by [14-C]AMG addition measured after... |
Bioorg Med Chem Lett 23: 5641-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.042
BindingDB Entry DOI: 10.7270/Q2TX3GSM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data