

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474150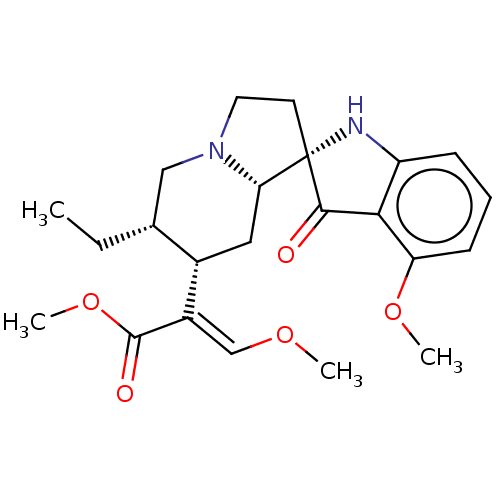 (CHEMBL58362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150028 ((5R,6R)-1-Benzoyl-5-benzyl-6-hydroxy-2,4-bis-(4-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192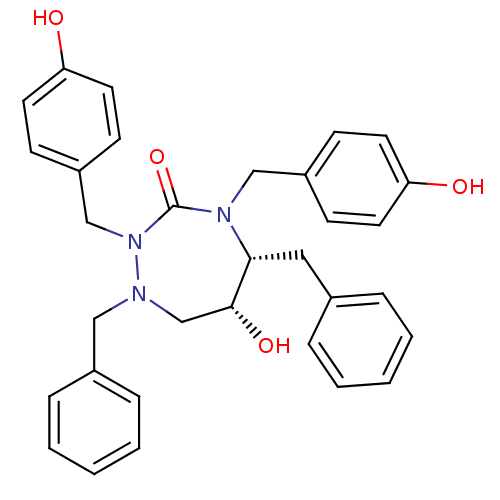 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50409919 (CHEMBL12282) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50409920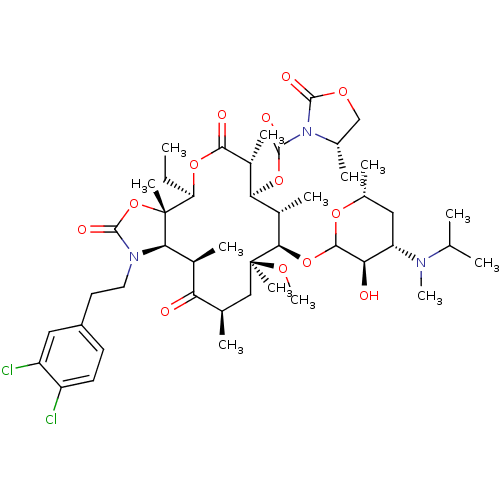 (CHEMBL273440) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409919 (CHEMBL12282) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to human luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409919 (CHEMBL12282) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50409921 (CHEMBL429956) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50409918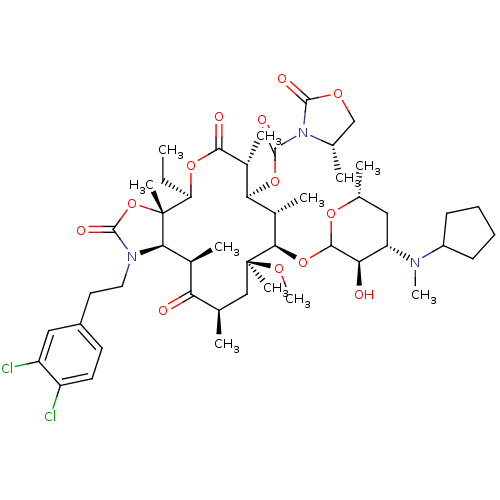 (CHEMBL275935) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224090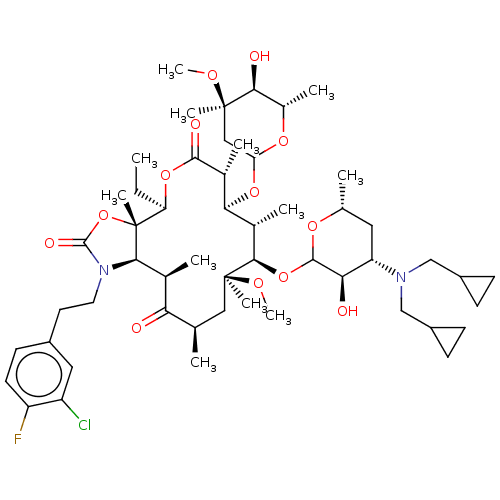 (CHEMBL410940) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001560 (CHEMBL273275) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224093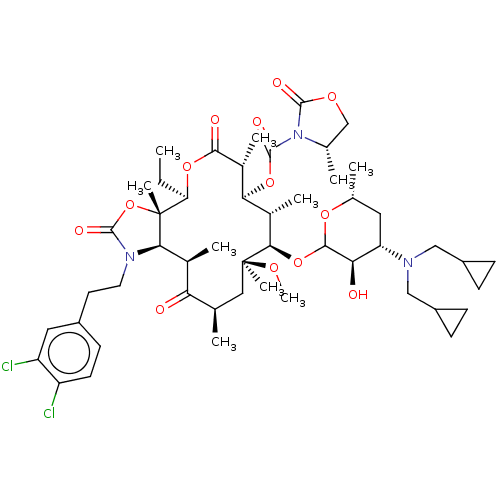 (CHEMBL408925) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150033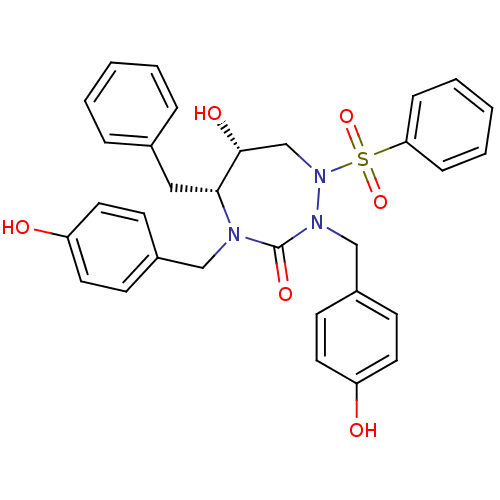 ((5R,6R)-1-Benzenesulfonyl-5-benzyl-6-hydroxy-2,4-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224095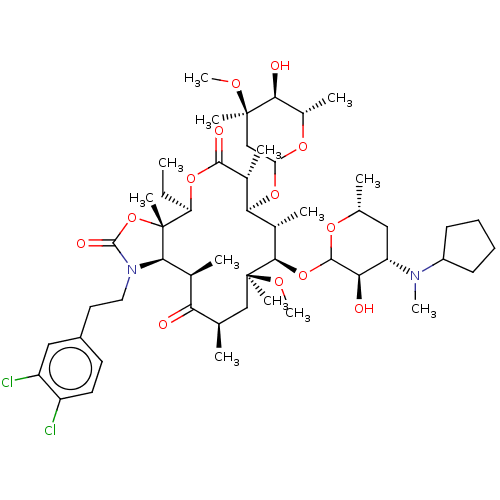 (CHEMBL264043) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403759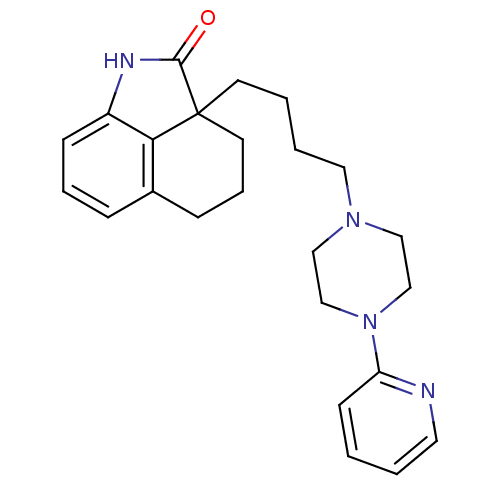 (CHEMBL428494) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409921 (CHEMBL429956) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to human luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50423641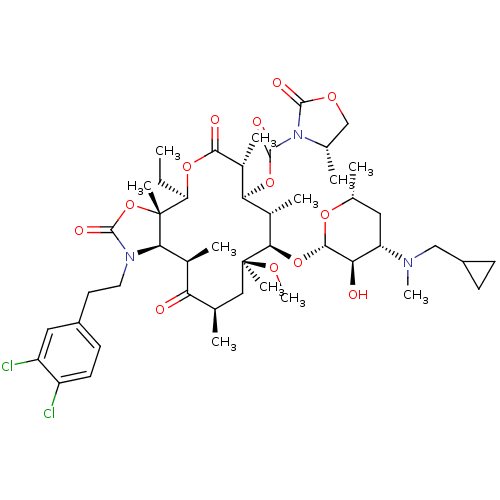 (A-198401 | CHEMBL303274) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50116963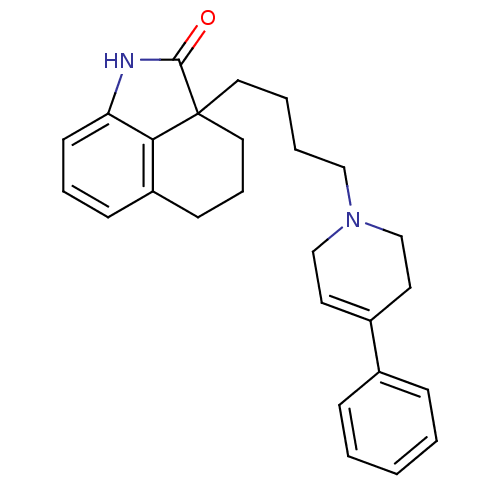 (2a-(4-(4-phenyl-5,6-dihydropyridin-1(2H)-yl)butyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001571 (CHEMBL273466) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473640 (CHEMBL71847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001566 (CHEMBL275467) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001563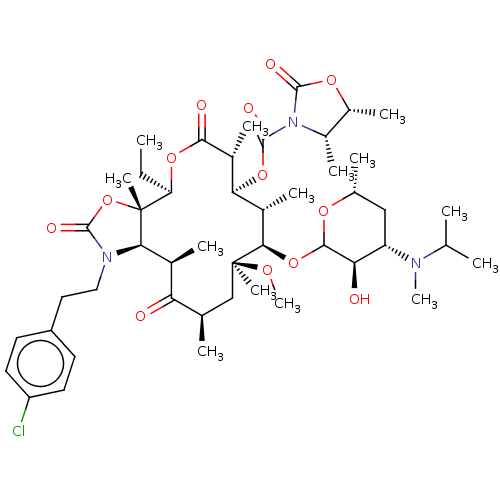 (CHEMBL273535) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474150 (CHEMBL58362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403770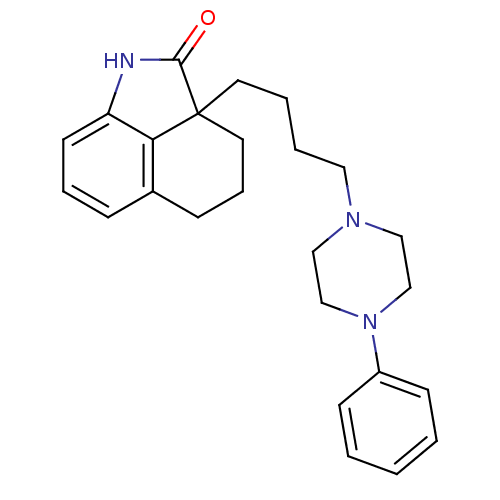 (CHEMBL12427) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opoid receptor in Dunkin-Hartley guinea pig brain after 1.5 hrs by liquid scintillation spectrometry | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130276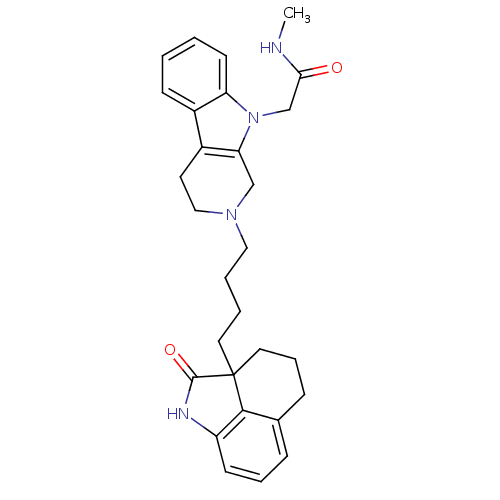 (CHEMBL553794 | N-Methyl-2-{2-[4-(2-oxo-1,2,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50220558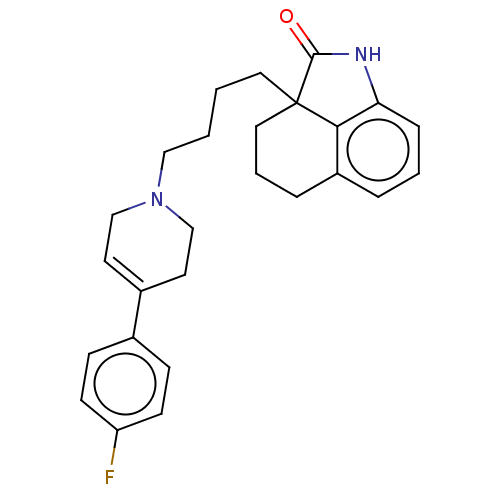 (CHEMBL418888) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403768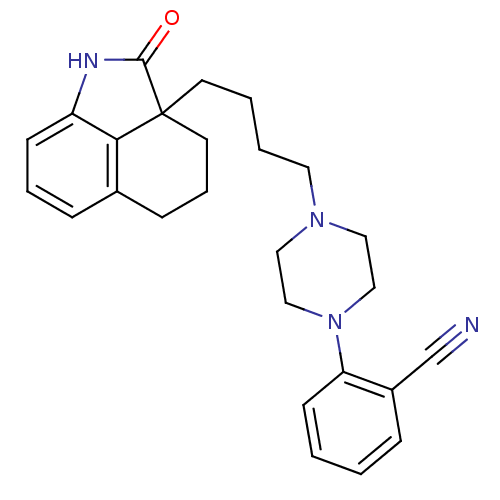 (CHEMBL273246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50001560 (CHEMBL273275) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403780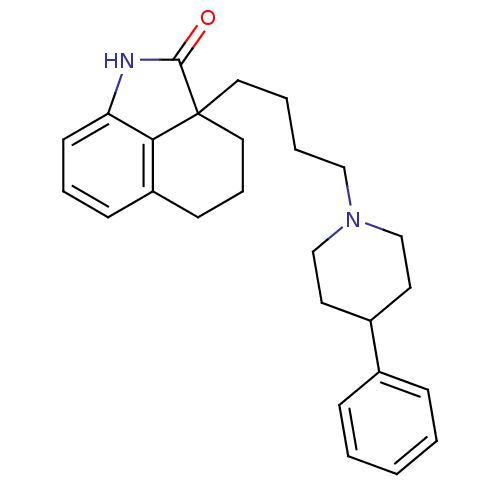 (CHEMBL12846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001573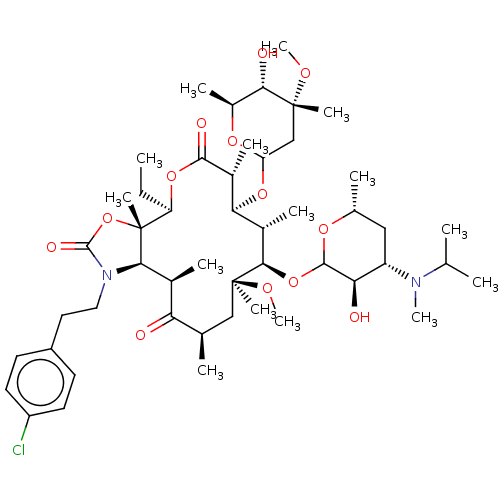 (CHEMBL273387) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473633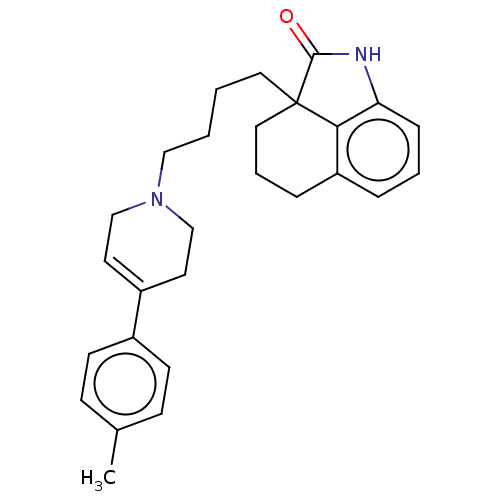 (CHEMBL304731) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50403777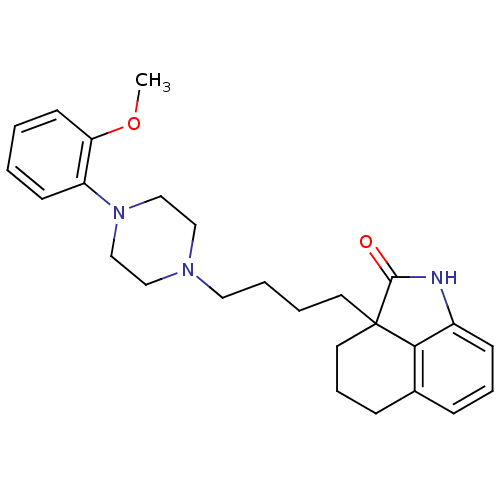 (CHEMBL12702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001564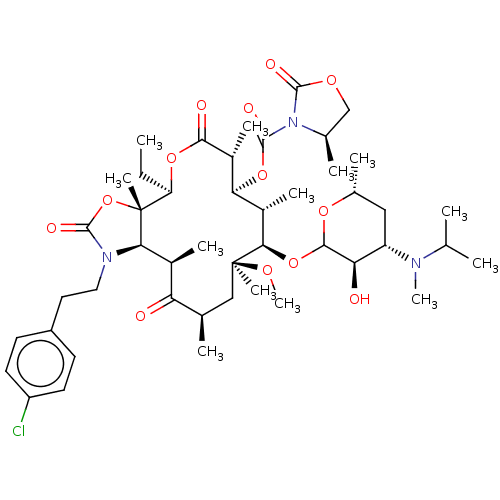 (CHEMBL429949) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409918 (CHEMBL275935) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to human luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473658 (CHEMBL71383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473644 (CHEMBL302766) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001548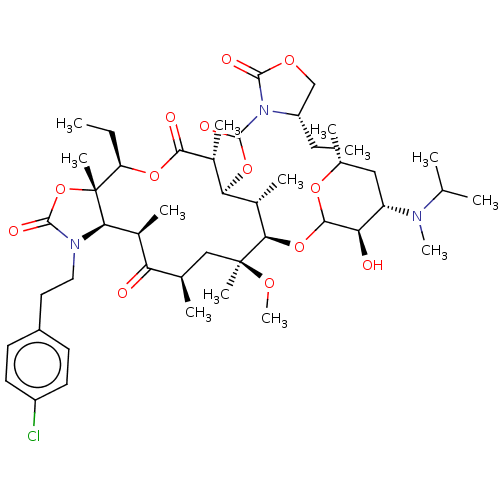 (CHEMBL412634) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473648 (CHEMBL71225) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492099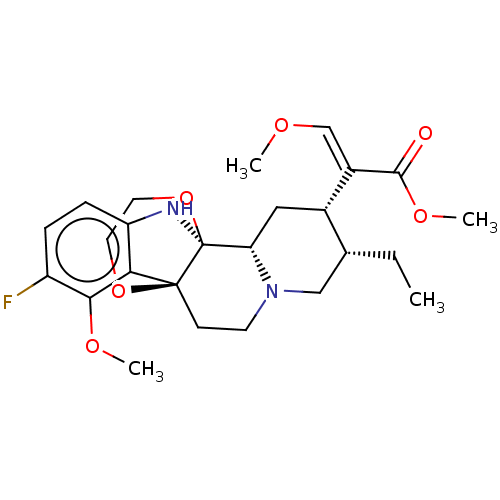 (CHEMBL2396991) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492099 (CHEMBL2396991) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473651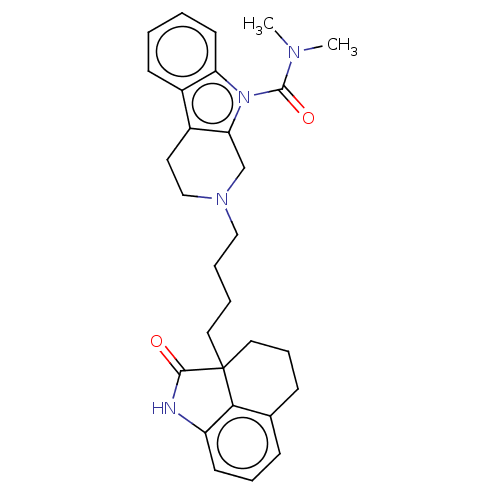 (CHEMBL71959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224097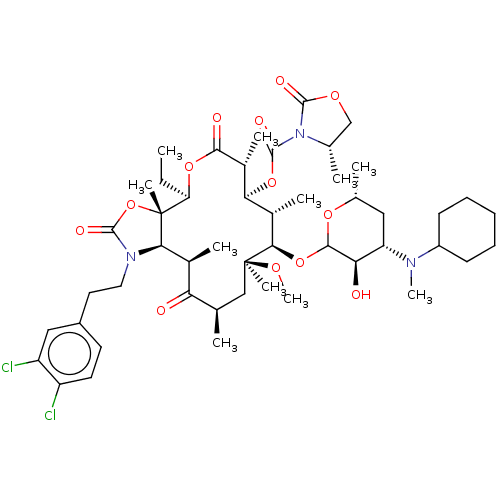 (CHEMBL418067) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474152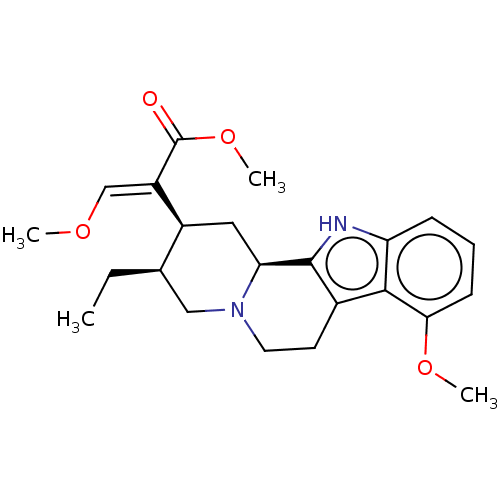 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opoid receptor in Dunkin-Hartley guinea pig brain after 1.5 hrs by liquid scintillation spectrometry | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224091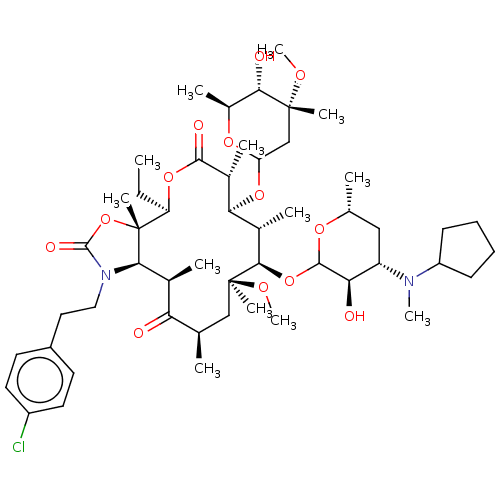 (CHEMBL442482) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001555 (CHEMBL267568) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat luteinizing hormone-releasing hormone (LHRH) receptor cloned in CHO cells | J Med Chem 47: 1085-97 (2004) Checked by Author Article DOI: 10.1021/jm030418i BindingDB Entry DOI: 10.7270/Q2ZG6TF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473639 (CHEMBL303166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473652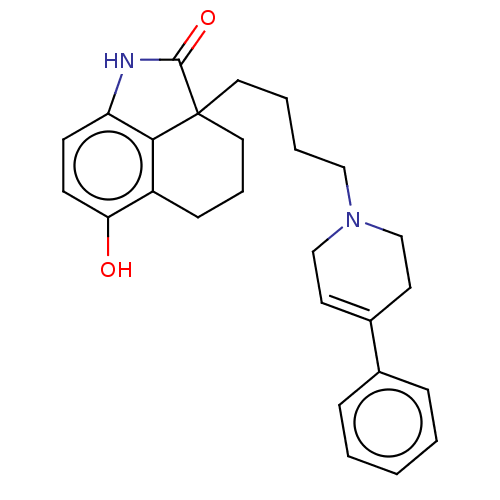 (CHEMBL422587) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50473657 (CHEMBL70889) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd. Curated by ChEMBL | Assay Description Binding affinity to human recombinant 5-hydroxytryptamine 7 receptor in mammalian cells using [3H]5-CT as radioligand | J Med Chem 45: 2197-206 (2002) Article DOI: 10.1021/jm0104264 BindingDB Entry DOI: 10.7270/Q2S46VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 977 total ) | Next | Last >> |