 Found 1903 hits with Last Name = 'akiyama' and Initial = 't'
Found 1903 hits with Last Name = 'akiyama' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM84958

(2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...)Show SMILES OC(=O)c1cccnc1SC[C@H](NC(=O)c1cc2ccccc2[nH]1)C(=O)N1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H33N5O4S/c41-32(29-22-26-14-7-8-16-28(26)37-29)38-30(23-45-33-27(35(43)44)15-9-17-36-33)34(42)40-20-18-39(19-21-40)31(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-17,22,30-31,37H,18-21,23H2,(H,38,41)(H,43,44)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50185261
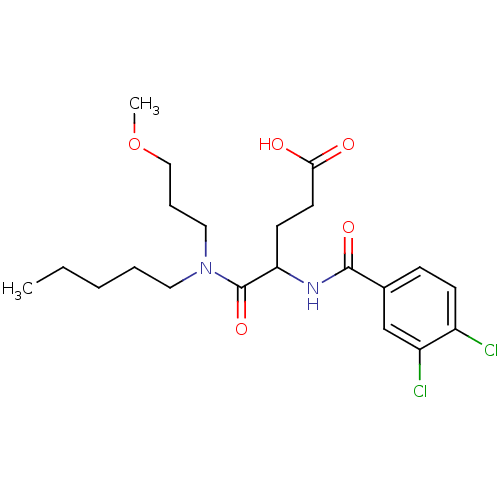
(4-(3,4-dichlorobenzamido)-5-((3-methoxypropyl)(pen...)Show SMILES CCCCCN(CCCOC)C(=O)C(CCC(O)=O)NC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H30Cl2N2O5/c1-3-4-5-11-25(12-6-13-30-2)21(29)18(9-10-19(26)27)24-20(28)15-7-8-16(22)17(23)14-15/h7-8,14,18H,3-6,9-13H2,1-2H3,(H,24,28)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase 2
(Homo sapiens (Human)) | BDBM50122309
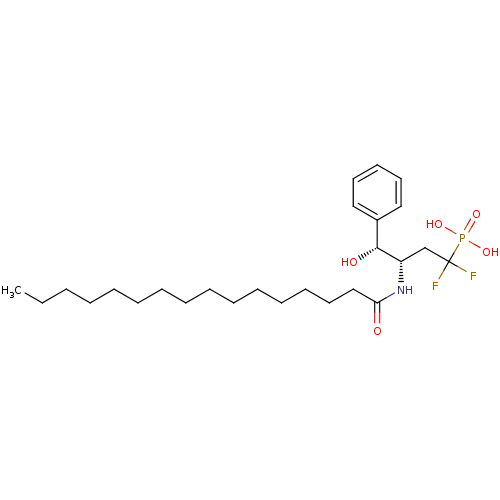
(((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CC(F)(F)P(O)(O)=O)[C@H](O)c1ccccc1 Show InChI InChI=1S/C26H44F2NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-24(30)29-23(21-26(27,28)35(32,33)34)25(31)22-18-15-14-16-19-22/h14-16,18-19,23,25,31H,2-13,17,20-21H2,1H3,(H,29,30)(H2,32,33,34)/t23-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against schyphostatin of neutral sphingomyelinase (N-SMase) from bovine brain microsome |
Bioorg Med Chem Lett 13: 229-36 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HFH |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM84958

(2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...)Show SMILES OC(=O)c1cccnc1SC[C@H](NC(=O)c1cc2ccccc2[nH]1)C(=O)N1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H33N5O4S/c41-32(29-22-26-14-7-8-16-28(26)37-29)38-30(23-45-33-27(35(43)44)15-9-17-36-33)34(42)40-20-18-39(19-21-40)31(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-17,22,30-31,37H,18-21,23H2,(H,38,41)(H,43,44)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 5
(Homo sapiens (Human)) | BDBM50437693
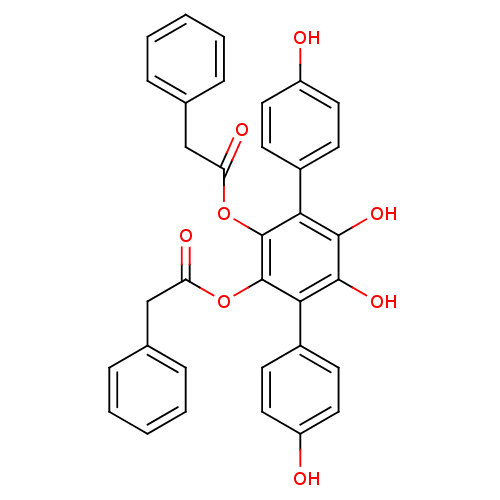
(VIALININ A)Show SMILES Oc1ccc(cc1)-c1c(O)c(O)c(-c2ccc(O)cc2)c(OC(=O)Cc2ccccc2)c1OC(=O)Cc1ccccc1 Show InChI InChI=1S/C34H26O8/c35-25-15-11-23(12-16-25)29-31(39)32(40)30(24-13-17-26(36)18-14-24)34(42-28(38)20-22-9-5-2-6-10-22)33(29)41-27(37)19-21-7-3-1-4-8-21/h1-18,35-36,39-40H,19-20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture
Curated by ChEMBL
| Assay Description
Inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate after 60 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 4328-31 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.093
BindingDB Entry DOI: 10.7270/Q2T15528 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50185261

(4-(3,4-dichlorobenzamido)-5-((3-methoxypropyl)(pen...)Show SMILES CCCCCN(CCCOC)C(=O)C(CCC(O)=O)NC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H30Cl2N2O5/c1-3-4-5-11-25(12-6-13-30-2)21(29)18(9-10-19(26)27)24-20(28)15-7-8-16(22)17(23)14-15/h7-8,14,18H,3-6,9-13H2,1-2H3,(H,24,28)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 5
(Homo sapiens (Human)) | BDBM50437693

(VIALININ A)Show SMILES Oc1ccc(cc1)-c1c(O)c(O)c(-c2ccc(O)cc2)c(OC(=O)Cc2ccccc2)c1OC(=O)Cc1ccccc1 Show InChI InChI=1S/C34H26O8/c35-25-15-11-23(12-16-25)29-31(39)32(40)30(24-13-17-26(36)18-14-24)34(42-28(38)20-22-9-5-2-6-10-22)33(29)41-27(37)19-21-7-3-1-4-8-21/h1-18,35-36,39-40H,19-20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate |
Bioorg Med Chem Lett 23: 4328-31 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.093
BindingDB Entry DOI: 10.7270/Q2T15528 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase 2
(Homo sapiens (Human)) | BDBM50122310
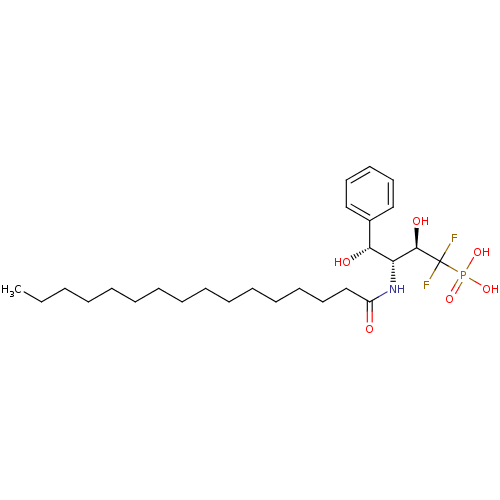
(((2R,3R,4R)-1,1-Difluoro-3-hexadecanoylamino-2,4-d...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@H]([C@H](O)c1ccccc1)[C@@H](O)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C26H44F2NO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-22(30)29-23(24(31)21-18-15-14-16-19-21)25(32)26(27,28)36(33,34)35/h14-16,18-19,23-25,31-32H,2-13,17,20H2,1H3,(H,29,30)(H2,33,34,35)/t23-,24-,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomes |
Bioorg Med Chem Lett 13: 229-36 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HFH |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328246
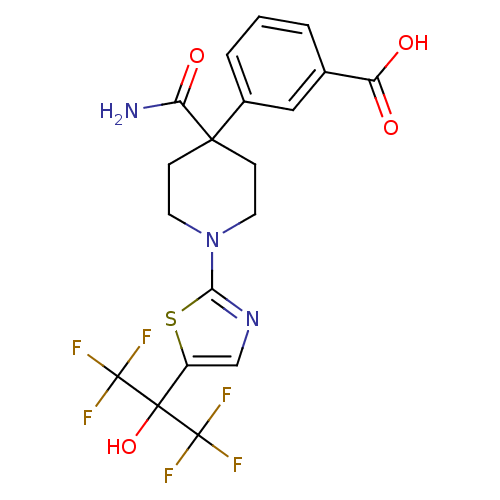
(3-(4-carbamoyl-1-(5-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES NC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1cccc(c1)C(O)=O Show InChI InChI=1S/C19H17F6N3O4S/c20-18(21,22)17(32,19(23,24)25)12-9-27-15(33-12)28-6-4-16(5-7-28,14(26)31)11-3-1-2-10(8-11)13(29)30/h1-3,8-9,32H,4-7H2,(H2,26,31)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468129
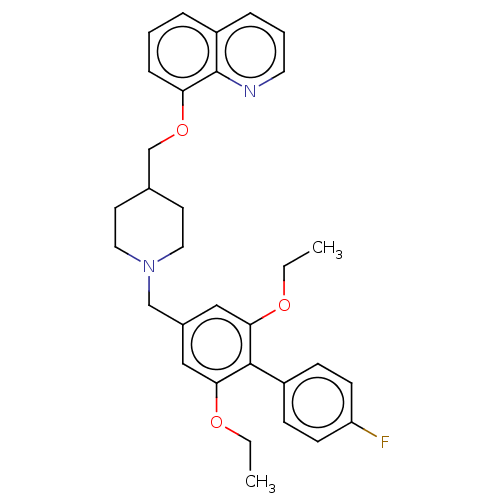
(CHEMBL4288848)Show SMILES CCOc1cc(CN2CCC(COc3cccc4cccnc34)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O3/c1-3-36-29-19-24(20-30(37-4-2)31(29)25-10-12-27(33)13-11-25)21-35-17-14-23(15-18-35)22-38-28-9-5-7-26-8-6-16-34-32(26)28/h5-13,16,19-20,23H,3-4,14-15,17-18,21-22H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328247
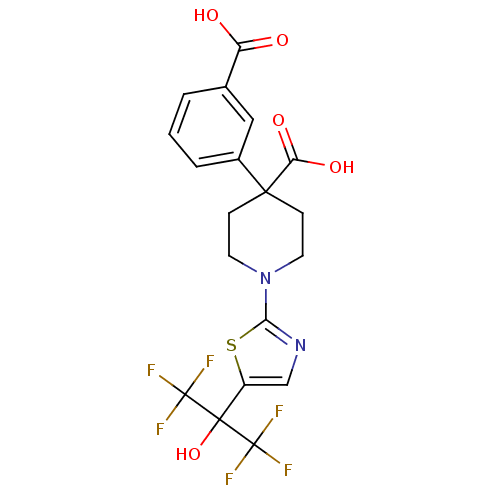
(4-(3-carboxyphenyl)-1-(5-(1,1,1,3,3,3-hexafluoro-2...)Show SMILES OC(=O)c1cccc(c1)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)C(O)=O Show InChI InChI=1S/C19H16F6N2O5S/c20-18(21,22)17(32,19(23,24)25)12-9-26-15(33-12)27-6-4-16(5-7-27,14(30)31)11-3-1-2-10(8-11)13(28)29/h1-3,8-9,32H,4-7H2,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468133
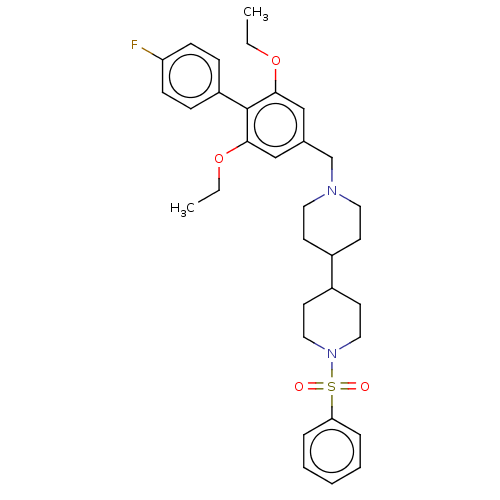
(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468133

(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328248
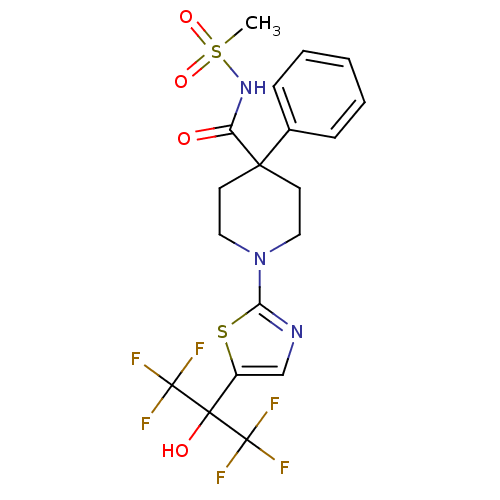
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES CS(=O)(=O)NC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C19H19F6N3O4S2/c1-34(31,32)27-14(29)16(12-5-3-2-4-6-12)7-9-28(10-8-16)15-26-11-13(33-15)17(30,18(20,21)22)19(23,24)25/h2-6,11,30H,7-10H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328235
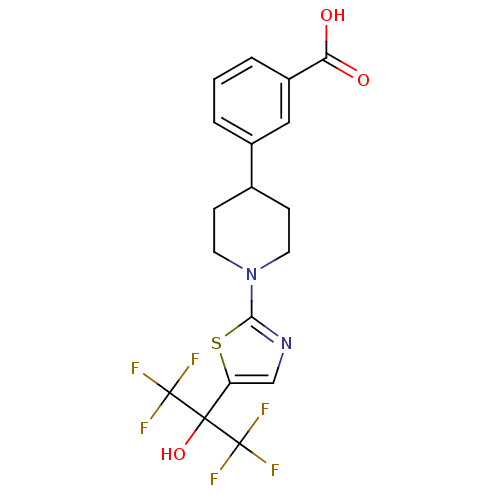
(3-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1cccc(c1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)13-9-25-15(30-13)26-6-4-10(5-7-26)11-2-1-3-12(8-11)14(27)28/h1-3,8-10,29H,4-7H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244702
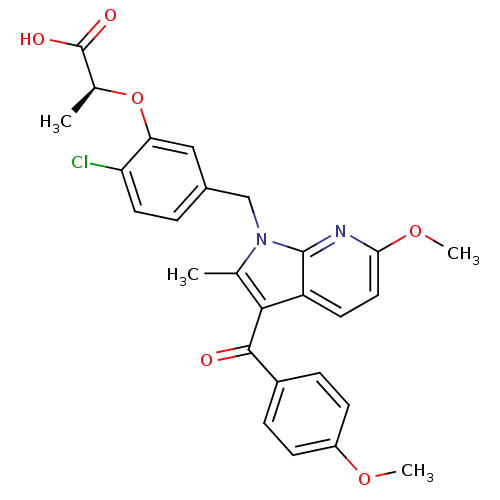
((S)-2-(2-chloro-5-((6-methoxy-3-(4-methoxybenzoyl)...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2ccc(Cl)c(O[C@@H](C)C(O)=O)c2)c2nc(OC)ccc12 |r| Show InChI InChI=1S/C27H25ClN2O6/c1-15-24(25(31)18-6-8-19(34-3)9-7-18)20-10-12-23(35-4)29-26(20)30(15)14-17-5-11-21(28)22(13-17)36-16(2)27(32)33/h5-13,16H,14H2,1-4H3,(H,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244700
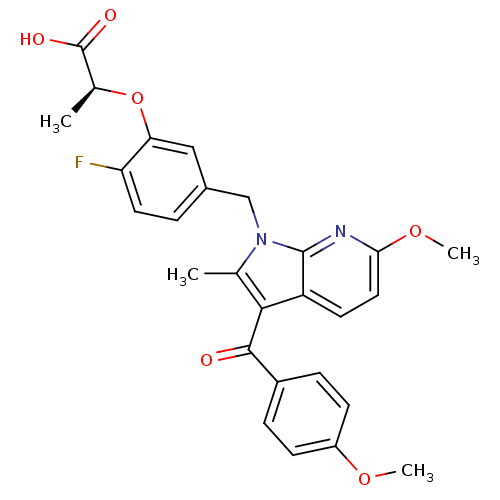
((S)-2-(2-fluoro-5-((6-methoxy-3-(4-methoxybenzoyl)...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2ccc(F)c(O[C@@H](C)C(O)=O)c2)c2nc(OC)ccc12 |r| Show InChI InChI=1S/C27H25FN2O6/c1-15-24(25(31)18-6-8-19(34-3)9-7-18)20-10-12-23(35-4)29-26(20)30(15)14-17-5-11-21(28)22(13-17)36-16(2)27(32)33/h5-13,16H,14H2,1-4H3,(H,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50322981
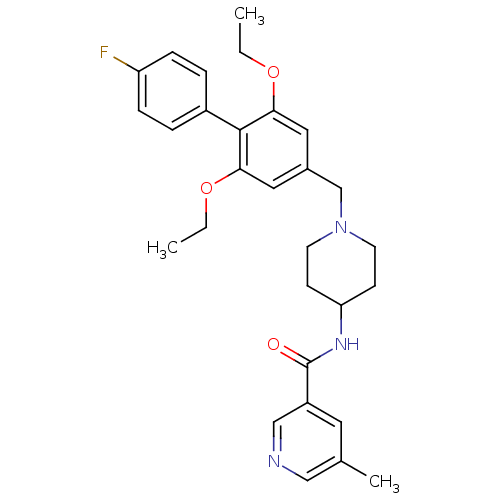
(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244556
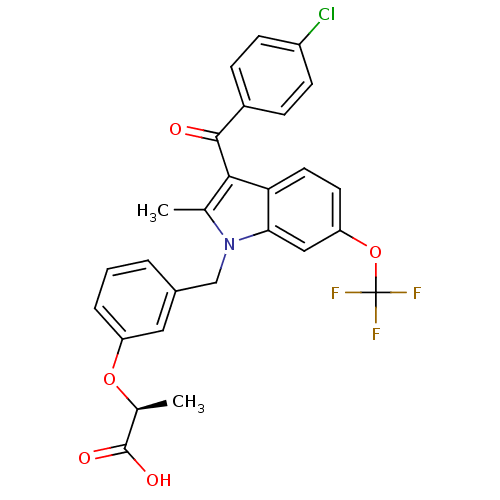
((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293854
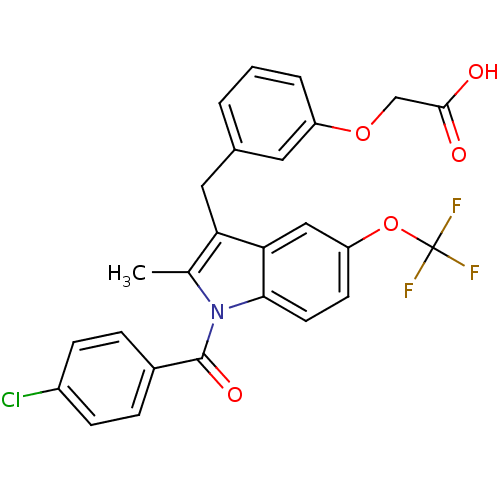
(2-(3-((1-(4-chlorobenzoyl)-2-methyl-5-(trifluorome...)Show SMILES Cc1c(Cc2cccc(OCC(O)=O)c2)c2cc(OC(F)(F)F)ccc2n1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H19ClF3NO5/c1-15-21(12-16-3-2-4-19(11-16)35-14-24(32)33)22-13-20(36-26(28,29)30)9-10-23(22)31(15)25(34)17-5-7-18(27)8-6-17/h2-11,13H,12,14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244556

((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267990
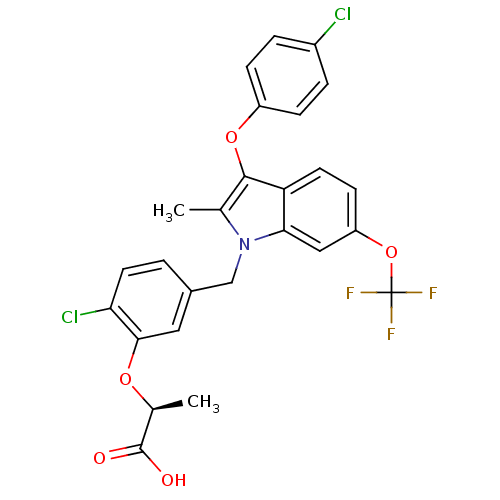
((S)-2-(2-chloro-5-((3-(4-chlorophenoxy)-2-methyl-6...)Show SMILES C[C@H](Oc1cc(Cn2c(C)c(Oc3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)ccc1Cl)C(O)=O |r| Show InChI InChI=1S/C26H20Cl2F3NO5/c1-14-24(36-18-6-4-17(27)5-7-18)20-9-8-19(37-26(29,30)31)12-22(20)32(14)13-16-3-10-21(28)23(11-16)35-15(2)25(33)34/h3-12,15H,13H2,1-2H3,(H,33,34)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50268271
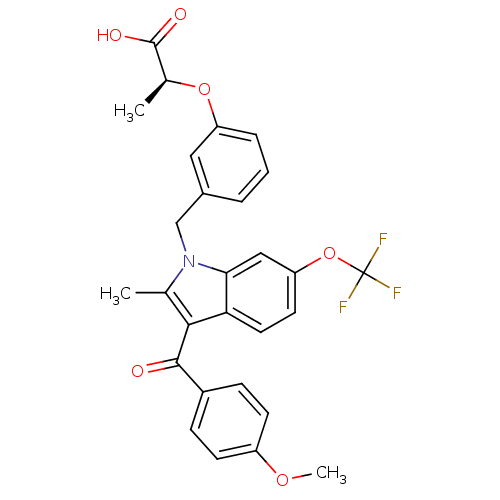
((S)-2-(3-((3-(4-methoxybenzoyl)-2-methyl-6-(triflu...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-25(26(33)19-7-9-20(36-3)10-8-19)23-12-11-22(38-28(29,30)31)14-24(23)32(16)15-18-5-4-6-21(13-18)37-17(2)27(34)35/h4-14,17H,15H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157917

((2S)-2-(3-{[1-(4-METHOXYBENZOYL)-2-METHYL-5-(TRIFL...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328241
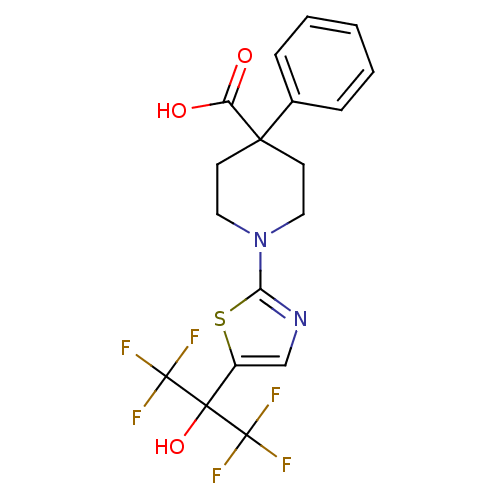
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES OC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)12-10-25-14(30-12)26-8-6-15(7-9-26,13(27)28)11-4-2-1-3-5-11/h1-5,10,29H,6-9H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322981

(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328222
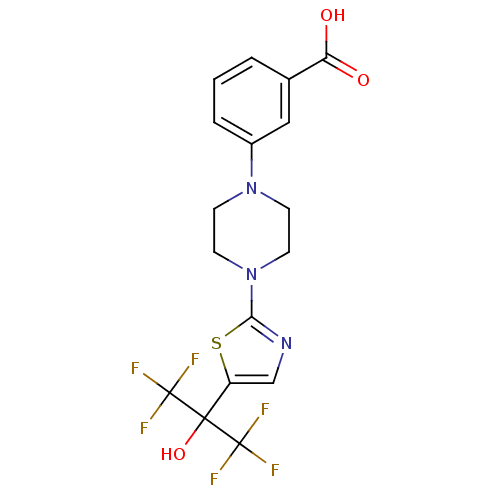
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O3S/c18-16(19,20)15(29,17(21,22)23)12-9-24-14(30-12)26-6-4-25(5-7-26)11-3-1-2-10(8-11)13(27)28/h1-3,8-9,29H,4-7H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328244
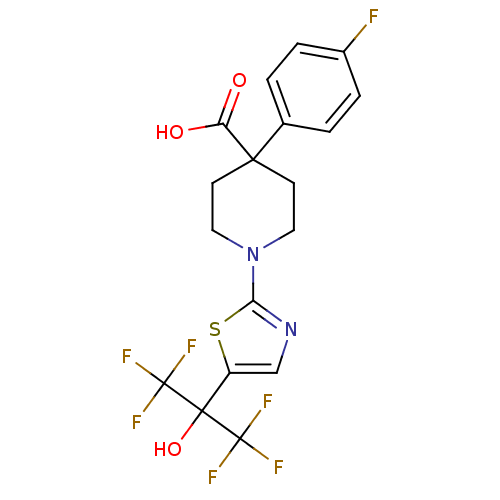
(4-(4-fluorophenyl)-1-(5-(1,1,1,3,3,3-hexafluoro-2-...)Show SMILES OC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 Show InChI InChI=1S/C18H15F7N2O3S/c19-11-3-1-10(2-4-11)15(13(28)29)5-7-27(8-6-15)14-26-9-12(31-14)16(30,17(20,21)22)18(23,24)25/h1-4,9,30H,5-8H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328245
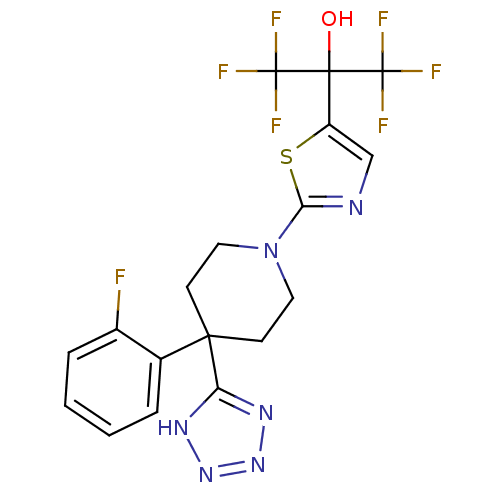
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(2-fluorophenyl)-4-...)Show SMILES OC(c1cnc(s1)N1CCC(CC1)(c1nnn[nH]1)c1ccccc1F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H15F7N6OS/c19-11-4-2-1-3-10(11)15(13-27-29-30-28-13)5-7-31(8-6-15)14-26-9-12(33-14)16(32,17(20,21)22)18(23,24)25/h1-4,9,32H,5-8H2,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468130

(CHEMBL4292255)Show SMILES CCOc1cc(CN2CCC3(CC(Cc4ccc(F)cc4)C(=O)O3)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35F2NO4/c1-3-37-28-18-23(19-29(38-4-2)30(28)24-7-11-27(34)12-8-24)21-35-15-13-32(14-16-35)20-25(31(36)39-32)17-22-5-9-26(33)10-6-22/h5-12,18-19,25H,3-4,13-17,20-21H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328226

(2-(2-(4-(3-(1H-tetrazol-5-yl)phenyl)piperazin-1-yl...)Show SMILES OC(c1cnc(s1)N1CCN(CC1)c1cccc(c1)-c1nnn[nH]1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6N7OS/c18-16(19,20)15(31,17(21,22)23)12-9-24-14(32-12)30-6-4-29(5-7-30)11-3-1-2-10(8-11)13-25-27-28-26-13/h1-3,8-9,31H,4-7H2,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328234
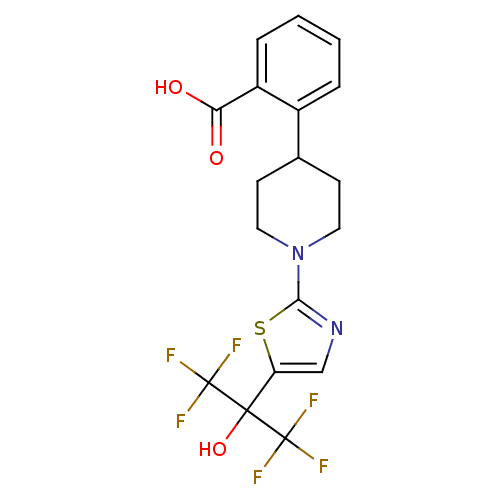
(2-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1ccccc1C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)13-9-25-15(30-13)26-7-5-10(6-8-26)11-3-1-2-4-12(11)14(27)28/h1-4,9-10,29H,5-8H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328237
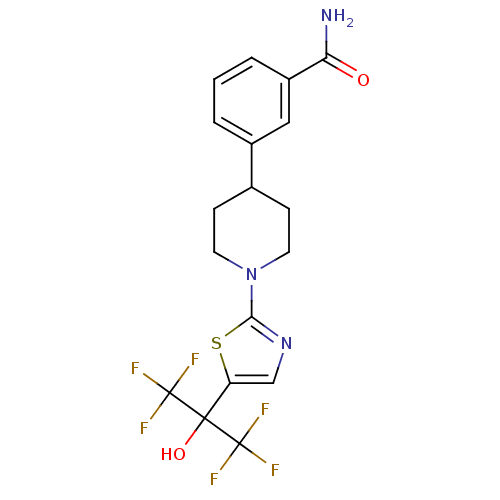
(3-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES NC(=O)c1cccc(c1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F6N3O2S/c19-17(20,21)16(29,18(22,23)24)13-9-26-15(30-13)27-6-4-10(5-7-27)11-2-1-3-12(8-11)14(25)28/h1-3,8-10,29H,4-7H2,(H2,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293853
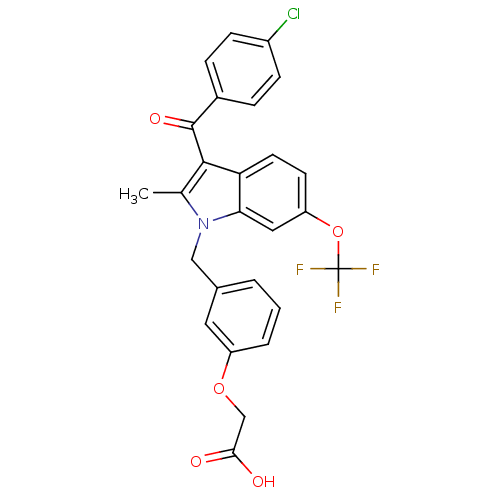
(2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(trifluorome...)Show SMILES Cc1c(C(=O)c2ccc(Cl)cc2)c2ccc(OC(F)(F)F)cc2n1Cc1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C26H19ClF3NO5/c1-15-24(25(34)17-5-7-18(27)8-6-17)21-10-9-20(36-26(28,29)30)12-22(21)31(15)13-16-3-2-4-19(11-16)35-14-23(32)33/h2-12H,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244701
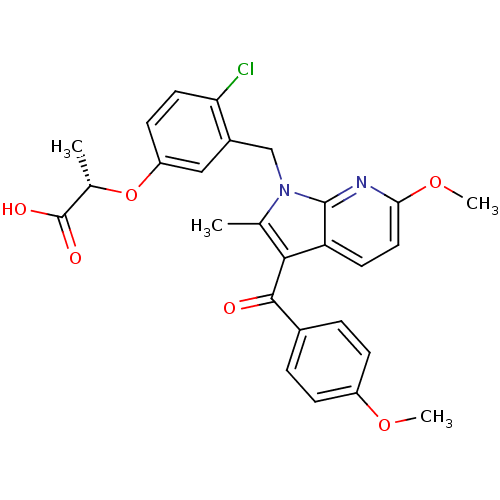
((S)-2-(4-chloro-3-((6-methoxy-3-(4-methoxybenzoyl)...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2cc(O[C@@H](C)C(O)=O)ccc2Cl)c2nc(OC)ccc12 |r| Show InChI InChI=1S/C27H25ClN2O6/c1-15-24(25(31)17-5-7-19(34-3)8-6-17)21-10-12-23(35-4)29-26(21)30(15)14-18-13-20(9-11-22(18)28)36-16(2)27(32)33/h5-13,16H,14H2,1-4H3,(H,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244699
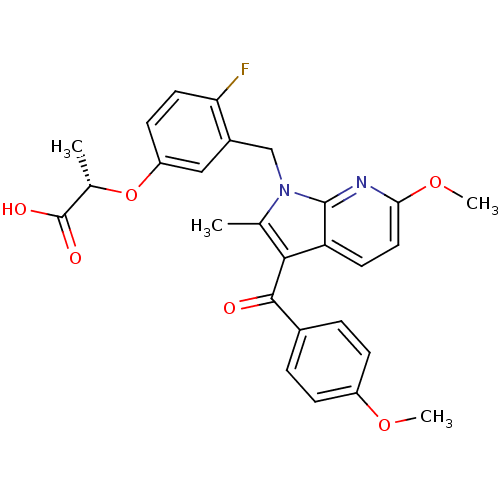
((S)-2-(4-fluoro-3-((6-methoxy-3-(4-methoxybenzoyl)...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2cc(O[C@@H](C)C(O)=O)ccc2F)c2nc(OC)ccc12 |r| Show InChI InChI=1S/C27H25FN2O6/c1-15-24(25(31)17-5-7-19(34-3)8-6-17)21-10-12-23(35-4)29-26(21)30(15)14-18-13-20(9-11-22(18)28)36-16(2)27(32)33/h5-13,16H,14H2,1-4H3,(H,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50173356
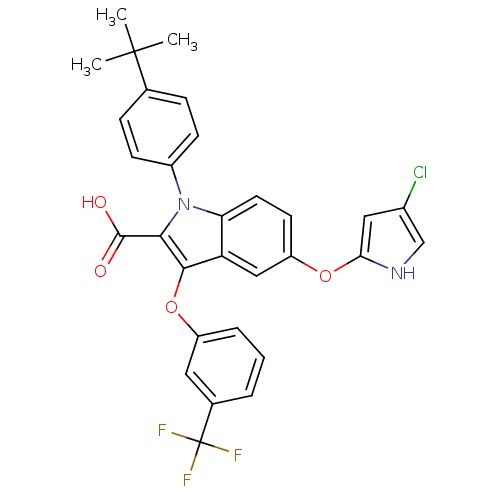
(1-(4-tert-Butyl-phenyl)-5-(4-chloro-1H-pyrrol-2-yl...)Show SMILES CC(C)(C)c1ccc(cc1)-n1c(C(O)=O)c(Oc2cccc(c2)C(F)(F)F)c2cc(Oc3cc(Cl)c[nH]3)ccc12 Show InChI InChI=1S/C30H24ClF3N2O4/c1-29(2,3)17-7-9-20(10-8-17)36-24-12-11-22(39-25-14-19(31)16-35-25)15-23(24)27(26(36)28(37)38)40-21-6-4-5-18(13-21)30(32,33)34/h4-16,35H,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay |
Bioorg Med Chem Lett 15: 5035-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.002
BindingDB Entry DOI: 10.7270/Q27H1J56 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267989

((2S)-2-(4-chloro-3-((3-(6-methoxybenzo[d]isoxazol-...)Show SMILES COc1ccc2c(noc2c1)-c1c(C)n(Cc2cc(O[C@@H](C)C(O)=O)ccc2Cl)c2cc(OC(F)(F)F)ccc12 |r,wU:20.22,(26.73,-43.65,;26.71,-42.11,;25.37,-41.35,;25.35,-39.81,;24.02,-39.06,;22.71,-39.84,;21.24,-39.37,;20.34,-40.62,;21.26,-41.86,;22.72,-41.37,;24.05,-42.13,;20.75,-37.92,;21.65,-36.65,;23.19,-36.64,;20.73,-35.4,;21.2,-33.94,;22.71,-33.61,;23.74,-34.74,;25.24,-34.41,;26.28,-35.55,;27.78,-35.22,;28.25,-33.76,;28.82,-36.36,;30.32,-36.03,;28.35,-37.83,;25.71,-32.95,;24.66,-31.8,;23.16,-32.14,;22.12,-31.01,;19.26,-35.89,;17.92,-35.13,;16.6,-35.9,;15.26,-35.13,;15.26,-33.59,;15.25,-32.05,;16.8,-33.58,;13.72,-33.6,;16.59,-37.44,;17.92,-38.22,;19.27,-37.44,)| Show InChI InChI=1S/C28H22ClF3N2O6/c1-14-25(26-21-8-4-17(37-3)12-24(21)40-33-26)20-7-5-19(39-28(30,31)32)11-23(20)34(14)13-16-10-18(6-9-22(16)29)38-15(2)27(35)36/h4-12,15H,13H2,1-3H3,(H,35,36)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328225
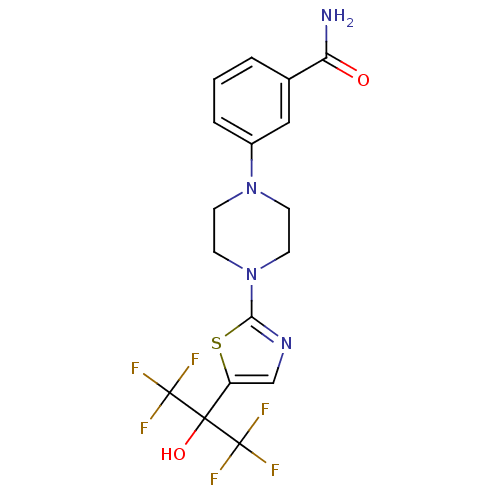
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES NC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N4O2S/c18-16(19,20)15(29,17(21,22)23)12-9-25-14(30-12)27-6-4-26(5-7-27)11-3-1-2-10(8-11)13(24)28/h1-3,8-9,29H,4-7H2,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328227
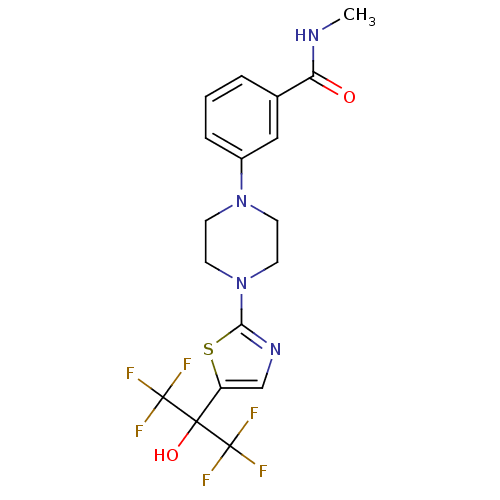
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES CNC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H18F6N4O2S/c1-25-14(29)11-3-2-4-12(9-11)27-5-7-28(8-6-27)15-26-10-13(31-15)16(30,17(19,20)21)18(22,23)24/h2-4,9-10,30H,5-8H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362957

(CHEMBL1946757)Show SMILES COc1ccc2c(noc2c1)-n1c2cc(ccc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O)C(F)(F)F |r,wU:27.30,(3.39,4.69,;2.06,5.47,;.72,4.7,;.72,3.15,;-.63,2.39,;-1.96,3.17,;-3.42,2.72,;-4.32,3.96,;-3.41,5.2,;-1.95,4.71,;-.61,5.48,;-4.18,1.38,;-5.65,.89,;-6.98,1.66,;-8.31,.89,;-8.31,-.66,;-6.98,-1.43,;-5.64,-.65,;-4.17,-1.12,;-4.14,-2.66,;-2.8,-3.41,;-2.78,-4.94,;-1.43,-5.69,;-.11,-4.9,;1.24,-5.64,;-.14,-3.35,;1.17,-2.55,;2.52,-3.3,;2.55,-4.84,;3.84,-2.5,;5.19,-3.24,;3.81,-.96,;-1.49,-2.61,;-3.27,.14,;-1.73,.15,;-9.64,1.66,;-9.64,3.2,;-10.97,.89,;-10.8,2.43,)| Show InChI InChI=1S/C26H19ClF3N3O6/c1-13(24(34)35)38-22-9-14(3-7-18(22)27)12-32-19-8-4-15(26(28,29)30)10-20(19)33(25(32)36)23-17-6-5-16(37-2)11-21(17)39-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468130

(CHEMBL4292255)Show SMILES CCOc1cc(CN2CCC3(CC(Cc4ccc(F)cc4)C(=O)O3)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35F2NO4/c1-3-37-28-18-23(19-29(38-4-2)30(28)24-7-11-27(34)12-8-24)21-35-15-13-32(14-16-35)20-25(31(36)39-32)17-22-5-9-26(33)10-6-22/h5-12,18-19,25H,3-4,13-17,20-21H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
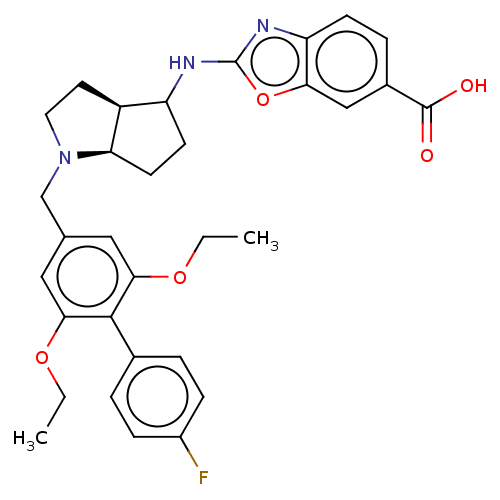
(Homo sapiens (Human)) | BDBM50468131
(CHEMBL4277106)Show SMILES [H][C@@]12CCN(Cc3cc(OCC)c(c(OCC)c3)-c3ccc(F)cc3)[C@]1([H])CCC2Nc1nc2ccc(cc2o1)C(O)=O |r| Show InChI InChI=1S/C32H34FN3O5/c1-3-39-28-15-19(16-29(40-4-2)30(28)20-5-8-22(33)9-6-20)18-36-14-13-23-24(11-12-26(23)36)34-32-35-25-10-7-21(31(37)38)17-27(25)41-32/h5-10,15-17,23-24,26H,3-4,11-14,18H2,1-2H3,(H,34,35)(H,37,38)/t23-,24?,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data