

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124354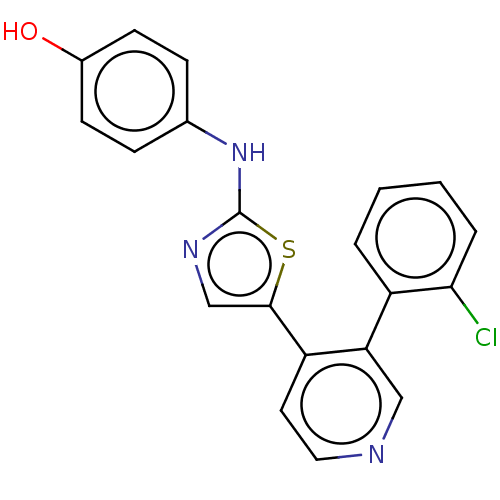 (CHEMBL3623439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50124354 (CHEMBL3623439) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LIMK2 | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124355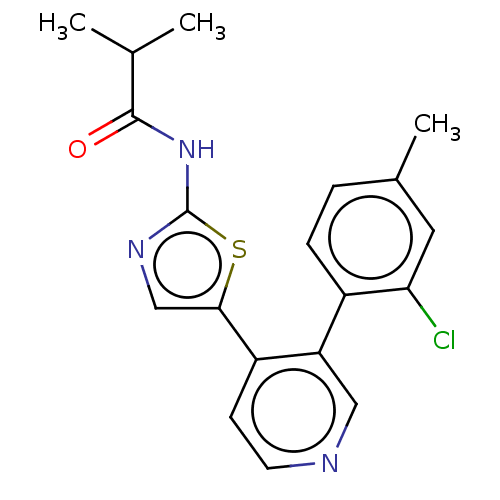 (CHEMBL3623442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50124355 (CHEMBL3623442) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LIMK2 | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124400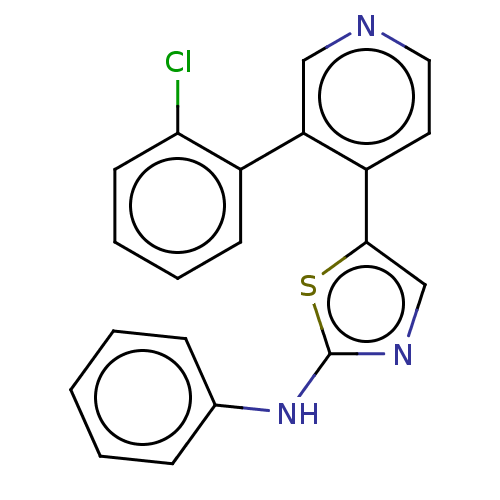 (CHEMBL3623438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124352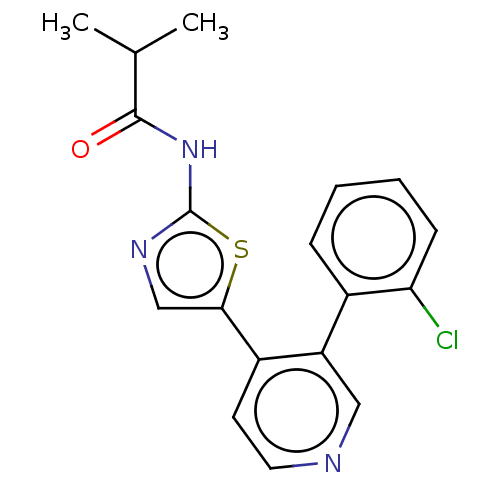 (CHEMBL3623441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124399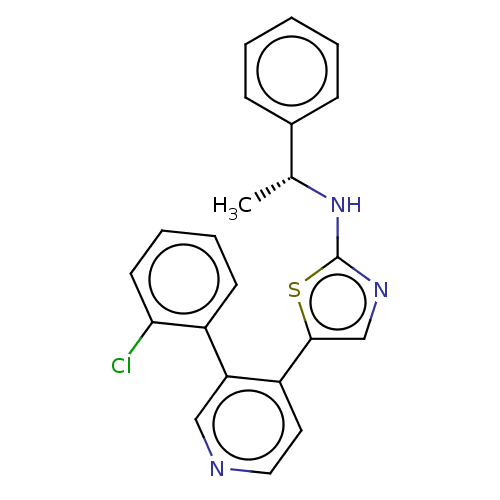 (CHEMBL3623437) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591272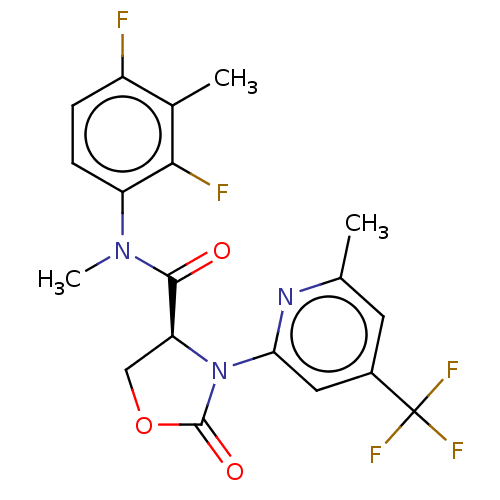 (CHEMBL5208956) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124397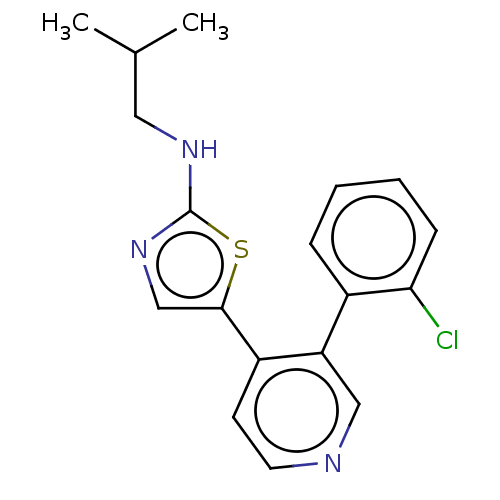 (CHEMBL3623435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591280 (CHEMBL5205456) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591283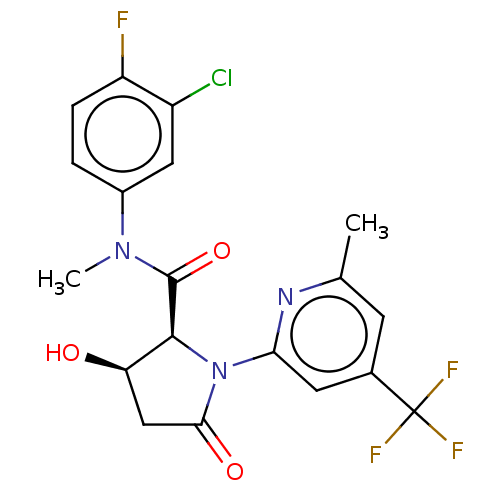 (CHEMBL5190089) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591246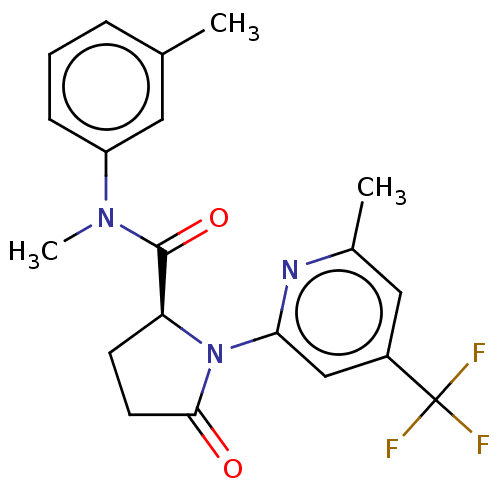 (CHEMBL5175531) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM109086 (US10793535, Cmpd ID 727 | US8604016, 670 | US99382...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514979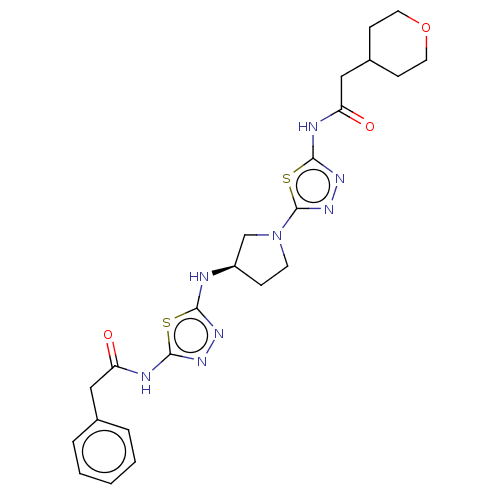 (CHEMBL4457936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591270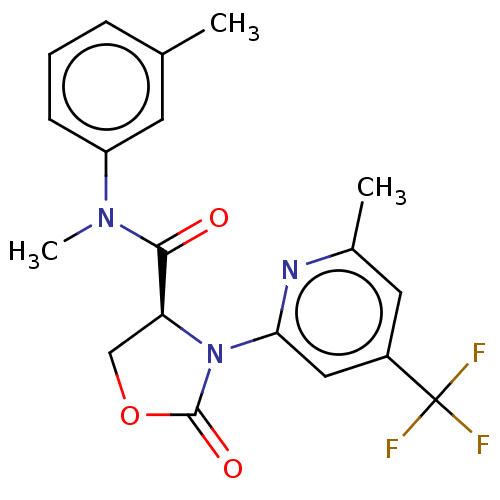 (CHEMBL5176919) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591285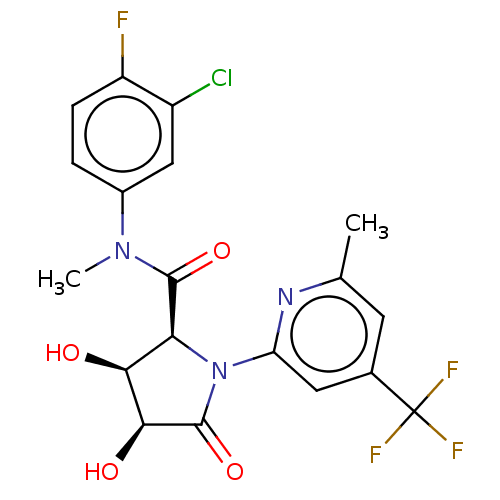 (CHEMBL5191330) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591248 (CHEMBL5206992) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124353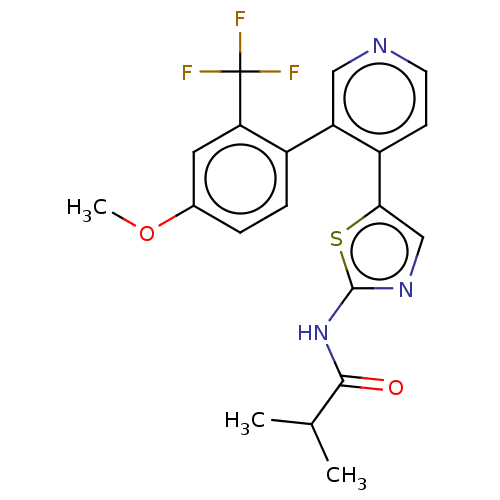 (CHEMBL3623443) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514986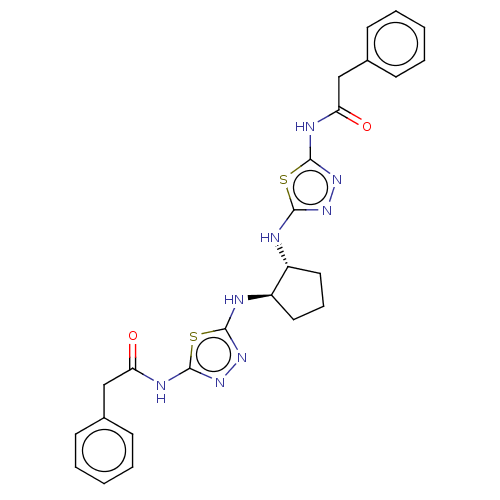 (CHEMBL4437956) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591255 (CHEMBL5180095) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591271 (CHEMBL5200410) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514985 (CHEMBL4573635) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124363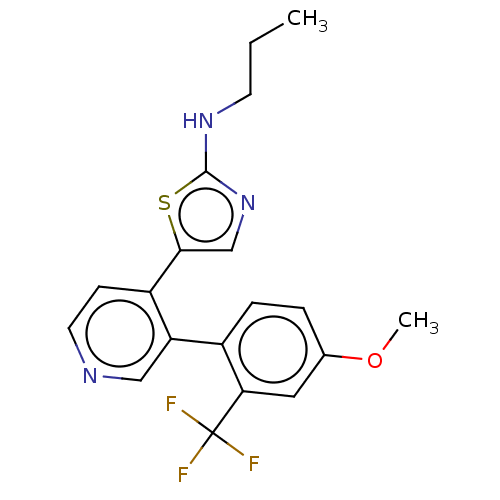 (CHEMBL3622874) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514975 (CHEMBL4473143) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50150109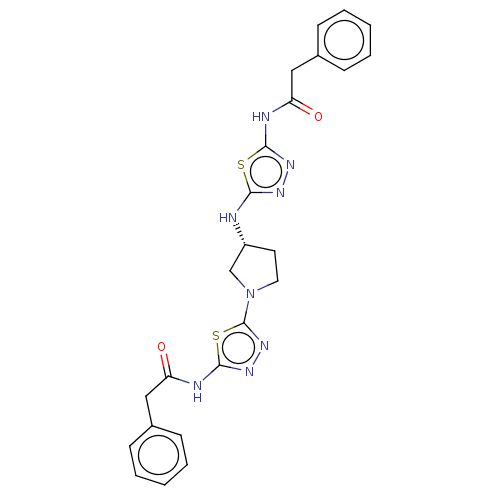 (CHEMBL3770355) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278409 ((2S)-2-[3-(Difluoromethoxy)phenyl]-2-methoxy-N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278409 ((2S)-2-[3-(Difluoromethoxy)phenyl]-2-methoxy-N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591279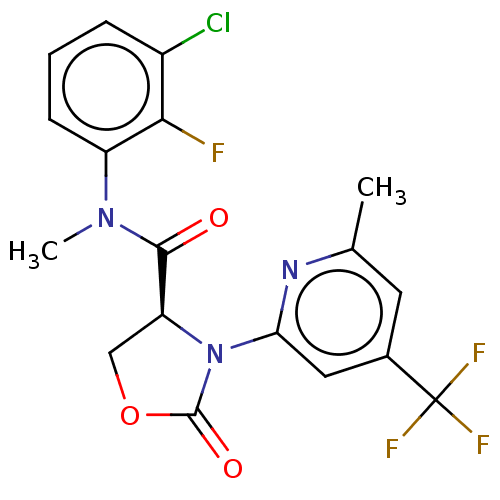 (CHEMBL5191696) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591273 (CHEMBL5205799) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591274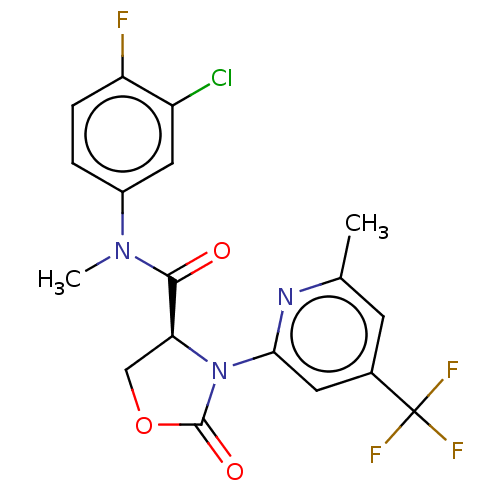 (CHEMBL5184660) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124356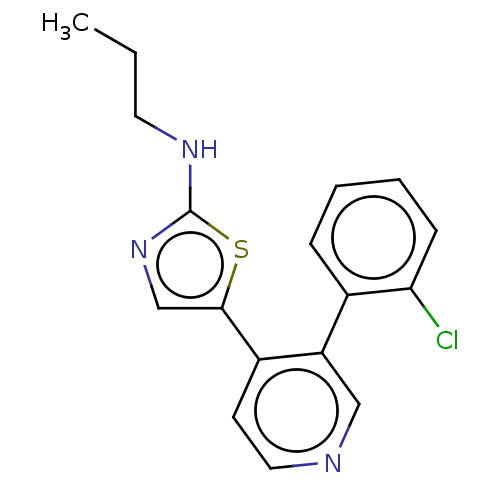 (CHEMBL3622867) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400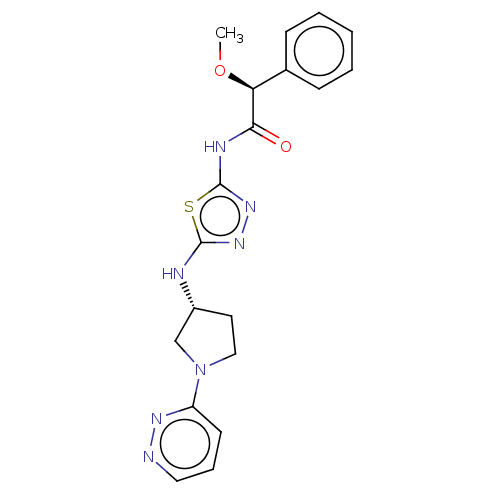 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278401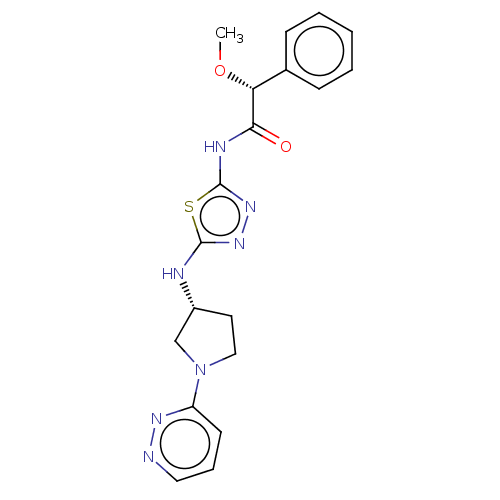 ((2R)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514977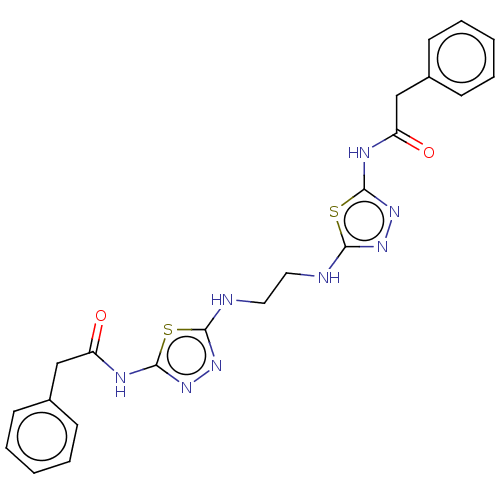 (CHEMBL4461749) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278401 ((2R)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514982 (CHEMBL4469711) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278413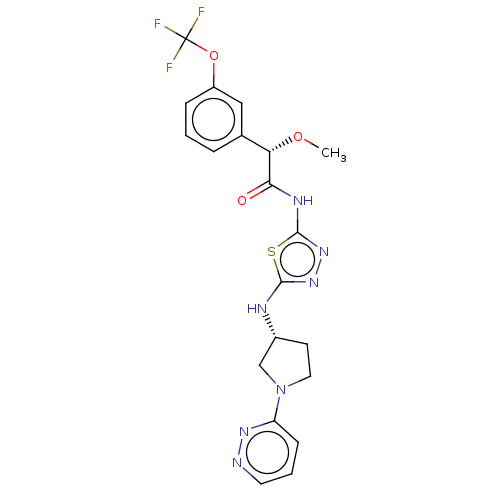 ((2S)-2-Methoxy-N-[5-[[(3R)-1-pyridazin-3-ylpyrroli...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278413 ((2S)-2-Methoxy-N-[5-[[(3R)-1-pyridazin-3-ylpyrroli...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124351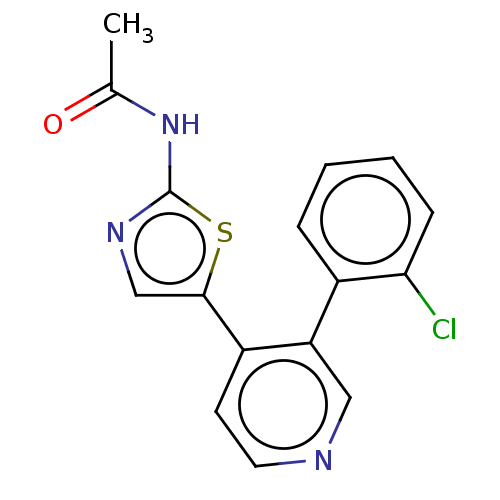 (CHEMBL3623440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591284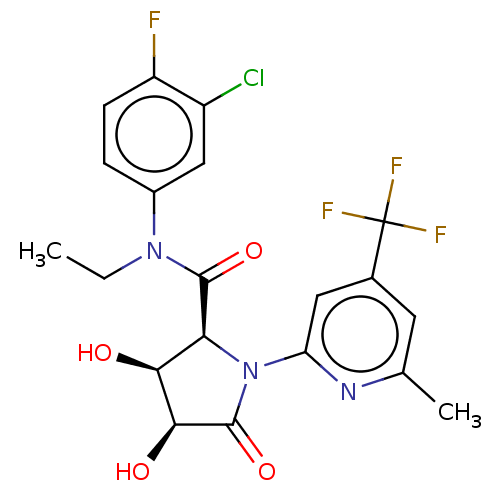 (CHEMBL5171679) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278450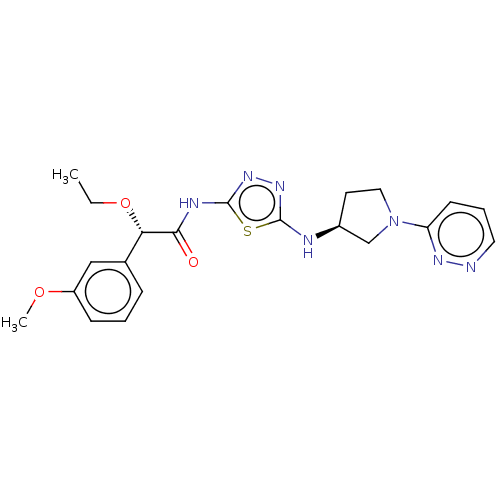 (US10040788, Example 30(b)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM387867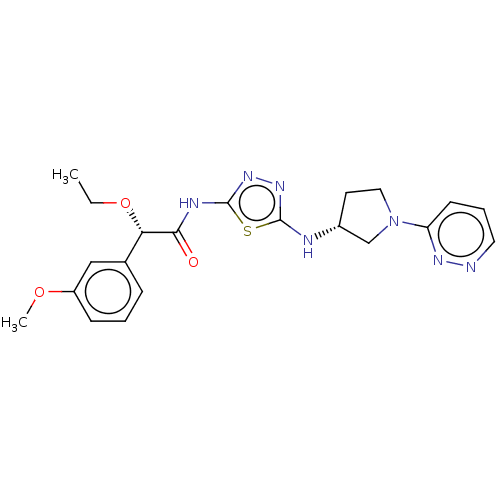 (US10294221, Example 30(b)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278405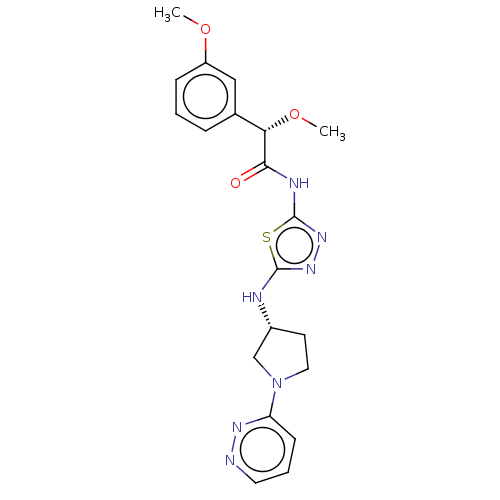 ((2S)-2-Methoxy-2-(3-methoxyphenyl)-N-[5-[[(3R)-1-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278405 ((2S)-2-Methoxy-2-(3-methoxyphenyl)-N-[5-[[(3R)-1-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM387835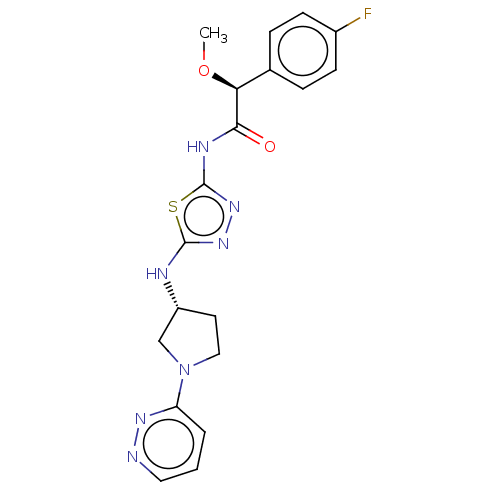 ((2S)-2-(4-Fluorophenyl)-2-methoxy-N-[5-[[(3R)-1-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278418 ((2S)-2-(4-Fluorophenyl)-2-methoxy-N-[5-[[(3R)-1-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 273 total ) | Next | Last >> |