 Found 469 hits with Last Name = 'jacks' and Initial = 't'
Found 469 hits with Last Name = 'jacks' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50029464
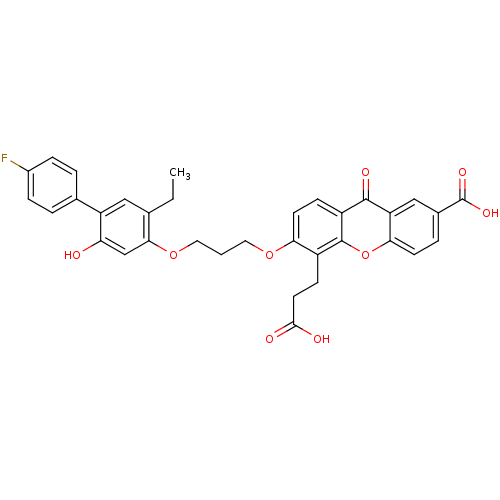
(5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...)Show SMILES CCc1cc(c(O)cc1OCCCOc1ccc2c(oc3ccc(cc3c2=O)C(O)=O)c1CCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FO9/c1-2-19-16-25(20-4-7-22(35)8-5-20)27(36)18-30(19)43-15-3-14-42-28-12-9-24-32(39)26-17-21(34(40)41)6-11-29(26)44-33(24)23(28)10-13-31(37)38/h4-9,11-12,16-18,36H,2-3,10,13-15H2,1H3,(H,37,38)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50283055
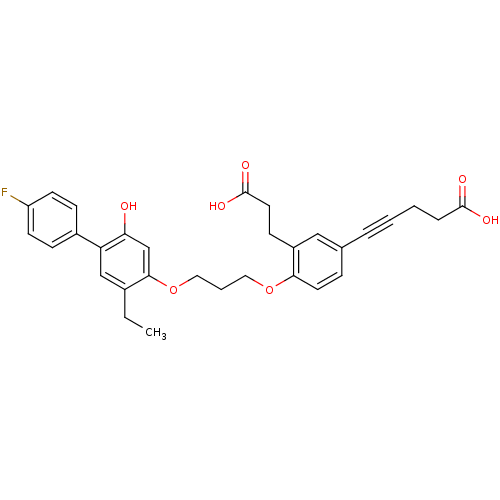
(5-{3-(2-Carboxy-ethyl)-4-[3-(5-ethyl-4'-fluoro-2-h...)Show SMILES CCc1cc(c(O)cc1OCCCOc1ccc(cc1CCC(O)=O)C#CCCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C31H31FO7/c1-2-22-19-26(23-9-12-25(32)13-10-23)27(33)20-29(22)39-17-5-16-38-28-14-8-21(6-3-4-7-30(34)35)18-24(28)11-15-31(36)37/h8-10,12-14,18-20,33H,2,4-5,7,11,15-17H2,1H3,(H,34,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50283055

(5-{3-(2-Carboxy-ethyl)-4-[3-(5-ethyl-4'-fluoro-2-h...)Show SMILES CCc1cc(c(O)cc1OCCCOc1ccc(cc1CCC(O)=O)C#CCCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C31H31FO7/c1-2-22-19-26(23-9-12-25(32)13-10-23)27(33)20-29(22)39-17-5-16-38-28-14-8-21(6-3-4-7-30(34)35)18-24(28)11-15-31(36)37/h8-10,12-14,18-20,33H,2,4-5,7,11,15-17H2,1H3,(H,34,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50013889
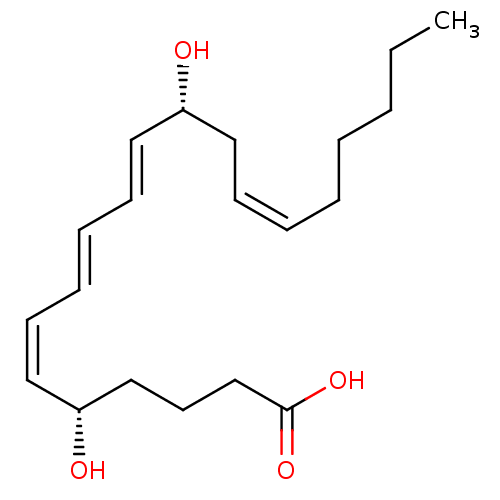
((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes |
Bioorg Med Chem Lett 3: 1981-1984 (1993)
Article DOI: 10.1016/S0960-894X(01)80999-9
BindingDB Entry DOI: 10.7270/Q2VH5P9R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50013889

((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50283054
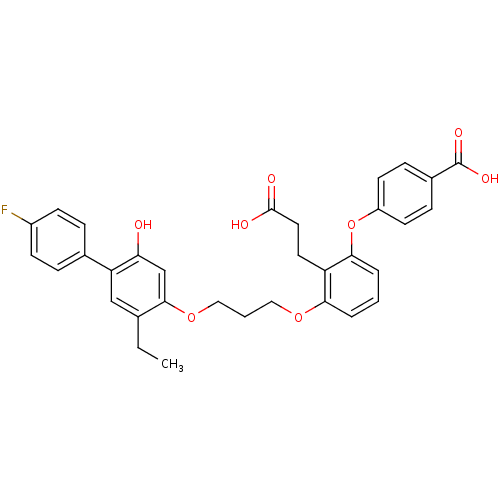
(4-{2-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...)Show SMILES CCc1cc(c(O)cc1OCCCOc1cccc(Oc2ccc(cc2)C(O)=O)c1CCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C33H31FO8/c1-2-21-19-27(22-7-11-24(34)12-8-22)28(35)20-31(21)41-18-4-17-40-29-5-3-6-30(26(29)15-16-32(36)37)42-25-13-9-23(10-14-25)33(38)39/h3,5-14,19-20,35H,2,4,15-18H2,1H3,(H,36,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50029464

(5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...)Show SMILES CCc1cc(c(O)cc1OCCCOc1ccc2c(oc3ccc(cc3c2=O)C(O)=O)c1CCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FO9/c1-2-19-16-25(20-4-7-22(35)8-5-20)27(36)18-30(19)43-15-3-14-42-28-12-9-24-32(39)26-17-21(34(40)41)6-11-29(26)44-33(24)23(28)10-13-31(37)38/h4-9,11-12,16-18,36H,2-3,10,13-15H2,1H3,(H,37,38)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096823
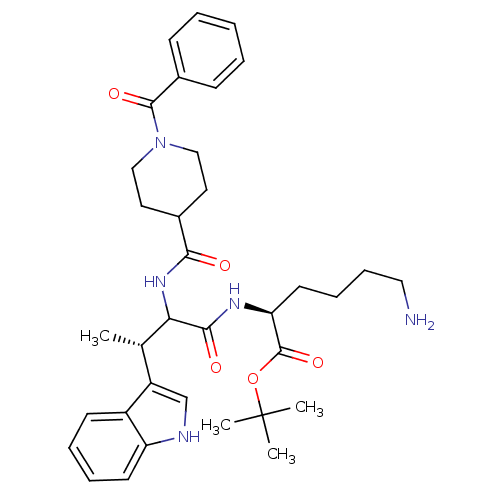
((S)-6-Amino-2-[(S)-2-[(1-benzoyl-piperidine-4-carb...)Show SMILES C[C@H](C(NC(=O)C1CCN(CC1)C(=O)c1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N5O5/c1-23(27-22-37-28-15-9-8-14-26(27)28)30(32(42)38-29(16-10-11-19-36)34(44)45-35(2,3)4)39-31(41)24-17-20-40(21-18-24)33(43)25-12-6-5-7-13-25/h5-9,12-15,22-24,29-30,37H,10-11,16-21,36H2,1-4H3,(H,38,42)(H,39,41)/t23-,29-,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound to Somatostatin receptor type 2 was determined |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2C receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075292
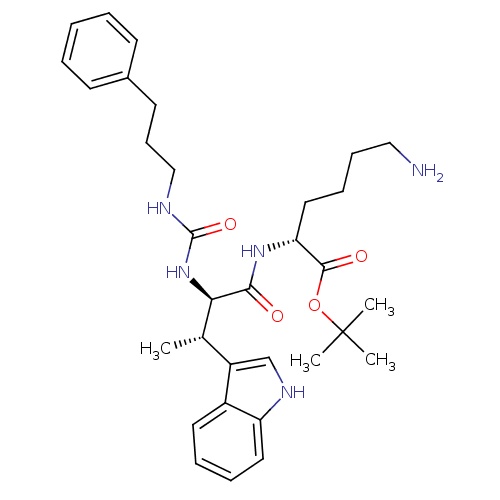
((R)-6-Amino-2-{(2R,3S)-3-(1H-indol-3-yl)-2-[3-(3-p...)Show SMILES C[C@H]([C@@H](NC(=O)NCCCc1ccccc1)C(=O)N[C@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C32H45N5O4/c1-22(25-21-35-26-17-9-8-16-24(25)26)28(37-31(40)34-20-12-15-23-13-6-5-7-14-23)29(38)36-27(18-10-11-19-33)30(39)41-32(2,3)4/h5-9,13-14,16-17,21-22,27-28,35H,10-12,15,18-20,33H2,1-4H3,(H,36,38)(H2,34,37,40)/t22-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50283054

(4-{2-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...)Show SMILES CCc1cc(c(O)cc1OCCCOc1cccc(Oc2ccc(cc2)C(O)=O)c1CCC(O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C33H31FO8/c1-2-21-19-27(22-7-11-24(34)12-8-22)28(35)20-31(21)41-18-4-17-40-29-5-3-6-30(26(29)15-16-32(36)37)42-25-13-9-23(10-14-25)33(38)39/h3,5-14,19-20,35H,2,4,15-18H2,1H3,(H,36,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50280884
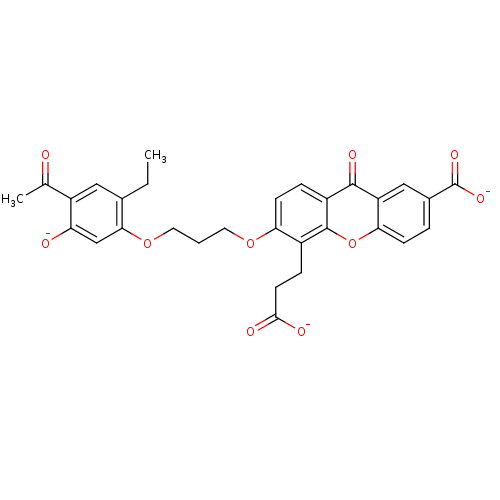
(3-{3-[3-(4-acetyl-2-ethyl-5-olatophenoxy)propoxy]-...)Show SMILES CCc1cc(C(C)=O)c([O-])cc1OCCCOc1ccc2c(oc3ccc(cc3c2=O)C([O-])=O)c1CCC([O-])=O Show InChI InChI=1S/C30H28O10/c1-3-17-13-21(16(2)31)23(32)15-26(17)39-12-4-11-38-24-9-6-20-28(35)22-14-18(30(36)37)5-8-25(22)40-29(20)19(24)7-10-27(33)34/h5-6,8-9,13-15,32H,3-4,7,10-12H2,1-2H3,(H,33,34)(H,36,37)/p-3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes |
Bioorg Med Chem Lett 3: 1981-1984 (1993)
Article DOI: 10.1016/S0960-894X(01)80999-9
BindingDB Entry DOI: 10.7270/Q2VH5P9R |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075271
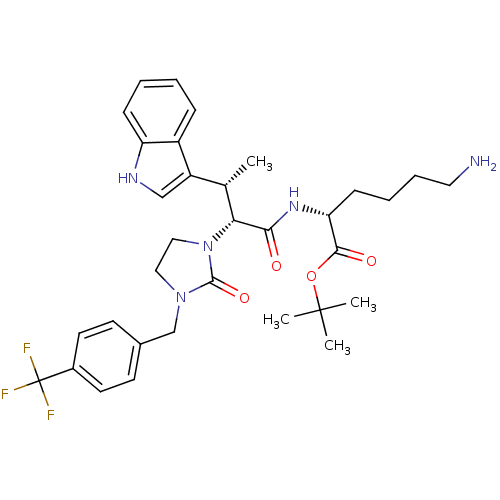
((R)-6-Amino-2-{(2R,3S)-3-(1H-indol-3-yl)-2-[2-oxo-...)Show SMILES C[C@H]([C@@H](N1CCN(Cc2ccc(cc2)C(F)(F)F)C1=O)C(=O)N[C@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C33H42F3N5O4/c1-21(25-19-38-26-10-6-5-9-24(25)26)28(29(42)39-27(11-7-8-16-37)30(43)45-32(2,3)4)41-18-17-40(31(41)44)20-22-12-14-23(15-13-22)33(34,35)36/h5-6,9-10,12-15,19,21,27-28,38H,7-8,11,16-18,20,37H2,1-4H3,(H,39,42)/t21-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D2 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075283

((R)-6-Amino-2-{(2R,3S)-3-(1H-indol-3-yl)-2-[2-oxo-...)Show SMILES C[C@H]([C@@H](N1CCN(C2CCCN(C2)c2ccccc2)C1=O)C(=O)N[C@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C36H50N6O4/c1-25(29-23-38-30-17-9-8-16-28(29)30)32(33(43)39-31(18-10-11-19-37)34(44)46-36(2,3)4)42-22-21-41(35(42)45)27-15-12-20-40(24-27)26-13-6-5-7-14-26/h5-9,13-14,16-17,23,25,27,31-32,38H,10-12,15,18-22,24,37H2,1-4H3,(H,39,43)/t25-,27?,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473
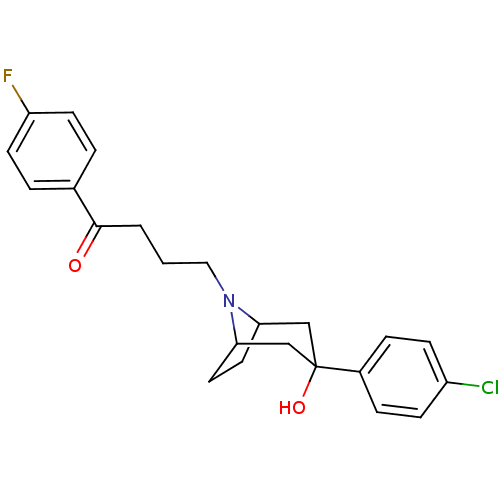
(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D2 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned histamine H1 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned muscarinic M1 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50013889

((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes |
Bioorg Med Chem Lett 4: 2077-2082 (1994)
Article DOI: 10.1016/S0960-894X(01)80105-0
BindingDB Entry DOI: 10.7270/Q27D2V2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50280292
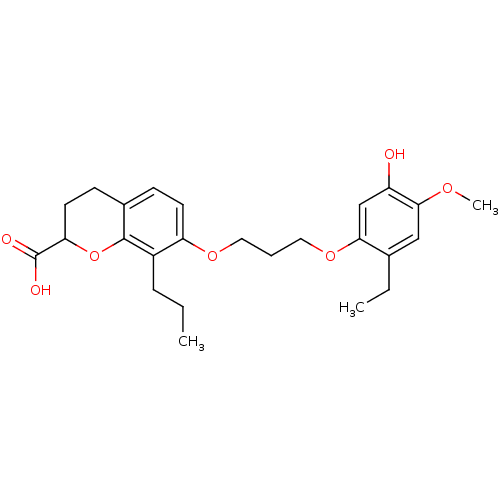
(7-[3-(2-Ethyl-5-hydroxy-4-methoxy-phenoxy)-propoxy...)Show SMILES CCCc1c(OCCCOc2cc(O)c(OC)cc2CC)ccc2CCC(Oc12)C(O)=O Show InChI InChI=1S/C25H32O7/c1-4-7-18-20(10-8-17-9-11-21(25(27)28)32-24(17)18)30-12-6-13-31-22-15-19(26)23(29-3)14-16(22)5-2/h8,10,14-15,21,26H,4-7,9,11-13H2,1-3H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity using [3H]-LTB4 radioligand binding to leukotriene B4 receptor in guinea pig lung membrane binding assay |
Bioorg Med Chem Lett 2: 1675-1680 (1992)
Article DOI: 10.1016/S0960-894X(00)80454-0
BindingDB Entry DOI: 10.7270/Q2JW8DT9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM50075278
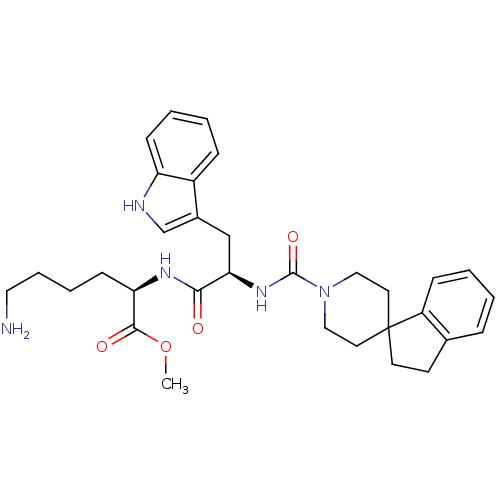
(CHEMBL147319 | methyl 6-amino-2-[2-(1H-3-indolyl)-...)Show SMILES COC(=O)[C@@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCC2(CCc3ccccc23)CC1 Show InChI InChI=1S/C32H41N5O4/c1-41-30(39)27(12-6-7-17-33)35-29(38)28(20-23-21-34-26-11-5-3-9-24(23)26)36-31(40)37-18-15-32(16-19-37)14-13-22-8-2-4-10-25(22)32/h2-5,8-11,21,27-28,34H,6-7,12-20,33H2,1H3,(H,35,38)(H,36,40)/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was evaluated against mouse Somatostatin receptor type 2 (mSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075273

((2R,3S)-N-(5-Amino-[1,3]dioxan-2-ylmethyl)-3-(1H-i...)Show SMILES C[C@H]([C@@H](N1CCN(C2CCCN(C2)c2ccccc2)C1=O)C(=O)N(C)C[C@H]1OC[C@H](N)CO1)c1c[nH]c2ccccc12 |wU:2.2,26.28,wD:29.32,1.0,(-1.79,4.28,;-1.77,2.74,;-.43,1.97,;.89,2.75,;.87,4.29,;3.54,4.3,;3.55,2.77,;4.9,2.04,;4.96,.5,;6.32,-.22,;7.63,.57,;7.58,2.11,;6.22,2.84,;8.89,2.93,;8.83,4.47,;10.14,5.28,;11.5,4.54,;11.54,3,;10.24,2.19,;2.22,2,;2.24,.46,;-.43,.44,;1.1,.43,;-1.21,-.89,;-2.75,-.87,;-.46,-2.22,;-1.24,-3.54,;-.47,-4.88,;-1.25,-6.21,;-2.8,-6.2,;-3.58,-7.51,;-3.56,-4.86,;-2.77,-3.53,;-3.1,1.96,;-3.09,.43,;-4.42,-.36,;-5.75,.41,;-7.08,-.36,;-8.43,.41,;-8.43,1.96,;-7.08,2.73,;-5.75,1.96,)| Show InChI InChI=1S/C32H42N6O4/c1-22(27-17-34-28-13-7-6-12-26(27)28)30(31(39)35(2)19-29-41-20-23(33)21-42-29)38-16-15-37(32(38)40)25-11-8-14-36(18-25)24-9-4-3-5-10-24/h3-7,9-10,12-13,17,22-23,25,29-30,34H,8,11,14-16,18-21,33H2,1-2H3/t22-,23-,25?,29-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was evaluated against human Somatostatin receptor type 2 in experiment 1 |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D2 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075286
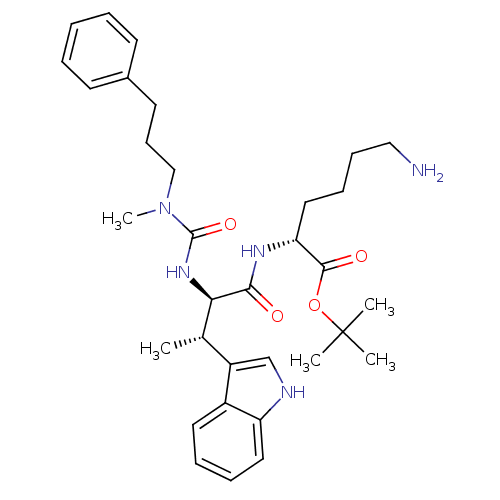
((R)-6-Amino-2-{(2R,3S)-3-(1H-indol-3-yl)-2-[3-meth...)Show SMILES C[C@H]([C@@H](NC(=O)N(C)CCCc1ccccc1)C(=O)N[C@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C33H47N5O4/c1-23(26-22-35-27-18-10-9-17-25(26)27)29(37-32(41)38(5)21-13-16-24-14-7-6-8-15-24)30(39)36-28(19-11-12-20-34)31(40)42-33(2,3)4/h6-10,14-15,17-18,22-23,28-29,35H,11-13,16,19-21,34H2,1-5H3,(H,36,39)(H,37,41)/t23-,28+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50262881
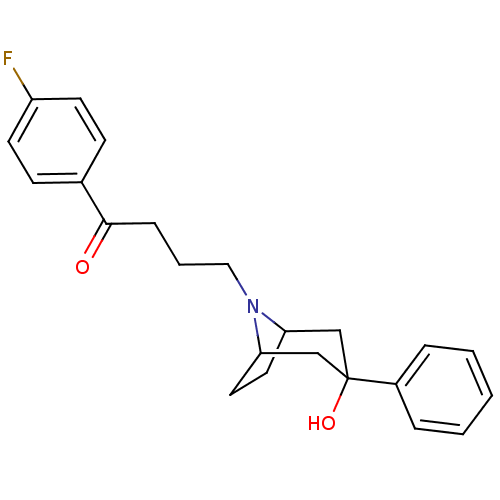
(1-(4-fluorophenyl)-4-(3-hydroxy-3-phenyl-8-aza-bic...)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccccc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H26FNO2/c24-19-10-8-17(9-11-19)22(26)7-4-14-25-20-12-13-21(25)16-23(27,15-20)18-5-2-1-3-6-18/h1-3,5-6,8-11,20-21,27H,4,7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D2 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096830

((S)-6-Amino-2-[(S)-2-({1-[2-(3,5-difluoro-phenyl)-...)Show SMILES C[C@H](C(NC(=O)C1CCCN(C1)C(=O)Cc1cc(F)cc(F)c1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C36H47F2N5O5/c1-22(28-20-40-29-12-6-5-11-27(28)29)32(34(46)41-30(13-7-8-14-39)35(47)48-36(2,3)4)42-33(45)24-10-9-15-43(21-24)31(44)18-23-16-25(37)19-26(38)17-23/h5-6,11-12,16-17,19-20,22,24,30,32,40H,7-10,13-15,18,21,39H2,1-4H3,(H,41,46)(H,42,45)/t22-,24?,30-,32?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT1A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D3 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096824

((S)-6-Amino-2-[(S)-2-[(1-benzyl-piperidine-4-carbo...)Show SMILES C[C@H](C(NC(=O)C1CCN(Cc2ccccc2)CC1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H49N5O4/c1-24(28-22-37-29-15-9-8-14-27(28)29)31(33(42)38-30(16-10-11-19-36)34(43)44-35(2,3)4)39-32(41)26-17-20-40(21-18-26)23-25-12-6-5-7-13-25/h5-9,12-15,22,24,26,30-31,37H,10-11,16-21,23,36H2,1-4H3,(H,38,42)(H,39,41)/t24-,30-,31?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound to Somatostatin receptor type 2 was determined |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096829

((S)-6-Amino-2-[(S)-2-{[1-(2-hydroxy-2,2-diphenyl-a...)Show SMILES C[C@H](C(NC(=O)C1CCCN(C1)C(=O)C(O)(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C42H53N5O6/c1-28(33-26-44-34-22-12-11-21-32(33)34)36(38(49)45-35(23-13-14-24-43)39(50)53-41(2,3)4)46-37(48)29-16-15-25-47(27-29)40(51)42(52,30-17-7-5-8-18-30)31-19-9-6-10-20-31/h5-12,17-22,26,28-29,35-36,44,52H,13-16,23-25,27,43H2,1-4H3,(H,45,49)(H,46,48)/t28-,29?,35-,36?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned histamine H1 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096835
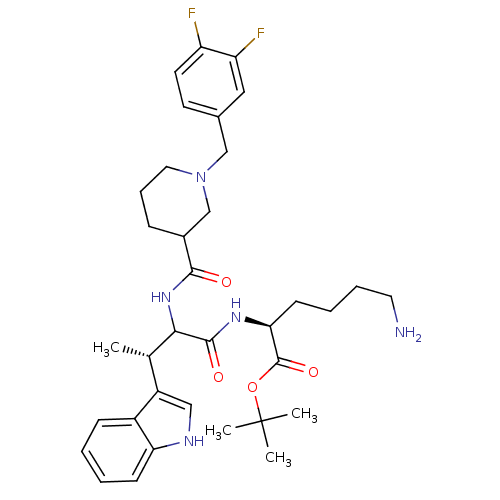
((S)-6-Amino-2-[(S)-2-{[1-(3,4-difluoro-benzyl)-pip...)Show SMILES C[C@H](C(NC(=O)C1CCCN(Cc2ccc(F)c(F)c2)C1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47F2N5O4/c1-22(26-19-39-29-12-6-5-11-25(26)29)31(33(44)40-30(13-7-8-16-38)34(45)46-35(2,3)4)41-32(43)24-10-9-17-42(21-24)20-23-14-15-27(36)28(37)18-23/h5-6,11-12,14-15,18-19,22,24,30-31,39H,7-10,13,16-17,20-21,38H2,1-4H3,(H,40,44)(H,41,43)/t22-,24?,30-,31?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D2 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D4 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50280291
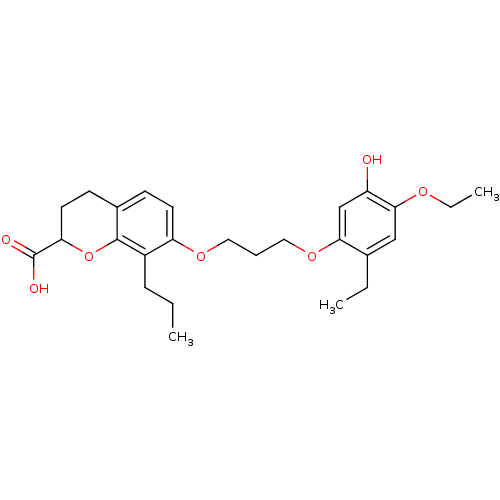
(7-[3-(4-Ethoxy-2-ethyl-5-hydroxy-phenoxy)-propoxy]...)Show SMILES CCCc1c(OCCCOc2cc(O)c(OCC)cc2CC)ccc2CCC(Oc12)C(O)=O Show InChI InChI=1S/C26H34O7/c1-4-8-19-21(11-9-18-10-12-22(26(28)29)33-25(18)19)31-13-7-14-32-23-16-20(27)24(30-6-3)15-17(23)5-2/h9,11,15-16,22,27H,4-8,10,12-14H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity using [3H]-LTB4 radioligand binding to leukotriene B4 receptor in guinea pig lung membrane binding assay |
Bioorg Med Chem Lett 2: 1675-1680 (1992)
Article DOI: 10.1016/S0960-894X(00)80454-0
BindingDB Entry DOI: 10.7270/Q2JW8DT9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075275
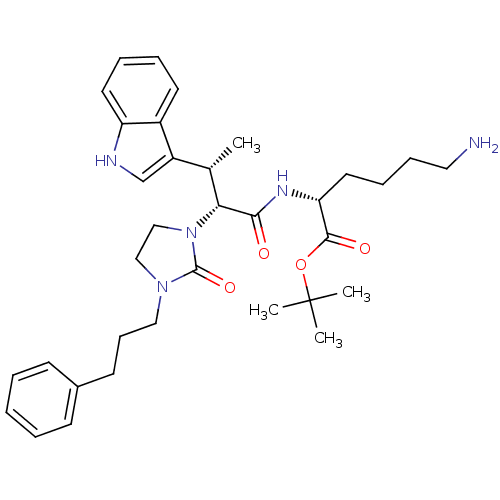
((R)-6-Amino-2-{(2R,3S)-3-(1H-indol-3-yl)-2-[2-oxo-...)Show SMILES C[C@H]([C@@H](N1CCN(CCCc2ccccc2)C1=O)C(=O)N[C@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C34H47N5O4/c1-24(27-23-36-28-17-9-8-16-26(27)28)30(31(40)37-29(18-10-11-19-35)32(41)43-34(2,3)4)39-22-21-38(33(39)42)20-12-15-25-13-6-5-7-14-25/h5-9,13-14,16-17,23-24,29-30,36H,10-12,15,18-22,35H2,1-4H3,(H,37,40)/t24-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50231624
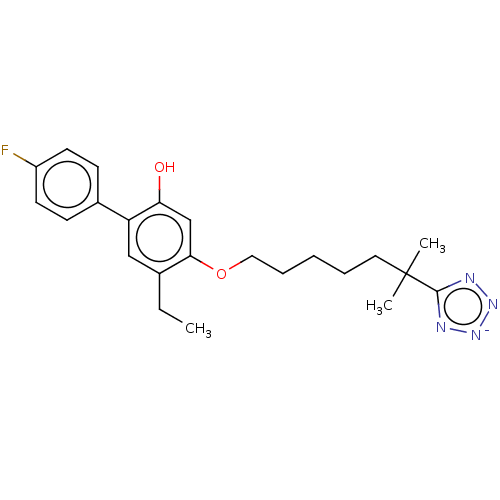
(CHEMBL129703)Show SMILES [Na+].CCc1cc(c(O)cc1OCCCCCC(C)(C)c1nn[n-]n1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H28FN4O2/c1-4-16-14-19(17-8-10-18(24)11-9-17)20(29)15-21(16)30-13-7-5-6-12-23(2,3)22-25-27-28-26-22/h8-11,14-15H,4-7,12-13H2,1-3H3,(H-,25,26,27,28,29)/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]LTB4 receptor binding in guinea pig lung membrane |
J Med Chem 36: 3978-81 (1993)
Article DOI: 10.1021/jm00076a029
BindingDB Entry DOI: 10.7270/Q20V8G1H |
More data for this
Ligand-Target Pair | |
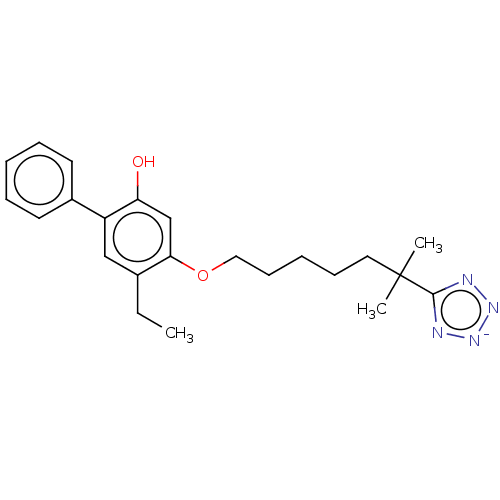
Leukotriene B4 receptor 1/2
(Homo sapiens (Human)) | BDBM50231626
(CHEMBL128013)Show SMILES [Na+].CCc1cc(c(O)cc1OCCCCCC(C)(C)c1nn[n-]n1)-c1ccccc1 Show InChI InChI=1S/C23H29N4O2/c1-4-17-15-19(18-11-7-5-8-12-18)20(28)16-21(17)29-14-10-6-9-13-23(2,3)22-24-26-27-25-22/h5,7-8,11-12,15-16H,4,6,9-10,13-14H2,1-3H3,(H-,24,25,26,27,28)/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]LTB4 receptor binding in guinea pig lung membrane |
J Med Chem 36: 3978-81 (1993)
Article DOI: 10.1021/jm00076a029
BindingDB Entry DOI: 10.7270/Q20V8G1H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50075290
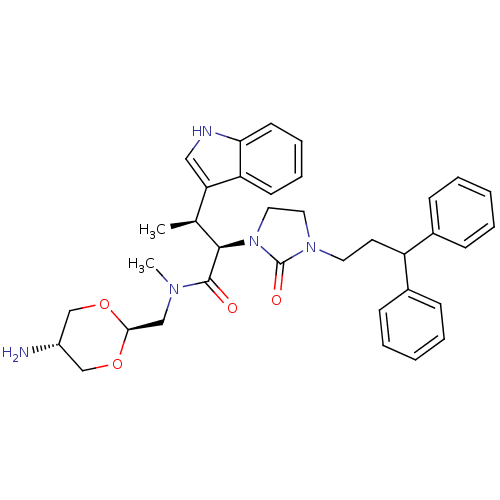
((2R,3S)-N-(5-Amino-[1,3]dioxan-2-ylmethyl)-2-[3-(3...)Show SMILES C[C@H]([C@@H](N1CCN(CCC(c2ccccc2)c2ccccc2)C1=O)C(=O)N(C)C[C@H]1OC[C@H](N)CO1)c1c[nH]c2ccccc12 |wU:2.2,29.31,wD:32.35,1.0,(-1.21,3.06,;-1.19,1.52,;.15,.76,;1.47,1.53,;1.45,3.07,;4.13,3.1,;4.14,1.56,;5.48,.79,;6.8,1.58,;8.14,.81,;9.47,1.59,;10.8,.83,;12.13,1.6,;12.12,3.14,;10.76,3.9,;9.45,3.12,;8.15,-.73,;6.82,-1.5,;6.84,-3.04,;8.17,-3.81,;9.5,-3.02,;9.48,-1.48,;2.81,.77,;2.82,-.77,;.15,-.78,;1.69,-.78,;-.63,-2.11,;-2.17,-2.08,;.12,-3.44,;-.65,-4.77,;.12,-6.11,;-.67,-7.44,;-2.22,-7.43,;-3,-8.75,;-2.96,-6.07,;-2.19,-4.76,;-2.52,.74,;-2.51,-.79,;-3.84,-1.57,;-5.17,-.8,;-6.51,-1.57,;-7.86,-.8,;-7.86,.74,;-6.51,1.51,;-5.17,.74,)| Show InChI InChI=1S/C36H43N5O4/c1-25(31-21-38-32-16-10-9-15-30(31)32)34(35(42)39(2)22-33-44-23-28(37)24-45-33)41-20-19-40(36(41)43)18-17-29(26-11-5-3-6-12-26)27-13-7-4-8-14-27/h3-16,21,25,28-29,33-34,38H,17-20,22-24,37H2,1-2H3/t25-,28-,33-,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was evaluated against human Somatostatin receptor type 2 (hSSTR-2) |
Bioorg Med Chem Lett 9: 491-6 (1999)
BindingDB Entry DOI: 10.7270/Q2FB5235 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096822

((S)-6-Amino-2-[(S)-2-[(1-benzyl-piperidine-3-carbo...)Show SMILES C[C@H](C(NC(=O)C1CCCN(Cc2ccccc2)C1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H49N5O4/c1-24(28-21-37-29-17-9-8-16-27(28)29)31(33(42)38-30(18-10-11-19-36)34(43)44-35(2,3)4)39-32(41)26-15-12-20-40(23-26)22-25-13-6-5-7-14-25/h5-9,13-14,16-17,21,24,26,30-31,37H,10-12,15,18-20,22-23,36H2,1-4H3,(H,38,42)(H,39,41)/t24-,26?,30-,31?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned muscarinic M1 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50281875

(CHEMBL15648 | {3-[3-(4-Acetyl-2-ethyl-5-hydroxy-ph...)Show SMILES CCCc1c(OCCCOc2cc(O)c(cc2CC)C(C)=O)cccc1OCC(O)=O Show InChI InChI=1S/C24H30O7/c1-4-8-18-21(9-6-10-22(18)31-15-24(27)28)29-11-7-12-30-23-14-20(26)19(16(3)25)13-17(23)5-2/h6,9-10,13-14,26H,4-5,7-8,11-12,15H2,1-3H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against LTB4 receptor in guinea pig membrane |
Bioorg Med Chem Lett 3: 1147-1152 (1993)
Article DOI: 10.1016/S0960-894X(00)80304-2
BindingDB Entry DOI: 10.7270/Q2M32VP9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096812
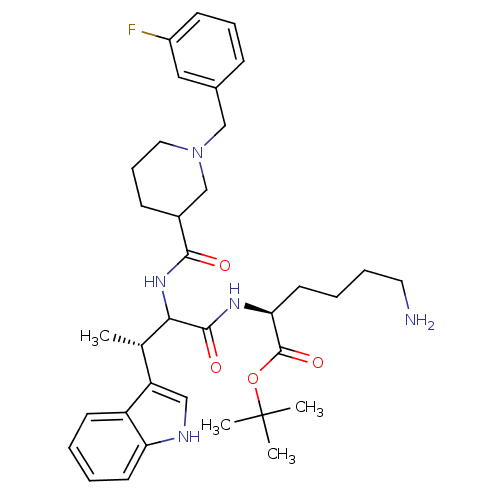
((S)-6-Amino-2-[(S)-2-{[1-(3-fluoro-benzyl)-piperid...)Show SMILES C[C@H](C(NC(=O)C1CCCN(Cc2cccc(F)c2)C1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H48FN5O4/c1-23(28-20-38-29-15-6-5-14-27(28)29)31(33(43)39-30(16-7-8-17-37)34(44)45-35(2,3)4)40-32(42)25-12-10-18-41(22-25)21-24-11-9-13-26(36)19-24/h5-6,9,11,13-15,19-20,23,25,30-31,38H,7-8,10,12,16-18,21-22,37H2,1-4H3,(H,39,43)(H,40,42)/t23-,25?,30-,31?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096810
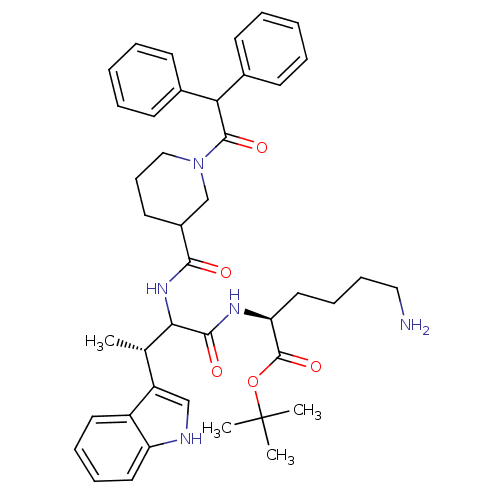
((S)-6-Amino-2-[(S)-2-[(1-diphenylacetyl-piperidine...)Show SMILES C[C@H](C(NC(=O)C1CCCN(C1)C(=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C42H53N5O5/c1-28(33-26-44-34-22-12-11-21-32(33)34)37(39(49)45-35(23-13-14-24-43)41(51)52-42(2,3)4)46-38(48)31-20-15-25-47(27-31)40(50)36(29-16-7-5-8-17-29)30-18-9-6-10-19-30/h5-12,16-19,21-22,26,28,31,35-37,44H,13-15,20,23-25,27,43H2,1-4H3,(H,45,49)(H,46,48)/t28-,31?,35-,37?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinant thymidine phosphorylase TP |
Bioorg Med Chem Lett 13: 107-10 (2002)
BindingDB Entry DOI: 10.7270/Q2GM87V9 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473

(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned dopamine D4 receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50096816

((S)-6-Amino-2-[(S)-2-{[1-(2,3-difluoro-benzyl)-pip...)Show SMILES C[C@H](C(NC(=O)C1CCCN(Cc2cccc(F)c2F)C1)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47F2N5O4/c1-22(26-19-39-28-15-6-5-13-25(26)28)31(33(44)40-29(16-7-8-17-38)34(45)46-35(2,3)4)41-32(43)24-12-10-18-42(21-24)20-23-11-9-14-27(36)30(23)37/h5-6,9,11,13-15,19,22,24,29,31,39H,7-8,10,12,16-18,20-21,38H2,1-4H3,(H,40,44)(H,41,43)/t22-,24?,29-,31?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human somatostatin 2 receptor |
Bioorg Med Chem Lett 11: 415-7 (2001)
BindingDB Entry DOI: 10.7270/Q2474949 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data