 Found 4667 hits with Last Name = 'sur' and Initial = 's'
Found 4667 hits with Last Name = 'sur' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364856

(CHEMBL1950167)Show SMILES O=S1(=O)c2ccccc2-c2ccc(cc12)N1CCN2CCC1CC2 |(9.73,-11.78,;8.95,-13.11,;8.19,-11.77,;7.7,-14.01,;6.2,-13.68,;5.16,-14.83,;5.64,-16.29,;7.13,-16.62,;8.17,-15.48,;9.71,-15.48,;10.72,-16.64,;12.24,-16.34,;12.73,-14.89,;11.72,-13.72,;10.21,-14.03,;14.25,-14.59,;15.21,-15.86,;16.72,-15.87,;17.72,-14.73,;17.48,-13.28,;16.07,-12.5,;14.65,-13.09,;15.41,-14.42,;16.75,-13.64,)| Show InChI InChI=1S/C19H20N2O2S/c22-24(23)18-4-2-1-3-16(18)17-6-5-15(13-19(17)24)21-12-11-20-9-7-14(21)8-10-20/h1-6,13-14H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364875
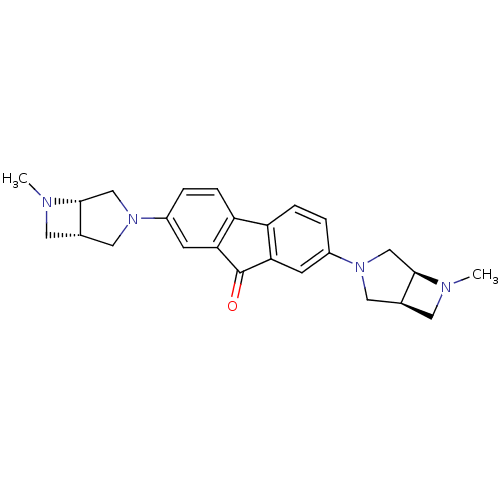
(CHEMBL1950156)Show SMILES CN1C[C@H]2CN(C[C@@H]12)c1ccc2-c3ccc(cc3C(=O)c2c1)N1C[C@@H]2CN(C)[C@@H]2C1 |r| Show InChI InChI=1S/C25H28N4O/c1-26-9-15-11-28(13-23(15)26)17-3-5-19-20-6-4-18(8-22(20)25(30)21(19)7-17)29-12-16-10-27(2)24(16)14-29/h3-8,15-16,23-24H,9-14H2,1-2H3/t15-,16-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364879

(CHEMBL1950160)Show SMILES O=C1c2ccccc2-c2ccc(cc12)N1CCN2CCC1CC2 |(21.84,6.38,;21.84,4.83,;20.58,3.93,;19.08,4.26,;18.04,3.11,;18.53,1.65,;20.02,1.33,;21.06,2.47,;22.59,2.47,;23.61,1.31,;25.12,1.6,;25.62,3.06,;24.61,4.22,;23.09,3.92,;27.13,3.35,;28.09,2.08,;29.6,2.07,;30.61,3.21,;30.36,4.67,;28.96,5.44,;27.53,4.85,;28.3,3.52,;29.63,4.3,)| Show InChI InChI=1S/C20H20N2O/c23-20-18-4-2-1-3-16(18)17-6-5-15(13-19(17)20)22-12-11-21-9-7-14(22)8-10-21/h1-6,13-14H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364874
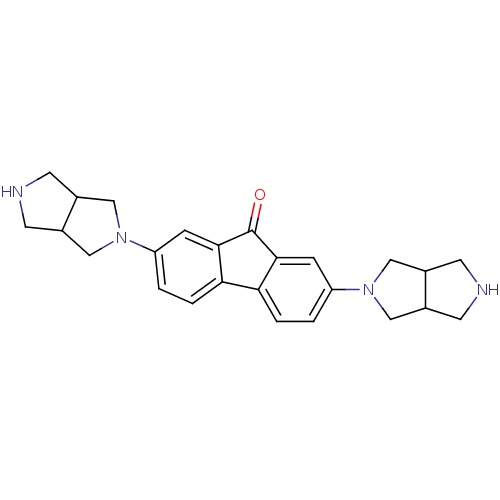
(CHEMBL1950155)Show SMILES O=C1c2cc(ccc2-c2ccc(cc12)N1CC2CNCC2C1)N1CC2CNCC2C1 Show InChI InChI=1S/C25H28N4O/c30-25-23-5-19(28-11-15-7-26-8-16(15)12-28)1-3-21(23)22-4-2-20(6-24(22)25)29-13-17-9-27-10-18(17)14-29/h1-6,15-18,26-27H,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364869
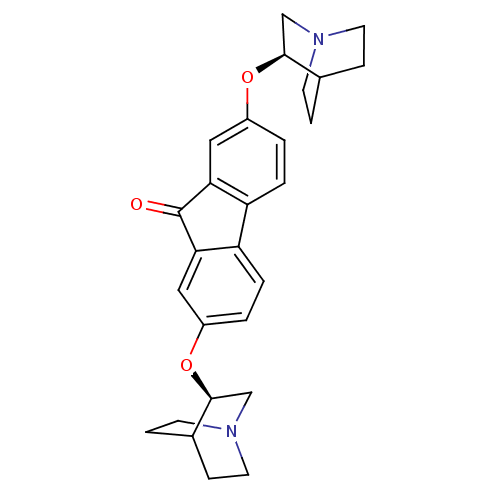
(CHEMBL1950150)Show SMILES O=C1c2cc(O[C@H]3CN4CCC3CC4)ccc2-c2ccc(O[C@H]3CN4CCC3CC4)cc12 |r,wD:6.5,22.24,(11.17,-6.37,;11.16,-7.92,;9.89,-8.83,;8.39,-8.51,;7.36,-9.65,;5.91,-9.1,;4.69,-9.8,;3.47,-9.08,;2.24,-9.77,;2.23,-11.2,;3.45,-11.92,;4.69,-11.22,;3.86,-10.06,;3.3,-11.36,;7.82,-11.13,;9.34,-11.46,;10.37,-10.31,;11.93,-10.32,;12.96,-11.48,;14.48,-11.15,;14.96,-9.67,;16.37,-9.47,;17.24,-10.58,;16.71,-11.89,;17.59,-13,;18.99,-12.8,;19.52,-11.48,;18.64,-10.37,;17.63,-11.36,;18.99,-11.72,;13.93,-8.53,;12.42,-8.83,)| Show InChI InChI=1S/C27H30N2O3/c30-27-23-13-19(31-25-15-28-9-5-17(25)6-10-28)1-3-21(23)22-4-2-20(14-24(22)27)32-26-16-29-11-7-18(26)8-12-29/h1-4,13-14,17-18,25-26H,5-12,15-16H2/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364889
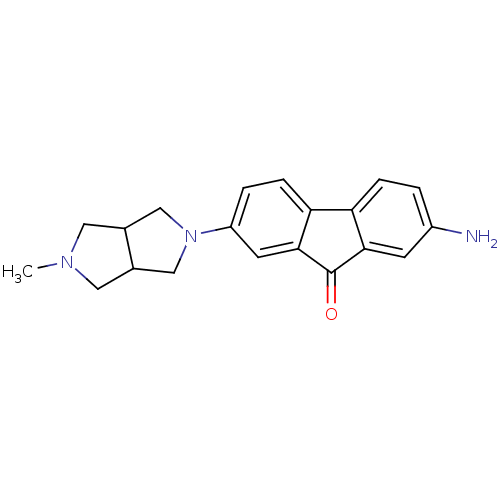
(CHEMBL1950172)Show InChI InChI=1S/C20H21N3O/c1-22-8-12-10-23(11-13(12)9-22)15-3-5-17-16-4-2-14(21)6-18(16)20(24)19(17)7-15/h2-7,12-13H,8-11,21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364873
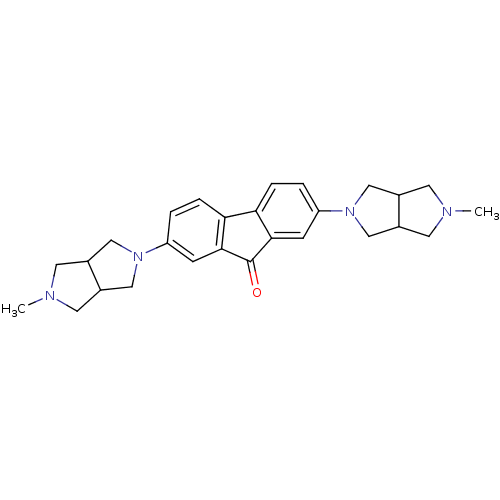
(CHEMBL1950154)Show SMILES CN1CC2CN(CC2C1)c1ccc2-c3ccc(cc3C(=O)c2c1)N1CC2CN(C)CC2C1 Show InChI InChI=1S/C27H32N4O/c1-28-9-17-13-30(14-18(17)10-28)21-3-5-23-24-6-4-22(8-26(24)27(32)25(23)7-21)31-15-19-11-29(2)12-20(19)16-31/h3-8,17-20H,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364870
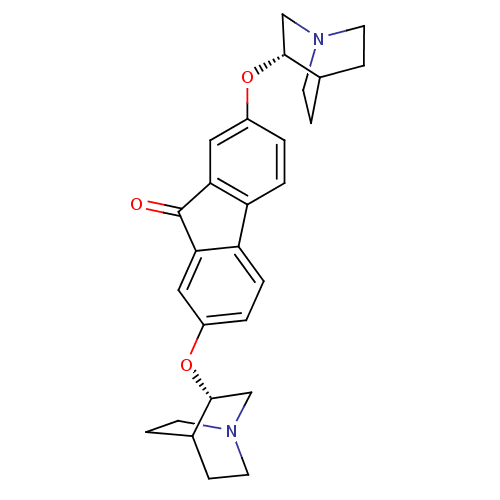
(CHEMBL1950151)Show SMILES O=C1c2cc(O[C@@H]3CN4CCC3CC4)ccc2-c2ccc(O[C@@H]3CN4CCC3CC4)cc12 |r,wU:6.5,22.24,(11.09,-6.31,;11.09,-7.85,;9.82,-8.77,;8.32,-8.45,;7.29,-9.58,;5.84,-9.03,;4.64,-9.76,;3.4,-9.08,;2.21,-9.81,;2.23,-11.22,;3.47,-11.9,;4.68,-11.17,;3.82,-10.05,;3.3,-11.35,;7.75,-11.05,;9.26,-11.38,;10.3,-10.24,;11.85,-10.24,;12.88,-11.4,;14.39,-11.09,;14.87,-9.61,;16.27,-9.4,;17.15,-10.51,;16.61,-11.81,;17.48,-12.91,;18.88,-12.72,;19.4,-11.41,;18.53,-10.3,;17.53,-11.28,;18.88,-11.64,;13.84,-8.46,;12.33,-8.77,)| Show InChI InChI=1S/C27H30N2O3/c30-27-23-13-19(31-25-15-28-9-5-17(25)6-10-28)1-3-21(23)22-4-2-20(14-24(22)27)32-26-16-29-11-7-18(26)8-12-29/h1-4,13-14,17-18,25-26H,5-12,15-16H2/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364877
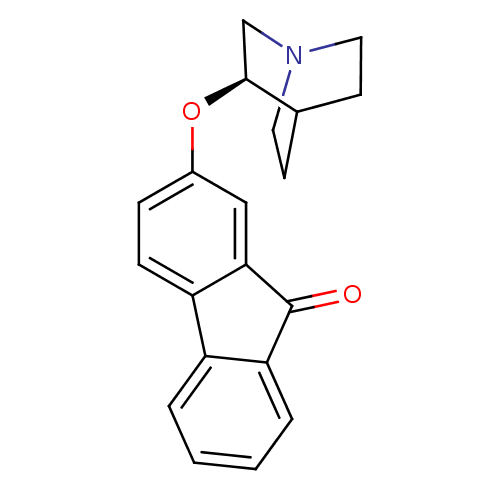
(CHEMBL1950158)Show SMILES O=C1c2ccccc2-c2ccc(O[C@H]3CN4CCC3CC4)cc12 |r,wD:13.13,(-6.3,5.83,;-6.31,4.29,;-7.56,3.39,;-9.06,3.71,;-10.1,2.57,;-9.61,1.11,;-8.12,.78,;-7.08,1.92,;-5.55,1.92,;-4.54,.76,;-3.02,1.06,;-2.53,2.51,;-1.02,2.81,;-0,1.65,;-.51,.19,;.5,-.97,;2.01,-.68,;2.52,.78,;1.5,1.95,;.41,.86,;1.74,.09,;-3.54,3.67,;-5.05,3.37,)| Show InChI InChI=1S/C20H19NO2/c22-20-17-4-2-1-3-15(17)16-6-5-14(11-18(16)20)23-19-12-21-9-7-13(19)8-10-21/h1-6,11,13,19H,7-10,12H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364884
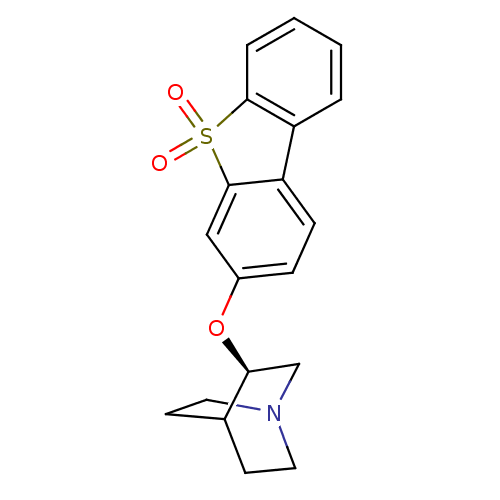
(CHEMBL1950165)Show SMILES O=S1(=O)c2ccccc2-c2ccc(O[C@H]3CN4CCC3CC4)cc12 |r,wD:14.14,(46.6,-11.14,;45.82,-12.47,;45.06,-11.13,;44.57,-13.37,;43.07,-13.04,;42.03,-14.19,;42.51,-15.65,;44.01,-15.98,;45.04,-14.84,;46.58,-14.84,;47.59,-16,;49.11,-15.7,;49.6,-14.25,;51.11,-13.95,;52.13,-15.11,;51.62,-16.57,;52.63,-17.73,;54.15,-17.44,;54.65,-15.98,;53.64,-14.81,;52.54,-15.89,;53.87,-16.67,;48.59,-13.08,;47.08,-13.39,)| Show InChI InChI=1S/C19H19NO3S/c21-24(22)18-4-2-1-3-15(18)16-6-5-14(11-19(16)24)23-17-12-20-9-7-13(17)8-10-20/h1-6,11,13,17H,7-10,12H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364857
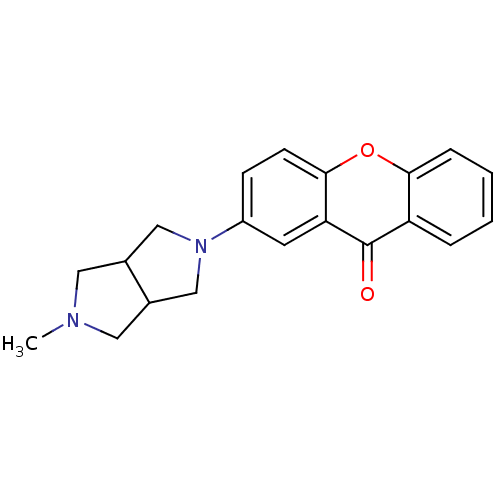
(CHEMBL1950171)Show InChI InChI=1S/C20H20N2O2/c1-21-9-13-11-22(12-14(13)10-21)15-6-7-19-17(8-15)20(23)16-4-2-3-5-18(16)24-19/h2-8,13-14H,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364864

(CHEMBL1950145)Show SMILES CN1CC[C@@H]1COc1ccc2-c3ccc(OC[C@H]4CCN4C)cc3C(=O)c2c1 |r| Show InChI InChI=1S/C23H26N2O3/c1-24-9-7-15(24)13-27-17-3-5-19-20-6-4-18(28-14-16-8-10-25(16)2)12-22(20)23(26)21(19)11-17/h3-6,11-12,15-16H,7-10,13-14H2,1-2H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364881
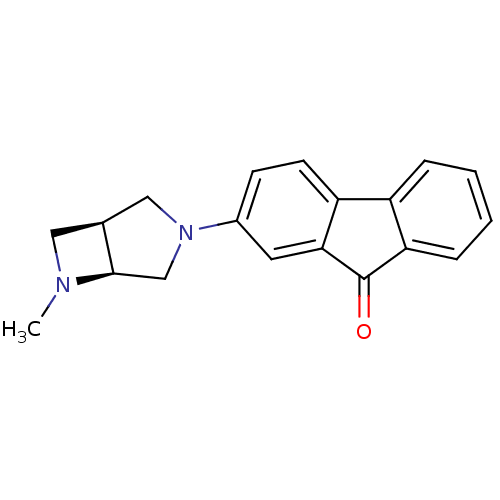
(CHEMBL1950162)Show SMILES CN1C[C@H]2CN(C[C@@H]12)c1ccc2-c3ccccc3C(=O)c2c1 |r| Show InChI InChI=1S/C19H18N2O/c1-20-9-12-10-21(11-18(12)20)13-6-7-15-14-4-2-3-5-16(14)19(22)17(15)8-13/h2-8,12,18H,9-11H2,1H3/t12-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364872
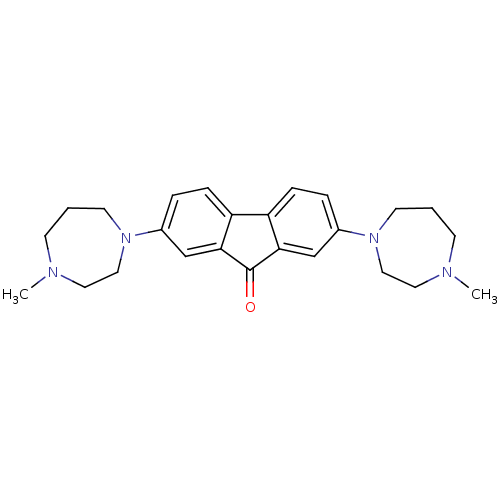
(CHEMBL1950153)Show SMILES CN1CCCN(CC1)c1ccc2-c3ccc(cc3C(=O)c2c1)N1CCCN(C)CC1 Show InChI InChI=1S/C25H32N4O/c1-26-9-3-11-28(15-13-26)19-5-7-21-22-8-6-20(18-24(22)25(30)23(21)17-19)29-12-4-10-27(2)14-16-29/h5-8,17-18H,3-4,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Escherichia coli (strain K12)) | BDBM50226181
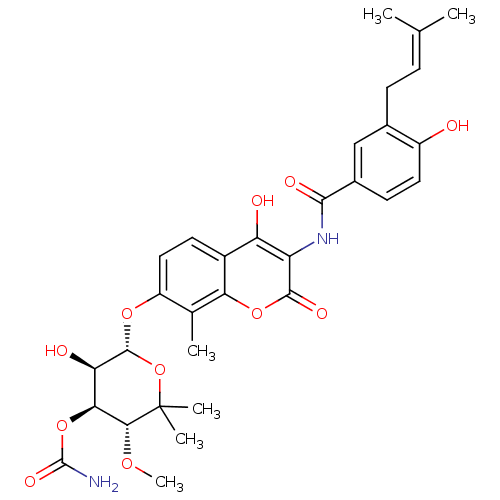
((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli H560 DNA gyrase B ATPase activity after 30 mins by ammonium molybdate/malachite green-based phosphate detection assay |
Eur J Med Chem 124: 160-185 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.034
BindingDB Entry DOI: 10.7270/Q2K64M38 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20458
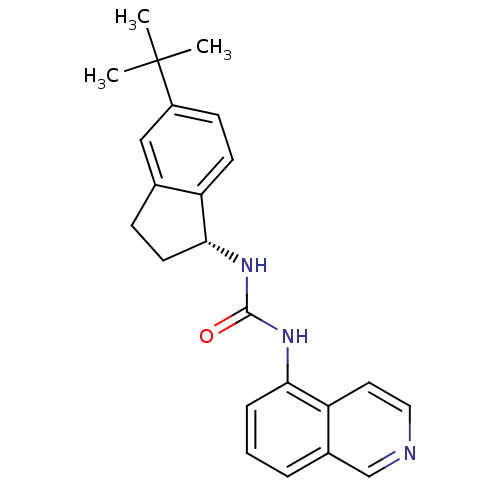
(1-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C23H25N3O/c1-23(2,3)17-8-9-18-15(13-17)7-10-21(18)26-22(27)25-20-6-4-5-16-14-24-12-11-19(16)20/h4-6,8-9,11-14,21H,7,10H2,1-3H3,(H2,25,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | 5 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364863
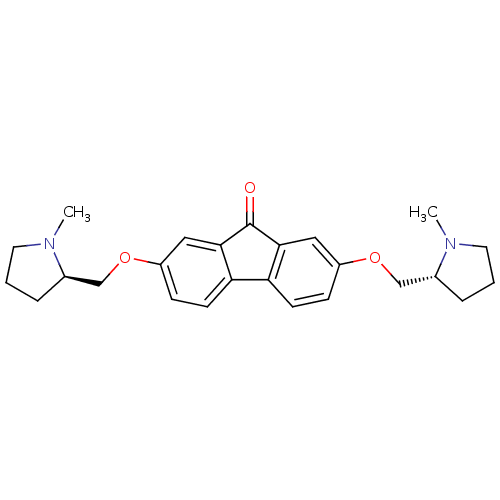
(CHEMBL1950144)Show SMILES CN1CCC[C@@H]1COc1ccc2-c3ccc(OC[C@H]4CCCN4C)cc3C(=O)c2c1 |r| Show InChI InChI=1S/C25H30N2O3/c1-26-11-3-5-17(26)15-29-19-7-9-21-22-10-8-20(14-24(22)25(28)23(21)13-19)30-16-18-6-4-12-27(18)2/h7-10,13-14,17-18H,3-6,11-12,15-16H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364887

(CHEMBL1950169)Show SMILES O=c1c2ccccc2oc2ccc(O[C@H]3CN4CCC3CC4)cc12 |r,wD:14.14,(-6.85,-19.93,;-6.89,-21.47,;-8.24,-22.21,;-9.55,-21.41,;-10.91,-22.15,;-10.93,-23.68,;-9.63,-24.48,;-8.28,-23.75,;-6.97,-24.55,;-5.58,-23.81,;-4.24,-24.57,;-2.91,-23.79,;-2.92,-22.26,;-1.59,-21.48,;-.25,-22.24,;-.25,-23.78,;1.08,-24.54,;2.42,-23.76,;2.41,-22.22,;1.07,-21.45,;.39,-22.84,;1.9,-23.13,;-4.26,-21.49,;-5.59,-22.28,)| Show InChI InChI=1S/C20H19NO3/c22-20-15-3-1-2-4-17(15)24-18-6-5-14(11-16(18)20)23-19-12-21-9-7-13(19)8-10-21/h1-6,11,13,19H,7-10,12H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364888
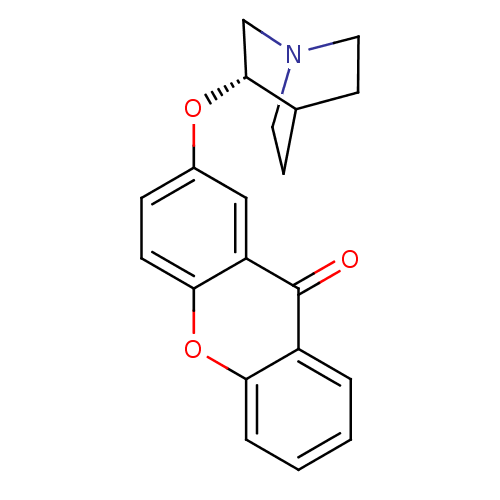
(CHEMBL1950170)Show SMILES O=c1c2ccccc2oc2ccc(O[C@@H]3CN4CCC3CC4)cc12 |r,wU:14.14,(8.61,-19.89,;8.6,-21.43,;7.28,-22.19,;5.96,-21.39,;4.61,-22.15,;4.6,-23.69,;5.91,-24.48,;7.26,-23.72,;8.6,-24.48,;9.91,-23.71,;11.25,-24.49,;12.59,-23.71,;12.58,-22.18,;13.92,-21.41,;15.25,-22.18,;15.25,-23.72,;16.57,-24.49,;17.92,-23.73,;17.92,-22.18,;16.58,-21.4,;15.89,-22.79,;17.4,-23.09,;11.25,-21.4,;9.92,-22.19,)| Show InChI InChI=1S/C20H19NO3/c22-20-15-3-1-2-4-17(15)24-18-6-5-14(11-16(18)20)23-19-12-21-9-7-13(19)8-10-21/h1-6,11,13,19H,7-10,12H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17786
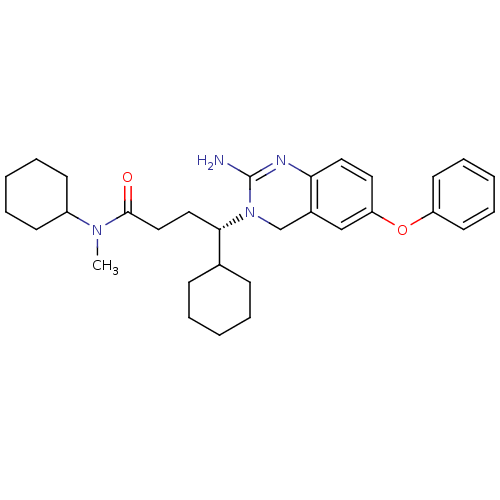
((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...)Show SMILES CN(C1CCCCC1)C(=O)CC[C@@H](C1CCCCC1)N1Cc2cc(Oc3ccccc3)ccc2N=C1N |r,c:38| Show InChI InChI=1S/C31H42N4O2/c1-34(25-13-7-3-8-14-25)30(36)20-19-29(23-11-5-2-6-12-23)35-22-24-21-27(17-18-28(24)33-31(35)32)37-26-15-9-4-10-16-26/h4,9-10,15-18,21,23,25,29H,2-3,5-8,11-14,19-20,22H2,1H3,(H2,32,33)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364878
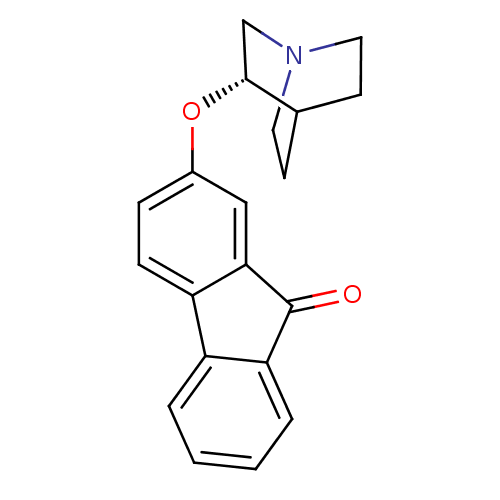
(CHEMBL1950159)Show SMILES O=C1c2ccccc2-c2ccc(O[C@@H]3CN4CCC3CC4)cc12 |r,wU:13.13,(7.41,6.4,;7.41,4.86,;6.15,3.96,;4.66,4.29,;3.62,3.14,;4.1,1.68,;5.59,1.36,;6.63,2.5,;8.16,2.5,;9.17,1.34,;10.69,1.63,;11.18,3.09,;12.7,3.38,;13.71,2.22,;13.2,.77,;14.21,-.39,;15.73,-.1,;16.23,1.36,;15.22,2.53,;14.12,1.44,;15.45,.67,;10.18,4.25,;8.66,3.94,)| Show InChI InChI=1S/C20H19NO2/c22-20-17-4-2-1-3-15(17)16-6-5-14(11-18(16)20)23-19-12-21-9-7-13(19)8-10-21/h1-6,11,13,19H,7-10,12H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364862
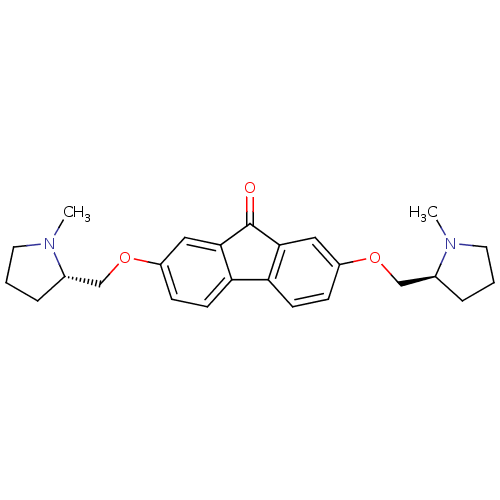
(CHEMBL1950143)Show SMILES CN1CCC[C@H]1COc1ccc2-c3ccc(OC[C@@H]4CCCN4C)cc3C(=O)c2c1 |r| Show InChI InChI=1S/C25H30N2O3/c1-26-11-3-5-17(26)15-29-19-7-9-21-22-10-8-20(14-24(22)25(28)23(21)13-19)30-16-18-6-4-12-27(18)2/h7-10,13-14,17-18H,3-6,11-12,15-16H2,1-2H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364866
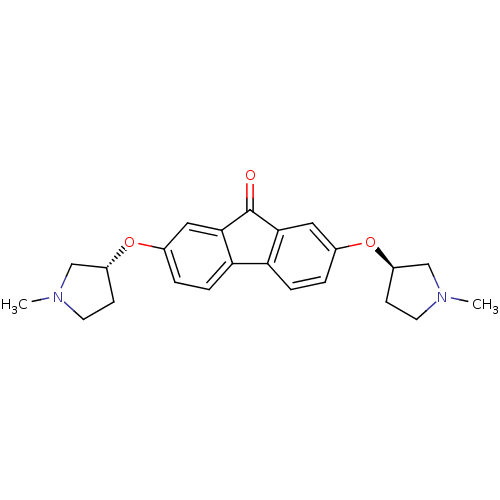
(CHEMBL1950147)Show SMILES CN1CC[C@H](C1)Oc1ccc2-c3ccc(O[C@@H]4CCN(C)C4)cc3C(=O)c2c1 |r| Show InChI InChI=1S/C23H26N2O3/c1-24-9-7-17(13-24)27-15-3-5-19-20-6-4-16(28-18-8-10-25(2)14-18)12-22(20)23(26)21(19)11-15/h3-6,11-12,17-18H,7-10,13-14H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364905
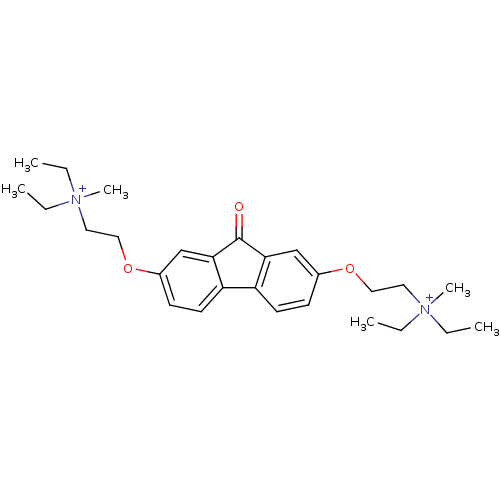
(CHEMBL1950136)Show SMILES CC[N+](C)(CC)CCOc1ccc2-c3ccc(OCC[N+](C)(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C27H40N2O3/c1-7-28(5,8-2)15-17-31-21-11-13-23-24-14-12-22(20-26(24)27(30)25(23)19-21)32-18-16-29(6,9-3)10-4/h11-14,19-20H,7-10,15-18H2,1-6H3/q+2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364876
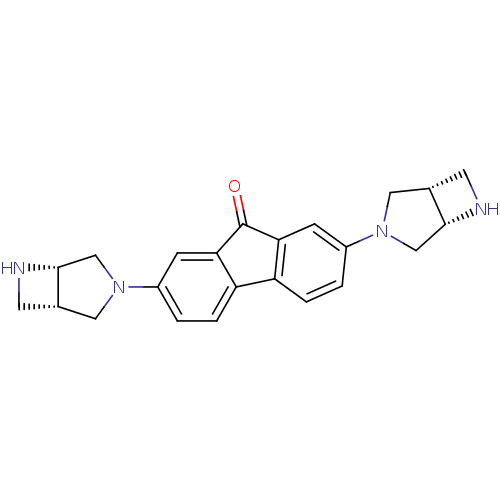
(CHEMBL1950157)Show SMILES O=C1c2cc(ccc2-c2ccc(cc12)N1C[C@@H]2CN[C@@H]2C1)N1C[C@@H]2CN[C@@H]2C1 |r| Show InChI InChI=1S/C23H24N4O/c28-23-19-5-15(26-9-13-7-24-21(13)11-26)1-3-17(19)18-4-2-16(6-20(18)23)27-10-14-8-25-22(14)12-27/h1-6,13-14,21-22,24-25H,7-12H2/t13-,14-,21+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364886
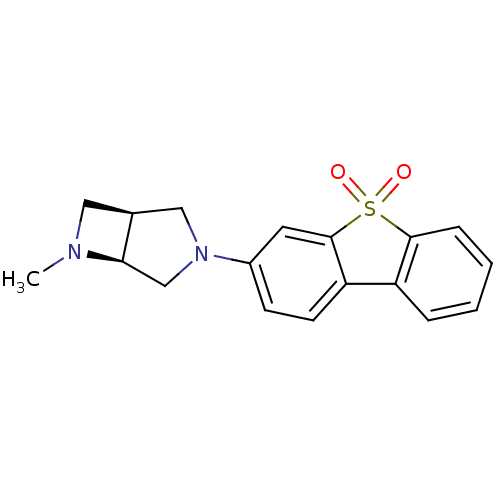
(CHEMBL1950168)Show SMILES CN1C[C@H]2CN(C[C@@H]12)c1ccc2-c3ccccc3S(=O)(=O)c2c1 |r| Show InChI InChI=1S/C18H18N2O2S/c1-19-9-12-10-20(11-16(12)19)13-6-7-15-14-4-2-3-5-17(14)23(21,22)18(15)8-13/h2-8,12,16H,9-11H2,1H3/t12-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20464
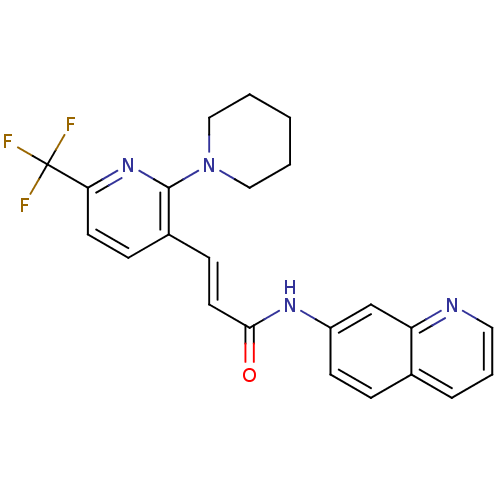
((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)Nc2ccc3cccnc3c2)c(n1)N1CCCCC1 Show InChI InChI=1S/C23H21F3N4O/c24-23(25,26)20-10-7-17(22(29-20)30-13-2-1-3-14-30)8-11-21(31)28-18-9-6-16-5-4-12-27-19(16)15-18/h4-12,15H,1-3,13-14H2,(H,28,31)/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | -43.2 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165996
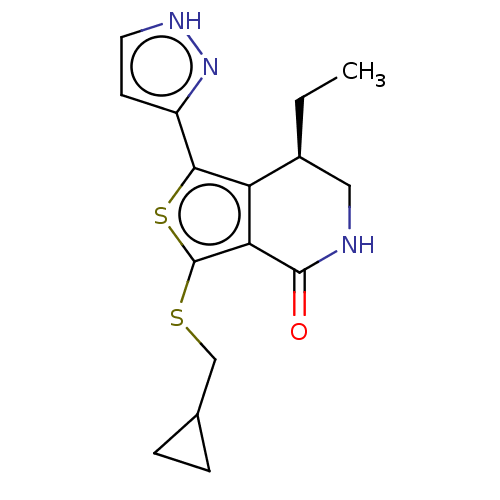
(CHEMBL3797298)Show SMILES CC[C@H]1CNC(=O)c2c(SCC3CC3)sc(-c3cc[nH]n3)c12 |r| Show InChI InChI=1S/C16H19N3OS2/c1-2-10-7-17-15(20)13-12(10)14(11-5-6-18-19-11)22-16(13)21-8-9-3-4-9/h5-6,9-10H,2-4,7-8H2,1H3,(H,17,20)(H,18,19)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364885
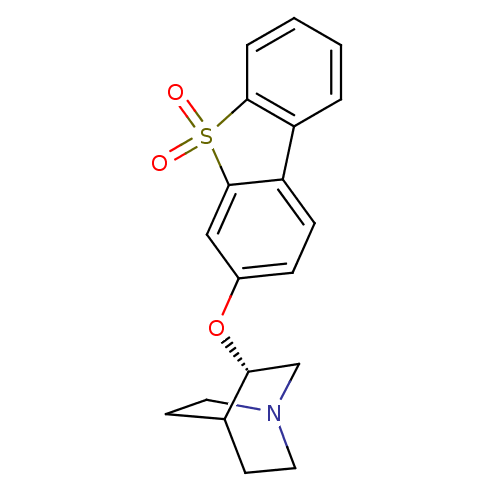
(CHEMBL1950166)Show SMILES O=S1(=O)c2ccccc2-c2ccc(O[C@@H]3CN4CCC3CC4)cc12 |r,wU:14.14,(-4.86,-11.54,;-5.64,-12.87,;-6.4,-11.53,;-6.89,-13.77,;-8.39,-13.44,;-9.43,-14.59,;-8.95,-16.05,;-7.45,-16.38,;-6.42,-15.23,;-4.88,-15.23,;-3.87,-16.4,;-2.35,-16.1,;-1.86,-14.64,;-.35,-14.35,;.67,-15.51,;.16,-16.96,;1.17,-18.12,;2.68,-17.83,;3.19,-16.37,;2.17,-15.2,;1.08,-16.29,;2.41,-17.06,;-2.87,-13.48,;-4.39,-13.79,)| Show InChI InChI=1S/C19H19NO3S/c21-24(22)18-4-2-1-3-15(18)16-6-5-14(11-19(16)24)23-17-12-20-9-7-13(17)8-10-20/h1-6,11,13,17H,7-10,12H2/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165997
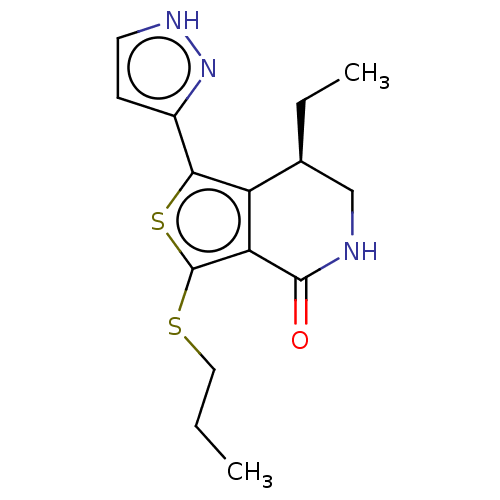
(CHEMBL3797431)Show SMILES CCCSc1sc(-c2cc[nH]n2)c2[C@@H](CC)CNC(=O)c12 |r| Show InChI InChI=1S/C15H19N3OS2/c1-3-7-20-15-12-11(9(4-2)8-16-14(12)19)13(21-15)10-5-6-17-18-10/h5-6,9H,3-4,7-8H2,1-2H3,(H,16,19)(H,17,18)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17785
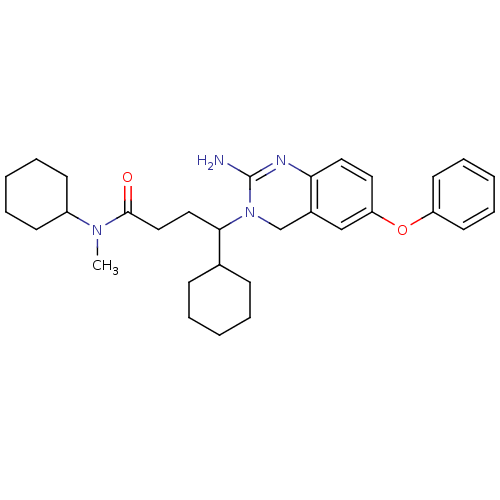
(2-aminoquinazoline, 3 | 4-(2-amino-6-phenoxy-3,4-d...)Show SMILES CN(C1CCCCC1)C(=O)CCC(C1CCCCC1)N1Cc2cc(Oc3ccccc3)ccc2N=C1N |c:38| Show InChI InChI=1S/C31H42N4O2/c1-34(25-13-7-3-8-14-25)30(36)20-19-29(23-11-5-2-6-12-23)35-22-24-21-27(17-18-28(24)33-31(35)32)37-26-15-9-4-10-16-26/h4,9-10,15-18,21,23,25,29H,2-3,5-8,11-14,19-20,22H2,1H3,(H2,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165998

(CHEMBL3800405)Show SMILES C[C@H]1CNC(=O)c2c(SCC3CC3)sc(-c3cc[nH]n3)c12 |r| Show InChI InChI=1S/C15H17N3OS2/c1-8-6-16-14(19)12-11(8)13(10-4-5-17-18-10)21-15(12)20-7-9-2-3-9/h4-5,8-9H,2-3,6-7H2,1H3,(H,16,19)(H,17,18)/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364867
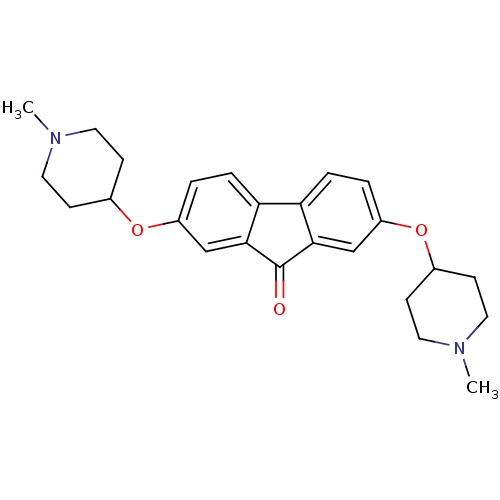
(CHEMBL1950148)Show SMILES CN1CCC(CC1)Oc1ccc2-c3ccc(OC4CCN(C)CC4)cc3C(=O)c2c1 Show InChI InChI=1S/C25H30N2O3/c1-26-11-7-17(8-12-26)29-19-3-5-21-22-6-4-20(16-24(22)25(28)23(21)15-19)30-18-9-13-27(2)14-10-18/h3-6,15-18H,7-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165999

(CHEMBL3797869)Show SMILES CCCSc1sc(-c2cc[nH]n2)c2[C@@H](C)CNC(=O)c12 |r| Show InChI InChI=1S/C14H17N3OS2/c1-3-6-19-14-11-10(8(2)7-15-13(11)18)12(20-14)9-4-5-16-17-9/h4-5,8H,3,6-7H2,1-2H3,(H,15,18)(H,16,17)/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50166012

(CHEMBL3800129)Show SMILES CC[C@H]1CNC(=O)c2c(SC)sc(-c3cc[nH]n3)c12 |r| Show InChI InChI=1S/C13H15N3OS2/c1-3-7-6-14-12(17)10-9(7)11(19-13(10)18-2)8-4-5-15-16-8/h4-5,7H,3,6H2,1-2H3,(H,14,17)(H,15,16)/t7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20334
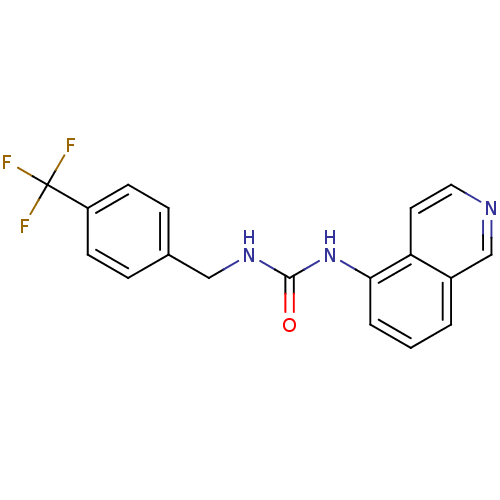
(1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-ur...)Show InChI InChI=1S/C18H14F3N3O/c19-18(20,21)14-6-4-12(5-7-14)10-23-17(25)24-16-3-1-2-13-11-22-9-8-15(13)16/h1-9,11H,10H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | -41.8 | n/a | n/a | 11 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364858

(CHEMBL1950139)Show InChI InChI=1S/C21H26N2O3/c1-22(2)9-11-25-15-5-7-17-18-8-6-16(26-12-10-23(3)4)14-20(18)21(24)19(17)13-15/h5-8,13-14H,9-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485783
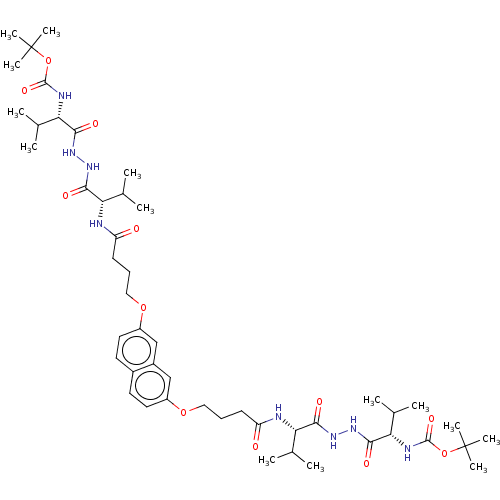
(CHEMBL2164408)Show SMILES CC(C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)NNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)cc2c1)C(=O)NNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C |r| Show InChI InChI=1S/C48H76N8O12/c1-27(2)37(41(59)53-55-43(61)39(29(5)6)51-45(63)67-47(9,10)11)49-35(57)17-15-23-65-33-21-19-31-20-22-34(26-32(31)25-33)66-24-16-18-36(58)50-38(28(3)4)42(60)54-56-44(62)40(30(7)8)52-46(64)68-48(12,13)14/h19-22,25-30,37-40H,15-18,23-24H2,1-14H3,(H,49,57)(H,50,58)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,61)(H,56,62)/t37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364860
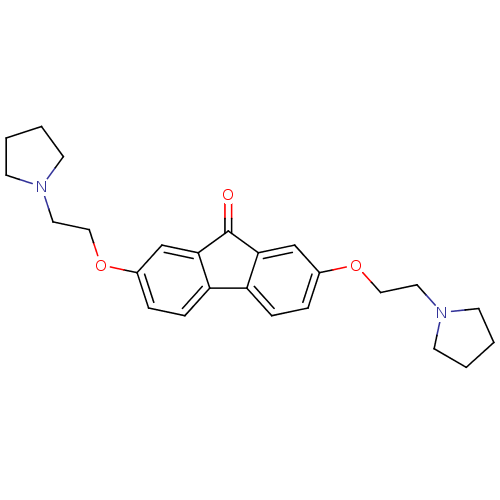
(CHEMBL1950141)Show SMILES O=C1c2cc(OCCN3CCCC3)ccc2-c2ccc(OCCN3CCCC3)cc12 Show InChI InChI=1S/C25H30N2O3/c28-25-23-17-19(29-15-13-26-9-1-2-10-26)5-7-21(23)22-8-6-20(18-24(22)25)30-16-14-27-11-3-4-12-27/h5-8,17-18H,1-4,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50166070

(CHEMBL3798994)Show SMILES CSc1sc(-c2cc[nH]n2)c2C[C@H](NC(=O)c12)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3OS2/c1-22-17-14-11(15(23-17)12-7-8-18-20-12)9-13(19-16(14)21)10-5-3-2-4-6-10/h2-8,13H,9H2,1H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364890
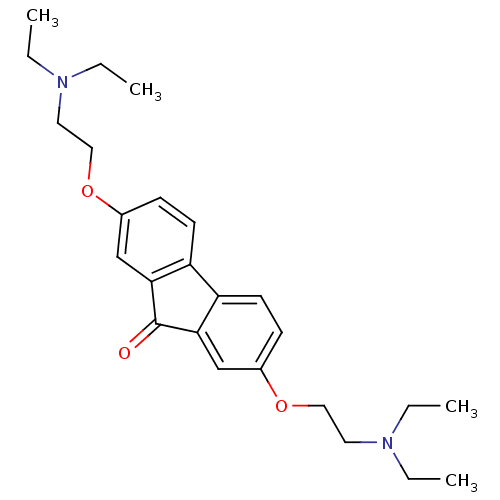
(Amixin IC | TILORONE | cid_5475)Show SMILES CCN(CC)CCOc1ccc2-c3ccc(OCCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C25H34N2O3/c1-5-26(6-2)13-15-29-19-9-11-21-22-12-10-20(30-16-14-27(7-3)8-4)18-24(22)25(28)23(21)17-19/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165996

(CHEMBL3797298)Show SMILES CC[C@H]1CNC(=O)c2c(SCC3CC3)sc(-c3cc[nH]n3)c12 |r| Show InChI InChI=1S/C16H19N3OS2/c1-2-10-7-17-15(20)13-12(10)14(11-5-6-18-19-11)22-16(13)21-8-9-3-4-9/h5-6,9-10H,2-4,7-8H2,1H3,(H,17,20)(H,18,19)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant wild type LRRK2 using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20465
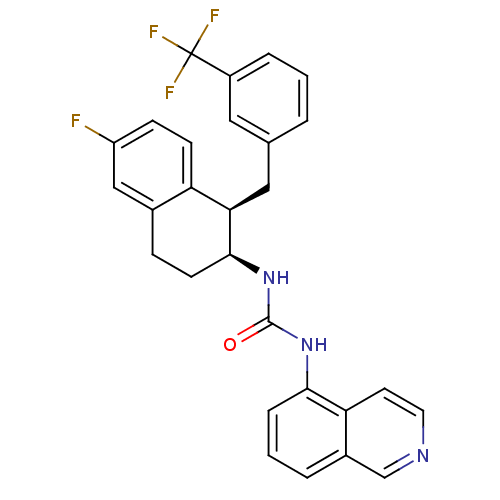
(1-[(1R,2S)-6-fluoro-1-{[3-(trifluoromethyl)phenyl]...)Show SMILES Fc1ccc2[C@@H](Cc3cccc(c3)C(F)(F)F)[C@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C28H23F4N3O/c29-21-8-9-22-18(15-21)7-10-26(24(22)14-17-3-1-5-20(13-17)28(30,31)32)35-27(36)34-25-6-2-4-19-16-33-12-11-23(19)25/h1-6,8-9,11-13,15-16,24,26H,7,10,14H2,(H2,34,35,36)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | 46 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364880
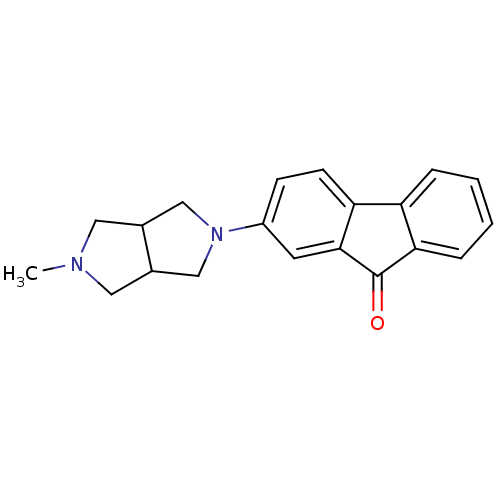
(CHEMBL1950161)Show InChI InChI=1S/C20H20N2O/c1-21-9-13-11-22(12-14(13)10-21)15-6-7-17-16-4-2-3-5-18(16)20(23)19(17)8-15/h2-8,13-14H,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165978

(CHEMBL3800284)Show SMILES CSc1sc(-c2cc[nH]n2)c2C[C@@H](NC(=O)c12)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3OS2/c1-22-17-14-11(15(23-17)12-7-8-18-20-12)9-13(19-16(14)21)10-5-3-2-4-6-10/h2-8,13H,9H2,1H3,(H,18,20)(H,19,21)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LRRK2 G2019S mutant using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20285
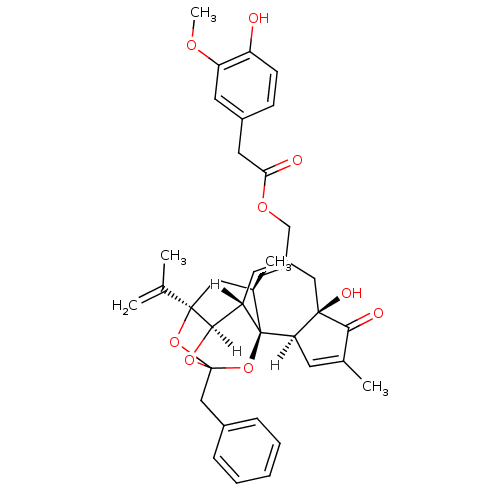
(Resiniferatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-b...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3ccc(O)c(OC)c3)=C[C@@]21[H])C(C)=C |c:47,t:23,TLB:11:3:12.14.13:44,THB:4:3:12.14.13:44| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35-,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | -41.0 | n/a | n/a | 24 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50165997

(CHEMBL3797431)Show SMILES CCCSc1sc(-c2cc[nH]n2)c2[C@@H](CC)CNC(=O)c12 |r| Show InChI InChI=1S/C15H19N3OS2/c1-3-7-20-15-12-11(9(4-2)8-16-14(12)19)13(21-15)10-5-6-17-18-10/h5-6,9H,3-4,7-8H2,1-2H3,(H,16,19)(H,17,18)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant wild type LRRK2 using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20466
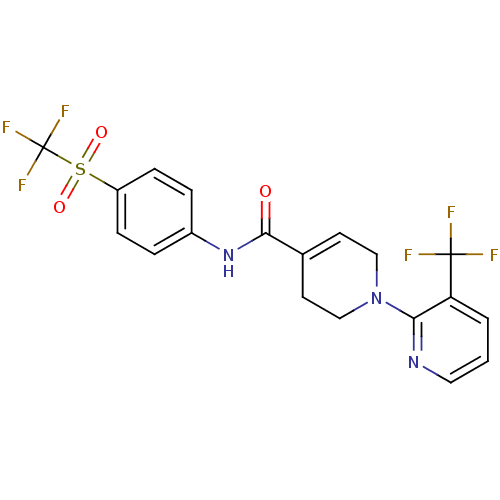
(A-784168 | CHEMBL482834 | N-[4-(trifluoromethane)s...)Show SMILES FC(F)(F)c1cccnc1N1CCC(=CC1)C(=O)Nc1ccc(cc1)S(=O)(=O)C(F)(F)F |c:14| Show InChI InChI=1S/C19H15F6N3O3S/c20-18(21,22)15-2-1-9-26-16(15)28-10-7-12(8-11-28)17(29)27-13-3-5-14(6-4-13)32(30,31)19(23,24)25/h1-7,9H,8,10-11H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | -40.8 | n/a | n/a | 74 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364865
(CHEMBL1950146)Show SMILES O=C1c2cc(O[C@@H]3CCNC3)ccc2-c2ccc(O[C@@H]3CCNC3)cc12 |r| Show InChI InChI=1S/C21H22N2O3/c24-21-19-9-13(25-15-5-7-22-11-15)1-3-17(19)18-4-2-14(10-20(18)21)26-16-6-8-23-12-16/h1-4,9-10,15-16,22-23H,5-8,11-12H2/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50166012

(CHEMBL3800129)Show SMILES CC[C@H]1CNC(=O)c2c(SC)sc(-c3cc[nH]n3)c12 |r| Show InChI InChI=1S/C13H15N3OS2/c1-3-7-6-14-12(17)10-9(7)11(19-13(10)18-2)8-4-5-15-16-8/h4-5,7H,3,6H2,1-2H3,(H,14,17)(H,15,16)/t7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant wild type LRRK2 using fluorescein- ERM as substrate after 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 26: 2631-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.021
BindingDB Entry DOI: 10.7270/Q2RV0QKC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data