

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252005 (2-(3-Benzoylamino-phenyl)-2-oxoethyl ethyl trithio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in HEK293 cells using variable substrate concentration by Lineweaver-Burk plot | J Med Chem 51: 3985-4001 (2008) Article DOI: 10.1021/jm800093c BindingDB Entry DOI: 10.7270/Q29886S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50252371 (CHEMBL520279 | Ethyl 2-oxo-2-[1-(phenyl-sulfonyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in HEK293 cells using variable substrate concentration by Lineweaver-Burk plot | J Med Chem 51: 3985-4001 (2008) Article DOI: 10.1021/jm800093c BindingDB Entry DOI: 10.7270/Q29886S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50252319 (CHEMBL479602 | Ethyl 2-oxo-2-{5-[(2-Phenoxy-ethylc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in HEK293 cells using variable substrate concentration by Lineweaver-Burk plot | J Med Chem 51: 3985-4001 (2008) Article DOI: 10.1021/jm800093c BindingDB Entry DOI: 10.7270/Q29886S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50217137 (CHEMBL388710 | Ethyl 2-(4-Methylphenyl)-2-oxoethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Altana Pharma AG Curated by ChEMBL | Assay Description Inhibition of HDAC1 in HeLa cells | Bioorg Med Chem Lett 17: 4746-52 (2007) Article DOI: 10.1016/j.bmcl.2007.06.063 BindingDB Entry DOI: 10.7270/Q26H4H45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50217137 (CHEMBL388710 | Ethyl 2-(4-Methylphenyl)-2-oxoethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in HEK293 cells using variable substrate concentration by Lineweaver-Burk plot | J Med Chem 51: 3985-4001 (2008) Article DOI: 10.1021/jm800093c BindingDB Entry DOI: 10.7270/Q29886S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196843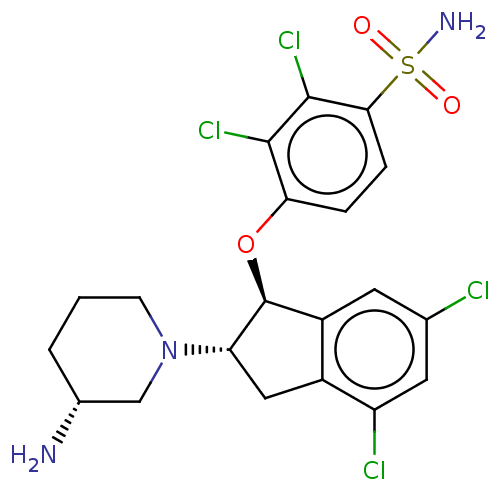 (CHEMBL3904640) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196811 (CHEMBL3967635) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332490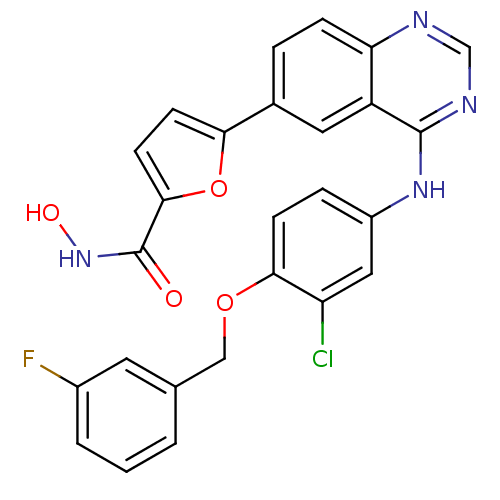 (5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)qu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332493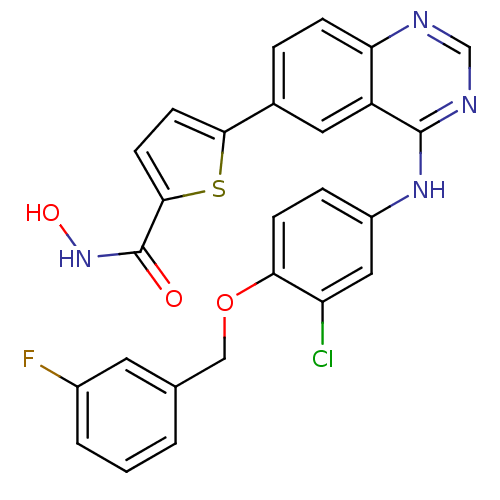 (5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332483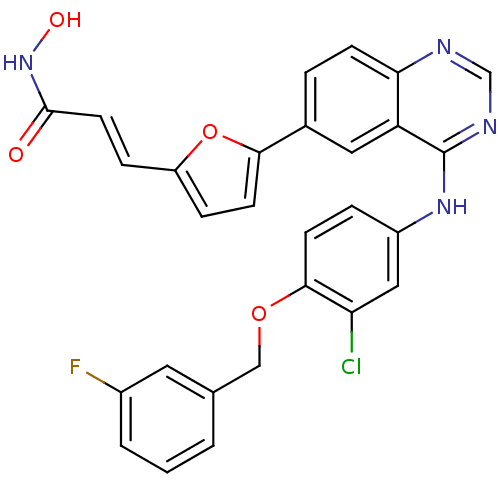 (3-{5-[4-(3-Chloro-4-(3-fluorobenzyloxy)phenylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332485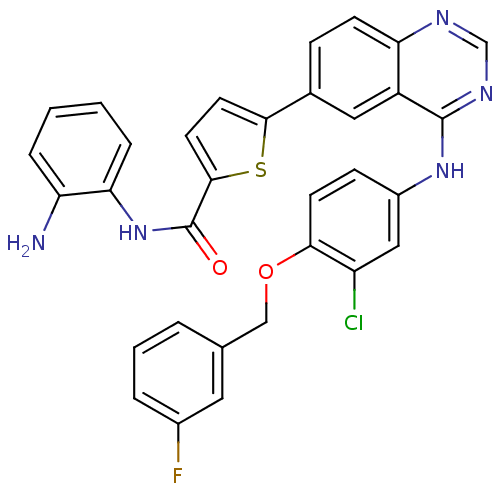 (CHEMBL1630112 | N-(2-aminophenyl)-5-(4-(3-chloro-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332484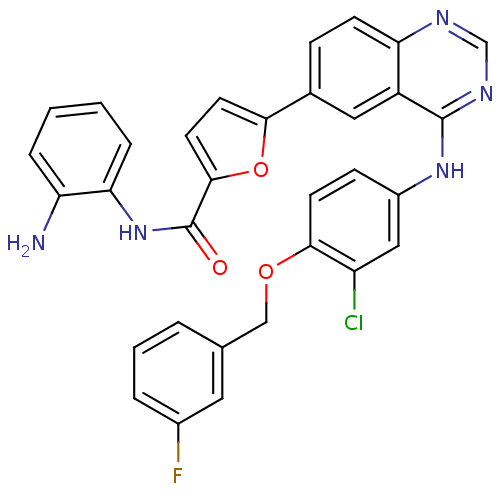 (CHEMBL1630111 | N-(2-aminophenyl)-5-(4-(3-chloro-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145937 (US8957064, 57.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145934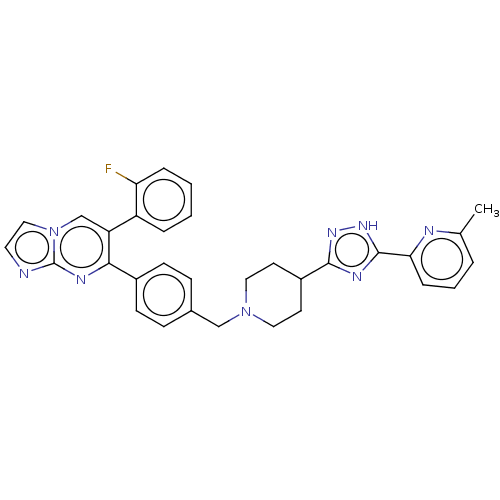 (US8957064, 54.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332493 (5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)qu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332491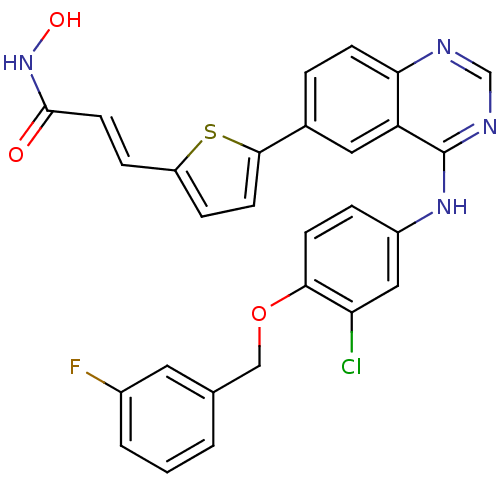 ((E)-3-(5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332490 (5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145872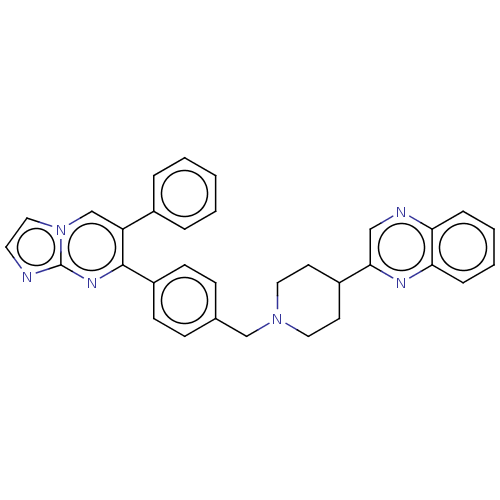 (US8957064, 10.0 | US8957064, 36.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145889 (US8957064, 26.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145958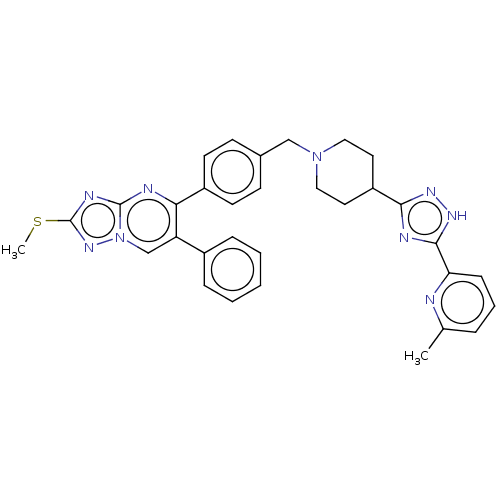 (US8957064, 72.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196812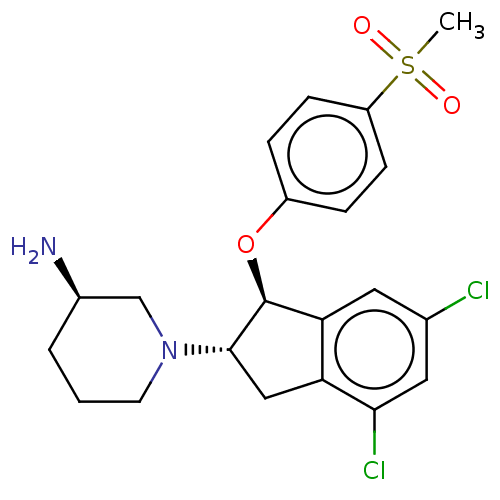 (CHEMBL3946835) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196805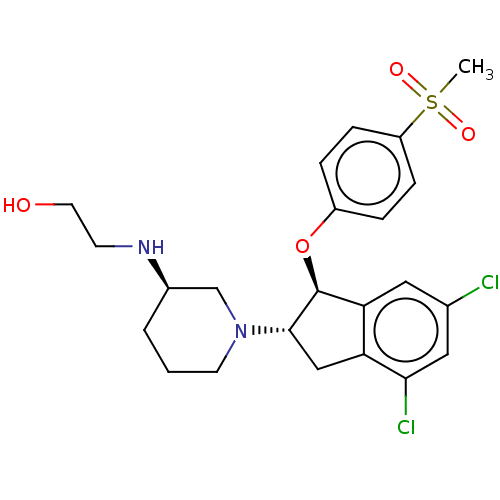 (CHEMBL3978001) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145889 (US8957064, 26.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145953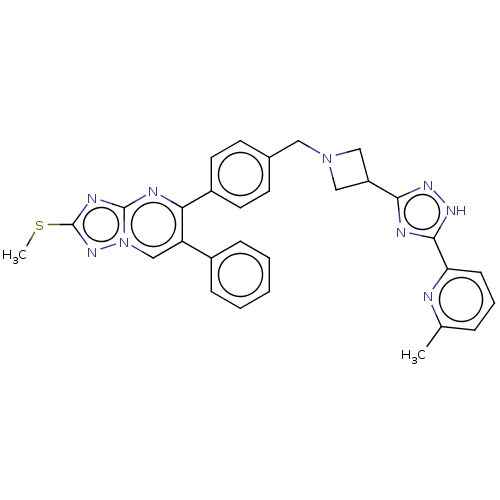 (US8957064, 71.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332483 (3-{5-[4-(3-Chloro-4-(3-fluorobenzyloxy)phenylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332489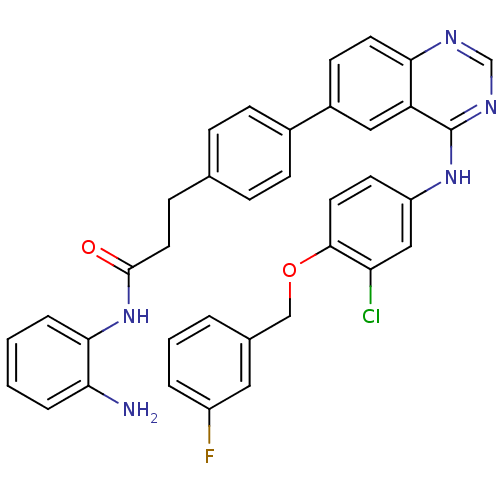 (CHEMBL1630116 | N-(2-aminophenyl)-3-(4-(4-(3-chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50332491 ((E)-3-(5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of EGFR by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332485 (CHEMBL1630112 | N-(2-aminophenyl)-5-(4-(3-chloro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145953 (US8957064, 71.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145958 (US8957064, 72.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145905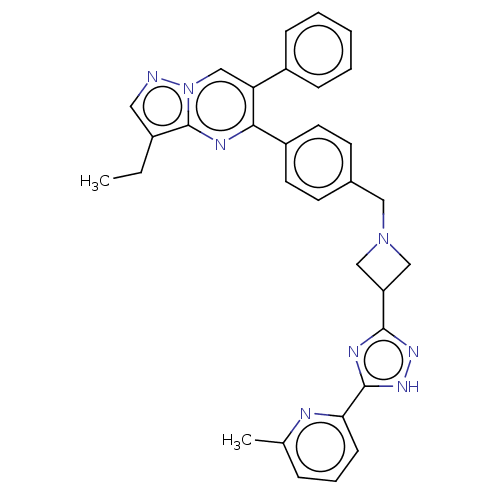 (US8957064, 42.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145954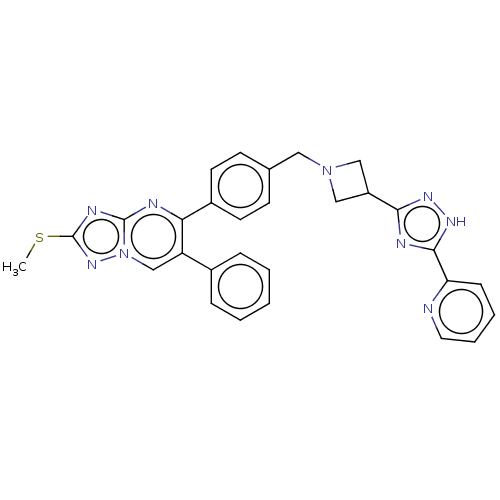 (US8957064, 72.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145952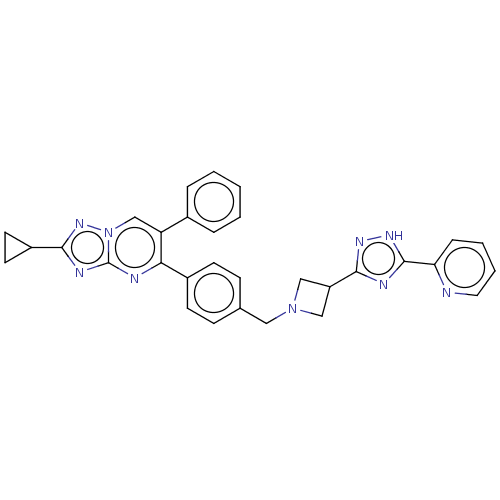 (US8957064, 70.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145920 (US8957064, 48.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50332484 (CHEMBL1630111 | N-(2-aminophenyl)-5-(4-(3-chloro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of HER2 by flash plate based radioactive enzyme assay | J Med Chem 53: 8546-55 (2010) Article DOI: 10.1021/jm100665z BindingDB Entry DOI: 10.7270/Q2J38STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145867 (US8957064, 5.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196829 (CHEMBL3914729) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145937 (US8957064, 57.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145935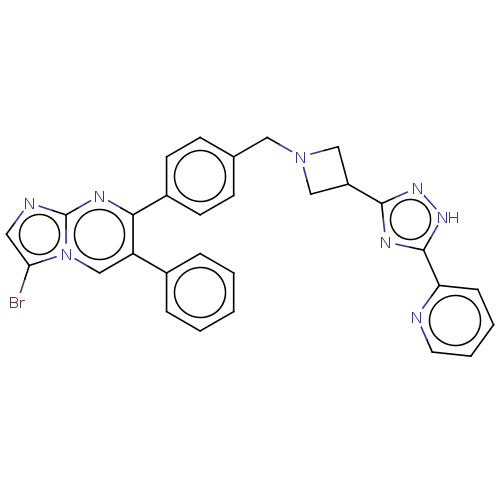 (US8957064, 55.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145952 (US8957064, 70.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145955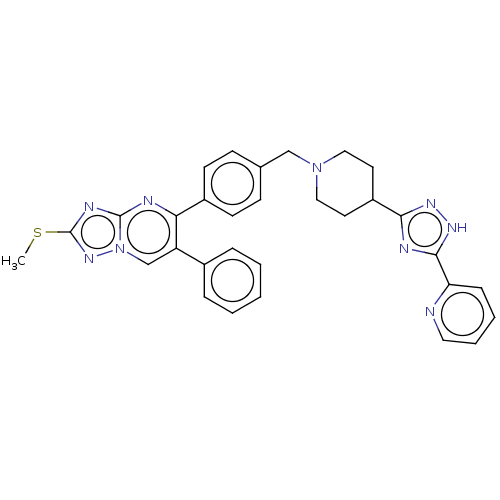 (US8957064, 72.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145932 (US8957064, 53.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145888 (US8957064, 25.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145895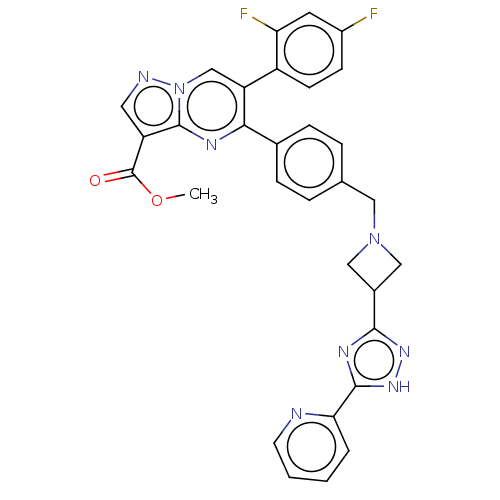 (US8957064, 32.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145909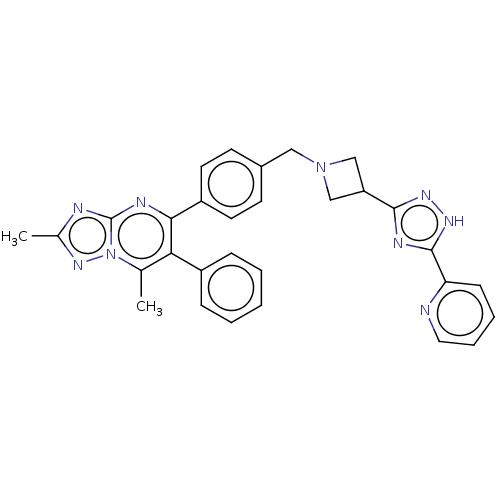 (US8957064, 44.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145919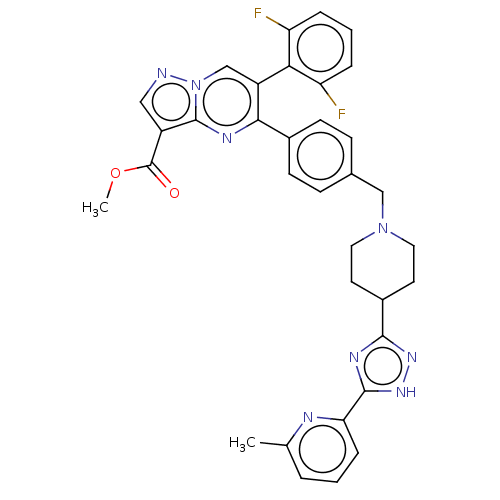 (US8957064, 48.0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt1 inhibitory activity of compounds of the present invention may be quantified0 employing the Akt1 TR-FRET assay as described in the following para... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger isoform 3 (Sus scrofa) | BDBM50196789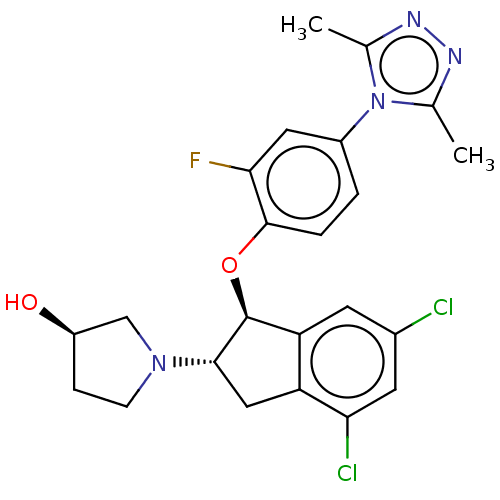 (CHEMBL3892788) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of pig NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM145910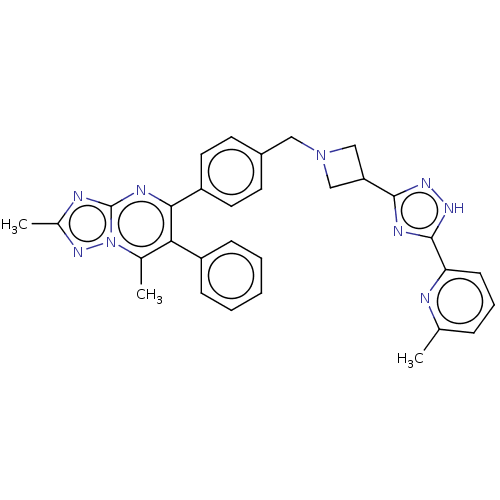 (US8957064, 44.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Akt2 inhibitory activity of compounds of the present invention was quantified employing the Akt2 TR-FRET assay as described in the following paragrap... | US Patent US8957064 (2015) BindingDB Entry DOI: 10.7270/Q2Z60MRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 750 total ) | Next | Last >> |