 Found 120 hits with Last Name = 'jones' and Initial = 'sb'
Found 120 hits with Last Name = 'jones' and Initial = 'sb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM31046
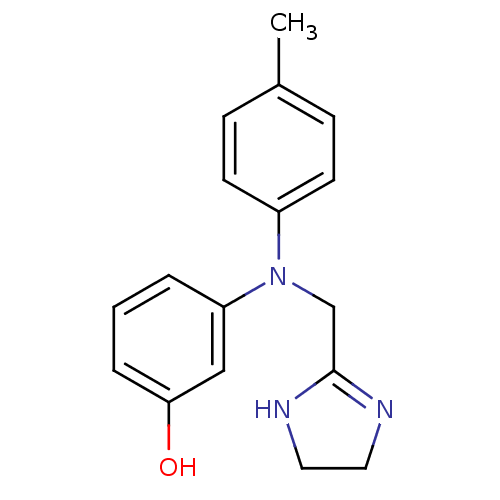
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM31046

(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM31046

(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM31046

(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM30712
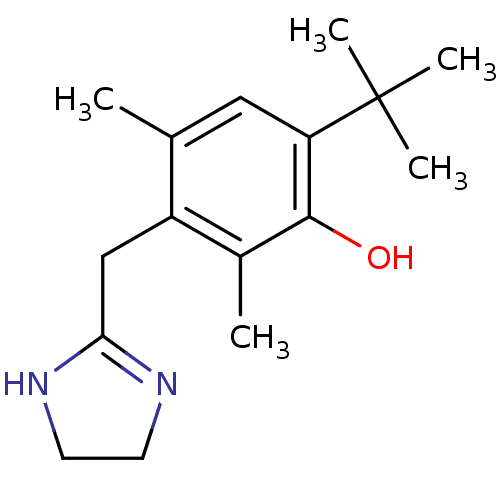
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM84342

(4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM50016897
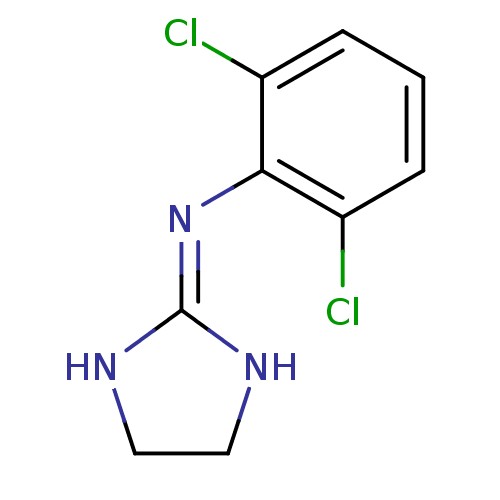
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM36024
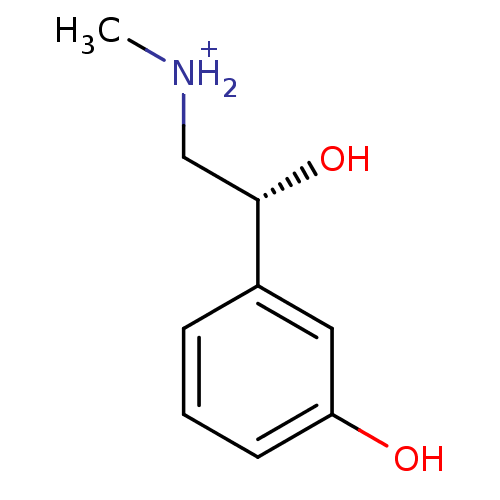
((R)-(-)-phenylephrine | PHENYLEPHRINE | Phenylephr...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/p+1/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM84342

(4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol...)Show InChI InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM36024

((R)-(-)-phenylephrine | PHENYLEPHRINE | Phenylephr...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/p+1/t9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM36024

((R)-(-)-phenylephrine | PHENYLEPHRINE | Phenylephr...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/p+1/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM36024

((R)-(-)-phenylephrine | PHENYLEPHRINE | Phenylephr...)Show InChI InChI=1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/p+1/t9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 901 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
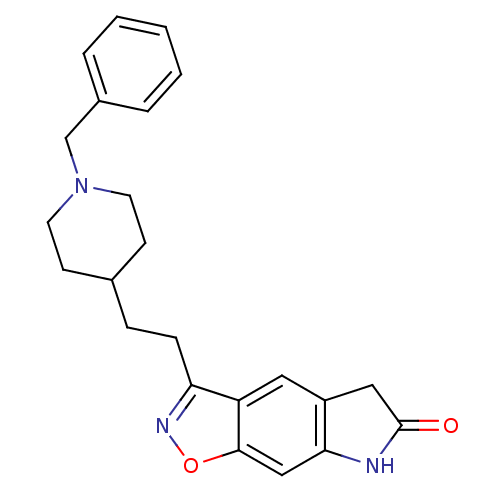
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032161
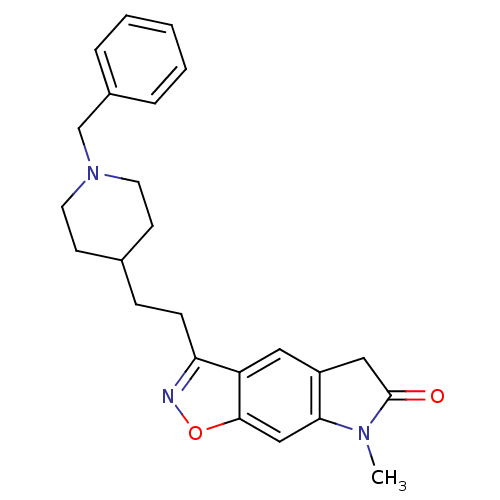
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-23-20(13-19(22)14-24(26)28)21(25-29-23)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
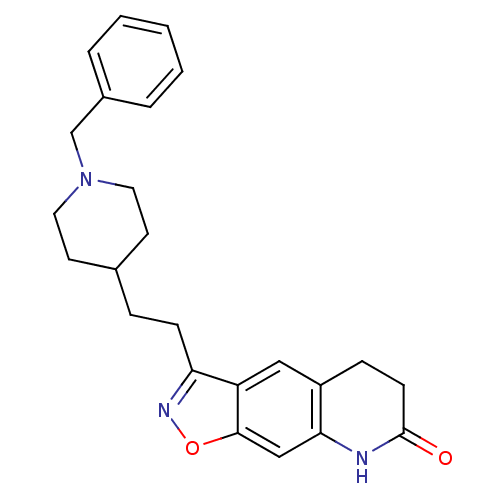
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039721
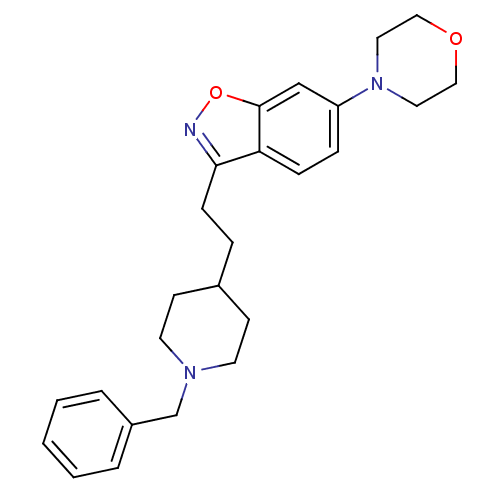
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...)Show SMILES C(Cc1noc2cc(ccc12)N1CCOCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O2/c1-2-4-21(5-3-1)19-27-12-10-20(11-13-27)6-9-24-23-8-7-22(18-25(23)30-26-24)28-14-16-29-17-15-28/h1-5,7-8,18,20H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
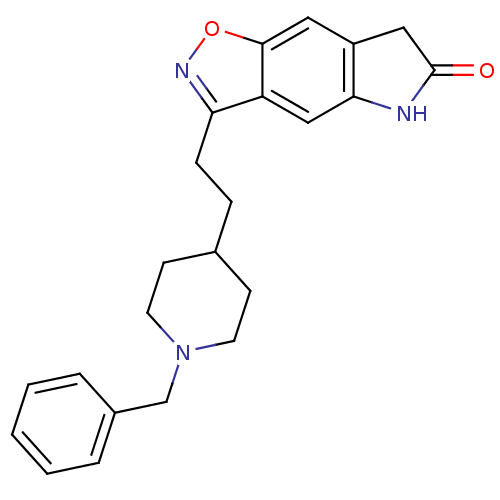
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535210
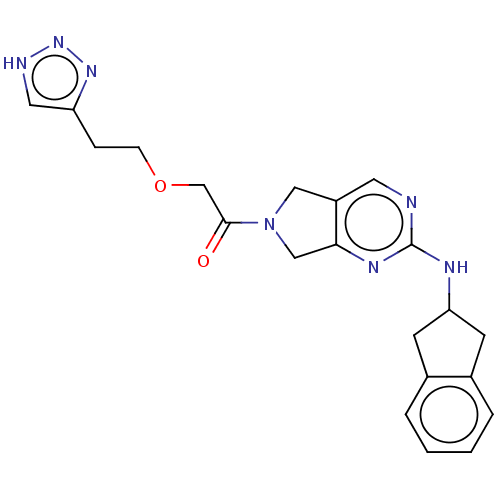
(CHEMBL4448598)Show SMILES O=C(COCCc1c[nH]nn1)N1Cc2cnc(NC3Cc4ccccc4C3)nc2C1 Show InChI InChI=1S/C21H23N7O2/c29-20(13-30-6-5-17-10-23-27-26-17)28-11-16-9-22-21(25-19(16)12-28)24-18-7-14-3-1-2-4-15(14)8-18/h1-4,9-10,18H,5-8,11-13H2,(H,22,24,25)(H,23,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535213
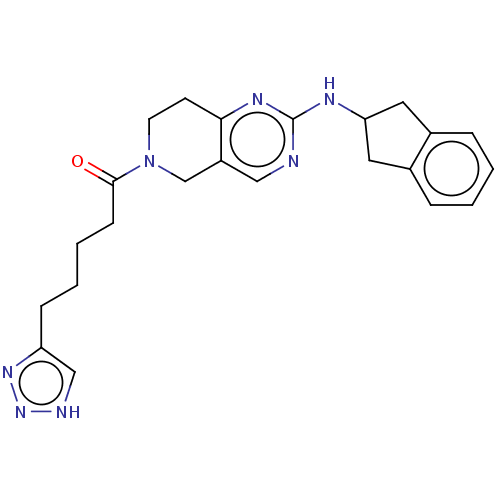
(CHEMBL4453084)Show SMILES O=C(CCCCc1c[nH]nn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-19-14-25-29-28-19)30-10-9-21-18(15-30)13-24-23(27-21)26-20-11-16-5-1-2-6-17(16)12-20/h1-2,5-6,13-14,20H,3-4,7-12,15H2,(H,24,26,27)(H,25,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535210

(CHEMBL4448598)Show SMILES O=C(COCCc1c[nH]nn1)N1Cc2cnc(NC3Cc4ccccc4C3)nc2C1 Show InChI InChI=1S/C21H23N7O2/c29-20(13-30-6-5-17-10-23-27-26-17)28-11-16-9-22-21(25-19(16)12-28)24-18-7-14-3-1-2-4-15(14)8-18/h1-4,9-10,18H,5-8,11-13H2,(H,22,24,25)(H,23,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535214
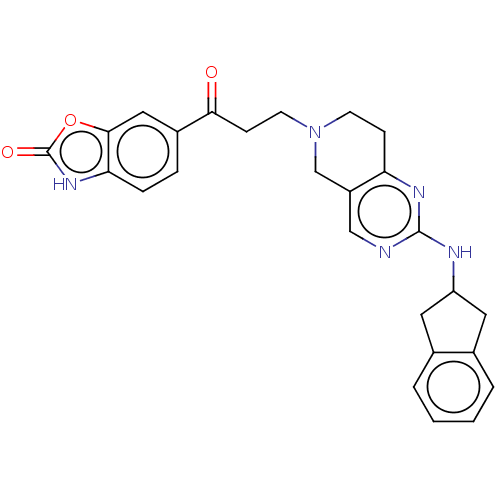
(CHEMBL4549771)Show SMILES O=C(CCN1CCc2nc(NC3Cc4ccccc4C3)ncc2C1)c1ccc2[nH]c(=O)oc2c1 Show InChI InChI=1S/C26H25N5O3/c32-23(18-5-6-22-24(13-18)34-26(33)30-22)8-10-31-9-7-21-19(15-31)14-27-25(29-21)28-20-11-16-3-1-2-4-17(16)12-20/h1-6,13-14,20H,7-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
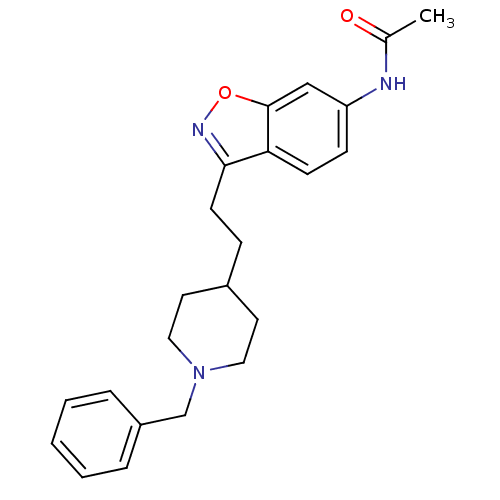
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165

(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535215
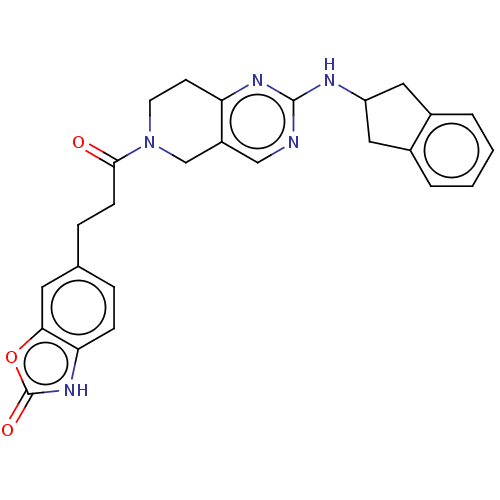
(CHEMBL4476558)Show SMILES O=C(CCc1ccc2[nH]c(=O)oc2c1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C26H25N5O3/c32-24(8-6-16-5-7-22-23(11-16)34-26(33)30-22)31-10-9-21-19(15-31)14-27-25(29-21)28-20-12-17-3-1-2-4-18(17)13-20/h1-5,7,11,14,20H,6,8-10,12-13,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
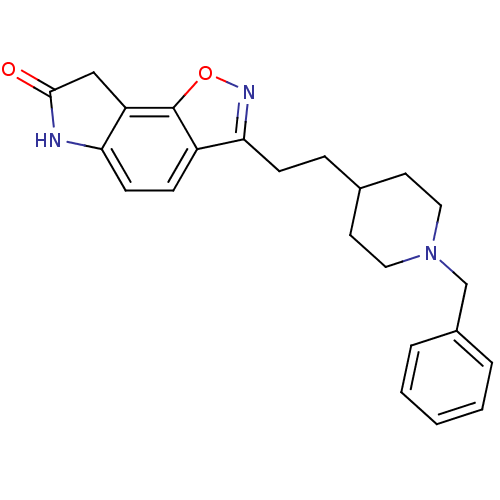
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535212

(CHEMBL4569141)Show SMILES Clc1ccc(CCNc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)cc1 Show InChI InChI=1S/C25H24ClN5O3/c26-19-5-1-16(2-6-19)9-11-27-24-28-14-18-15-31(12-10-20(18)29-24)23(32)8-4-17-3-7-21-22(13-17)34-25(33)30-21/h1-3,5-7,13-14H,4,8-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034001
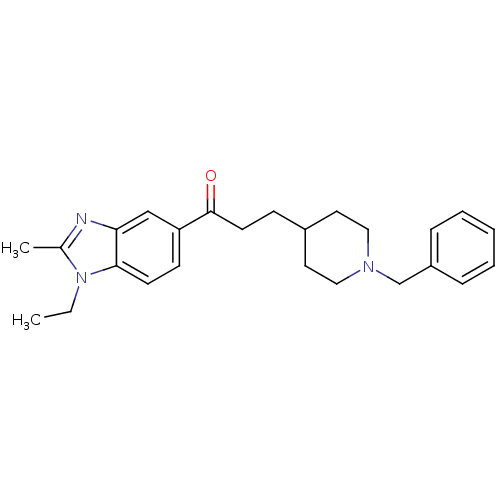
(3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...)Show SMILES CCn1c(C)nc2cc(ccc12)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O/c1-3-28-19(2)26-23-17-22(10-11-24(23)28)25(29)12-9-20-13-15-27(16-14-20)18-21-7-5-4-6-8-21/h4-8,10-11,17,20H,3,9,12-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535220

(CHEMBL4454442)Show SMILES O=C(CCCCn1ccnc1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(7-3-4-10-29-12-9-25-17-29)30-11-8-22-20(16-30)15-26-24(28-22)27-21-13-18-5-1-2-6-19(18)14-21/h1-2,5-6,9,12,15,17,21H,3-4,7-8,10-11,13-14,16H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039729
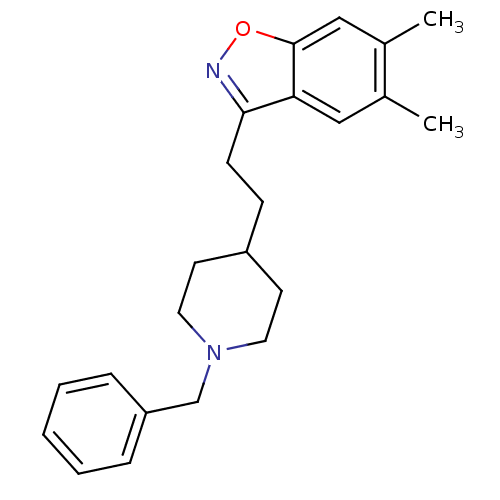
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...)Show InChI InChI=1S/C23H28N2O/c1-17-14-21-22(24-26-23(21)15-18(17)2)9-8-19-10-12-25(13-11-19)16-20-6-4-3-5-7-20/h3-7,14-15,19H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Butyrylcholinesterase obtained from human serum was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535215

(CHEMBL4476558)Show SMILES O=C(CCc1ccc2[nH]c(=O)oc2c1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C26H25N5O3/c32-24(8-6-16-5-7-22-23(11-16)34-26(33)30-22)31-10-9-21-19(15-31)14-27-25(29-21)28-20-12-17-3-1-2-4-18(17)13-20/h1-5,7,11,14,20H,6,8-10,12-13,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Butyrylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535213

(CHEMBL4453084)Show SMILES O=C(CCCCc1c[nH]nn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-19-14-25-29-28-19)30-10-9-21-18(15-30)13-24-23(27-21)26-20-11-16-5-1-2-6-17(16)12-20/h1-2,5-6,13-14,20H,3-4,7-12,15H2,(H,24,26,27)(H,25,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034002

(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-benzothiaz...)Show InChI InChI=1S/C23H26N2OS/c1-17-24-21-9-8-20(15-23(21)27-17)22(26)10-7-18-11-13-25(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
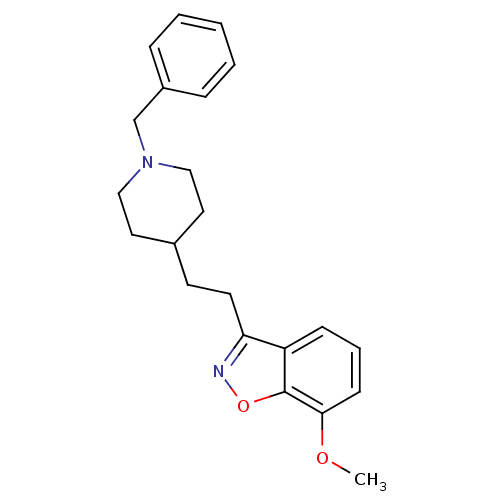
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535220

(CHEMBL4454442)Show SMILES O=C(CCCCn1ccnc1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(7-3-4-10-29-12-9-25-17-29)30-11-8-22-20(16-30)15-26-24(28-22)27-21-13-18-5-1-2-6-19(18)14-21/h1-2,5-6,9,12,15,17,21H,3-4,7-8,10-11,13-14,16H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data