

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50251208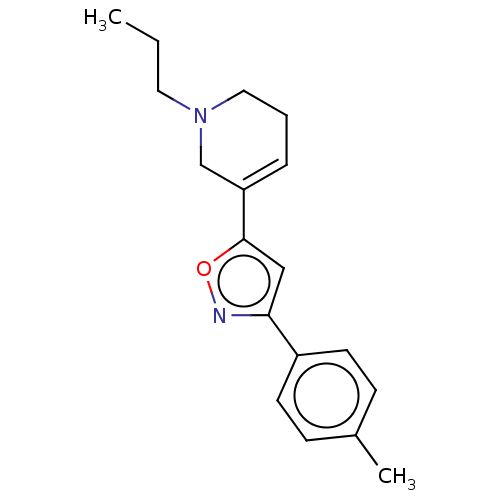 (CHEMBL4088272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021126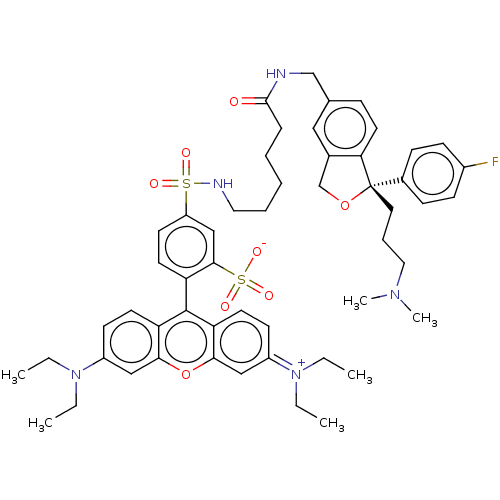 (CHEMBL3287656) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-S-citalopram from human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to [3H]-S-citalopram addit... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50302225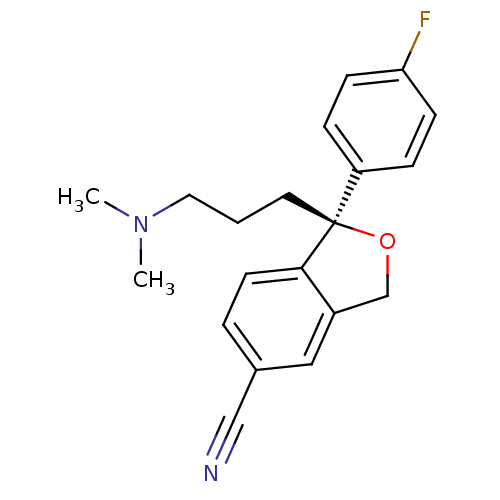 ((1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021111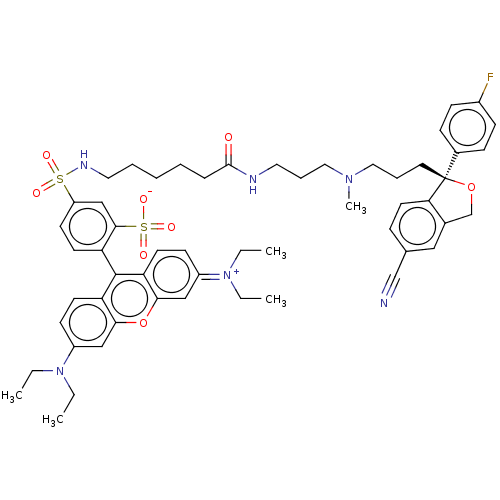 (CHEMBL3287653) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-S-citalopram from human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to [3H]-S-citalopram addit... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021113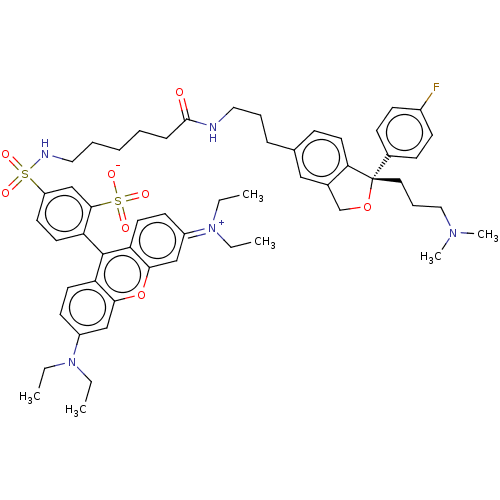 (CHEMBL3287655) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-S-citalopram from human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to [3H]-S-citalopram addit... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177767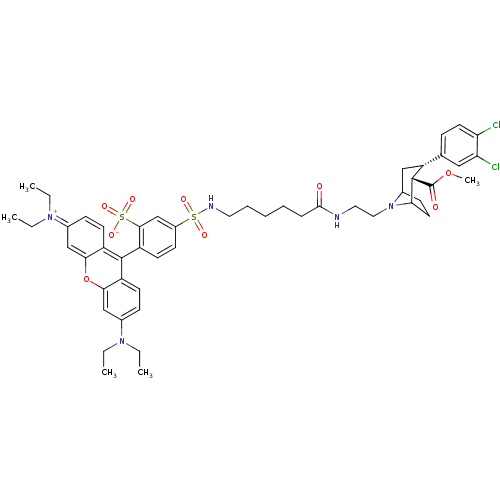 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 2,5,6-[3H]-dopamine uptake at human DAT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand additio... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021126 (CHEMBL3287656) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021113 (CHEMBL3287655) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM22985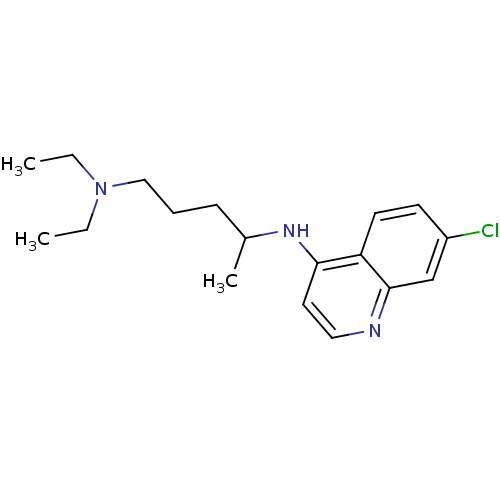 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 2,5,6-[3H]-dopamine uptake at human NET expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand additio... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-sensitive adenylate cyclase (Bacillus anthracis) | BDBM50304913 ((2R,3R,4R,5R)-2-(6-amino-2-oxopyrimidin-1(2H)-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis edema factor catalytic domain by radiometric assay | Bioorg Med Chem Lett 20: 232-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.125 BindingDB Entry DOI: 10.7270/Q2CF9Q5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50467780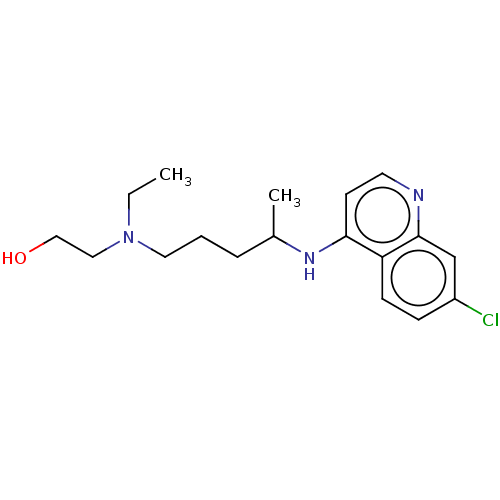 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021111 (CHEMBL3287653) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha-2A (ADRA2A) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021112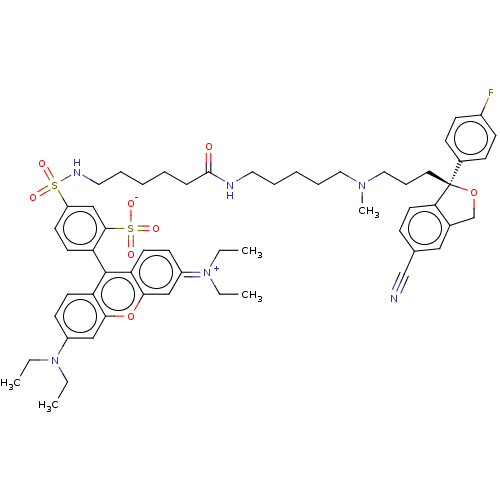 (CHEMBL3287654) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-S-citalopram from human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to [3H]-S-citalopram addit... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha 2C (ADRA2C) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50021112 (CHEMBL3287654) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of 5-hydroxy[3H]tryptamine uptake at human SERT expressed in African green monkey COS7 cells incubated for 10 mins prior to radioligand ad... | ACS Med Chem Lett 5: 696-9 (2014) Article DOI: 10.1021/ml5000806 BindingDB Entry DOI: 10.7270/Q2XW4MCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha 2C (ADRA2C) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha 2B (ADRA2B) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM22985 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50251208 (CHEMBL4088272) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-sensitive adenylate cyclase (Bacillus anthracis) | BDBM50206817 ((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis edema factor catalytic domain by radiometric assay | Bioorg Med Chem Lett 20: 232-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.125 BindingDB Entry DOI: 10.7270/Q2CF9Q5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50467780 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM24226 (1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha-2A (ADRA2A) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50467780 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM22985 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM22985 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM22985 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Alpha-2A (ADRA2A) adrenergic receptor by displacement of [3H]-rauwolscine | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM22985 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50467780 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50467780 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 558 total ) | Next | Last >> |