 Found 1186 hits with Last Name = 'zhou' and Initial = 't'
Found 1186 hits with Last Name = 'zhou' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM153
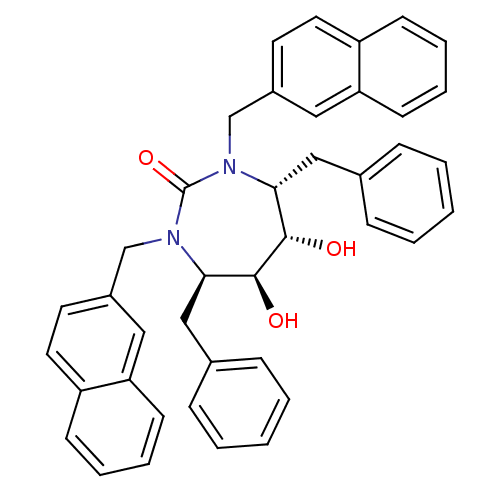
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(n...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3ccccc3c2)C(=O)N(Cc2ccc3ccccc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H38N2O3/c44-39-37(25-29-11-3-1-4-12-29)42(27-31-19-21-33-15-7-9-17-35(33)23-31)41(46)43(38(40(39)45)26-30-13-5-2-6-14-30)28-32-20-22-34-16-8-10-18-36(34)24-32/h1-24,37-40,44-45H,25-28H2/t37-,38-,39+,40+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 4280-8 (2008)
Article DOI: 10.1021/jm800242q
BindingDB Entry DOI: 10.7270/Q2S1858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478961

(CHEMBL443355)Show SMILES [H][C@]1(N[C@@H](C(=O)N[C@H](Cc2ccccc2)[C@H](O)CC(=O)NCc2nc3ccccc3[nH]2)C(C)(C)S1)[C@H](NC(=O)Cc1ccccc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C42H47N7O5S/c1-42(2)38(49-41(55-42)37(39(53)44-25-29-18-10-5-11-19-29)48-36(52)23-28-16-8-4-9-17-28)40(54)47-32(22-27-14-6-3-7-15-27)33(50)24-35(51)43-26-34-45-30-20-12-13-21-31(30)46-34/h3-21,32-33,37-38,41,49-50H,22-26H2,1-2H3,(H,43,51)(H,44,53)(H,45,46)(H,47,54)(H,48,52)/t32-,33-,37-,38+,41-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 4280-8 (2008)
Article DOI: 10.1021/jm800242q
BindingDB Entry DOI: 10.7270/Q2S1858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50473786
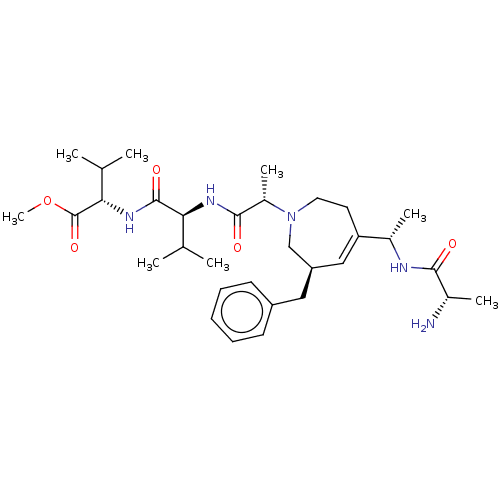
(CHEMBL506279 | SB-203238)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C)N1CCC(=C[C@@H](Cc2ccccc2)C1)[C@H](C)NC(=O)[C@H](C)N)C(C)C)C(C)C |r,c:17| Show InChI InChI=1S/C32H51N5O5/c1-19(2)27(31(40)36-28(20(3)4)32(41)42-8)35-30(39)23(7)37-15-14-26(22(6)34-29(38)21(5)33)17-25(18-37)16-24-12-10-9-11-13-24/h9-13,17,19-23,25,27-28H,14-16,18,33H2,1-8H3,(H,34,38)(H,35,39)(H,36,40)/t21-,22-,23-,25+,27-,28-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 4280-8 (2008)
Article DOI: 10.1021/jm800242q
BindingDB Entry DOI: 10.7270/Q2S1858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50368642

(ACETYLPEPSTATIN)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(C)=O)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C31H57N5O9/c1-15(2)11-21(35-30(44)28(18(7)8)36-31(45)27(17(5)6)33-20(10)37)23(38)13-25(40)32-19(9)29(43)34-22(12-16(3)4)24(39)14-26(41)42/h15-19,21-24,27-28,38-39H,11-14H2,1-10H3,(H,32,40)(H,33,37)(H,34,43)(H,35,44)(H,36,45)(H,41,42)/t19-,21-,22-,23-,24-,27-,28-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 4280-8 (2008)
Article DOI: 10.1021/jm800242q
BindingDB Entry DOI: 10.7270/Q2S1858S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478960

(CHEMBL455734)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)NC(Cc1ccccc1)[C@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36?,37-,38-,39+,40-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 4280-8 (2008)
Article DOI: 10.1021/jm800242q
BindingDB Entry DOI: 10.7270/Q2S1858S |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50174577
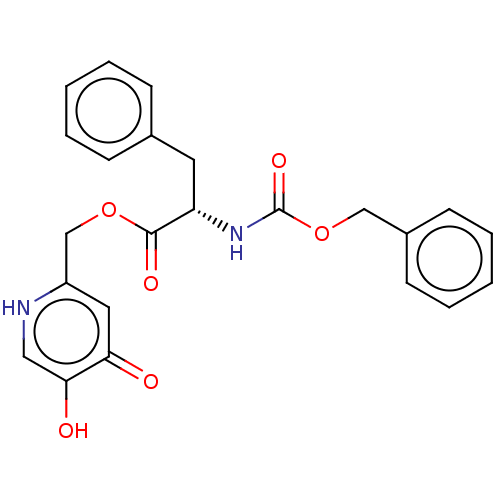
(CHEMBL3810334)Show SMILES Oc1c[nH]c(COC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C23H22N2O6/c26-20-12-18(24-13-21(20)27)15-30-22(28)19(11-16-7-3-1-4-8-16)25-23(29)31-14-17-9-5-2-6-10-17/h1-10,12-13,19,27H,11,14-15H2,(H,24,26)(H,25,29)/t19-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University
Curated by ChEMBL
| Assay Description
Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor complex incubated for 10 mins by Lin... |
Bioorg Med Chem Lett 26: 3103-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.006
BindingDB Entry DOI: 10.7270/Q2JM2CJ8 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50174577

(CHEMBL3810334)Show SMILES Oc1c[nH]c(COC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)cc1=O |r| Show InChI InChI=1S/C23H22N2O6/c26-20-12-18(24-13-21(20)27)15-30-22(28)19(11-16-7-3-1-4-8-16)25-23(29)31-14-17-9-5-2-6-10-17/h1-10,12-13,19,27H,11,14-15H2,(H,24,26)(H,25,29)/t19-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University
Curated by ChEMBL
| Assay Description
Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor complex incubated for 10 m... |
Bioorg Med Chem Lett 26: 3103-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.006
BindingDB Entry DOI: 10.7270/Q2JM2CJ8 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
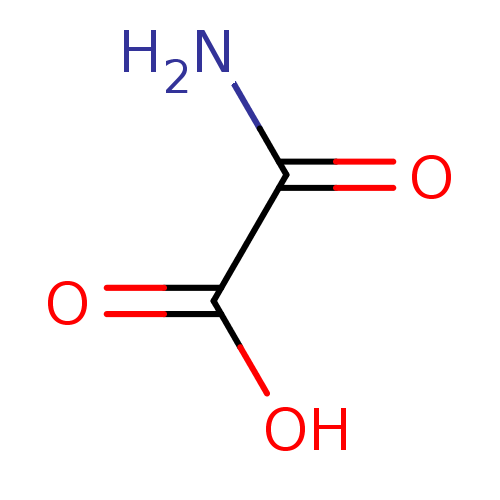
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50174582
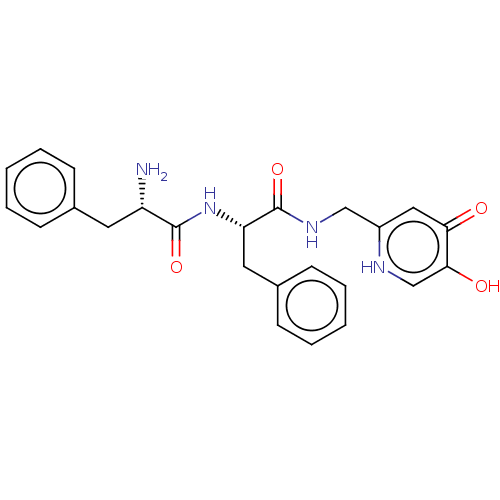
(CHEMBL3809975)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1cc(=O)c(O)c[nH]1 |r| Show InChI InChI=1S/C24H26N4O4/c25-19(11-16-7-3-1-4-8-16)23(31)28-20(12-17-9-5-2-6-10-17)24(32)27-14-18-13-21(29)22(30)15-26-18/h1-10,13,15,19-20,30H,11-12,14,25H2,(H,26,29)(H,27,32)(H,28,31)/t19-,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University
Curated by ChEMBL
| Assay Description
Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor complex incubated for 10 mins by Lin... |
Bioorg Med Chem Lett 26: 3103-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.006
BindingDB Entry DOI: 10.7270/Q2JM2CJ8 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50174582

(CHEMBL3809975)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1cc(=O)c(O)c[nH]1 |r| Show InChI InChI=1S/C24H26N4O4/c25-19(11-16-7-3-1-4-8-16)23(31)28-20(12-17-9-5-2-6-10-17)24(32)27-14-18-13-21(29)22(30)15-26-18/h1-10,13,15,19-20,30H,11-12,14,25H2,(H,26,29)(H,27,32)(H,28,31)/t19-,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University
Curated by ChEMBL
| Assay Description
Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor complex incubated for 10 m... |
Bioorg Med Chem Lett 26: 3103-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.006
BindingDB Entry DOI: 10.7270/Q2JM2CJ8 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259656
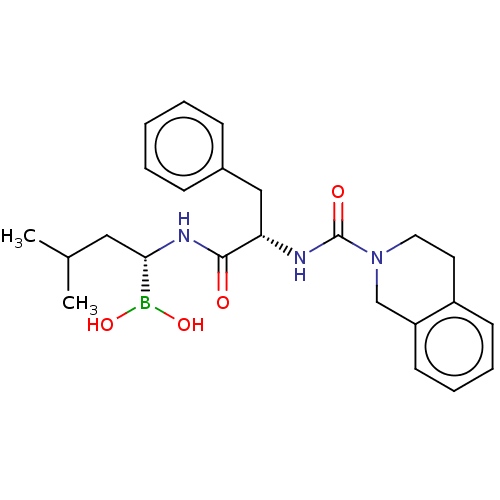
(CHEMBL4089402)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1)B(O)O |r| Show InChI InChI=1S/C24H32BN3O4/c1-17(2)14-22(25(31)32)27-23(29)21(15-18-8-4-3-5-9-18)26-24(30)28-13-12-19-10-6-7-11-20(19)16-28/h3-11,17,21-22,31-32H,12-16H2,1-2H3,(H,26,30)(H,27,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50062357

(AP26113 | CHEMBL3397300)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N(C)C Show InChI InChI=1S/C26H34ClN6O2P/c1-32(2)18-12-14-33(15-13-18)19-10-11-21(23(16-19)35-3)30-26-28-17-20(27)25(31-26)29-22-8-6-7-9-24(22)36(4,5)34/h6-11,16-18H,12-15H2,1-5H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50591813
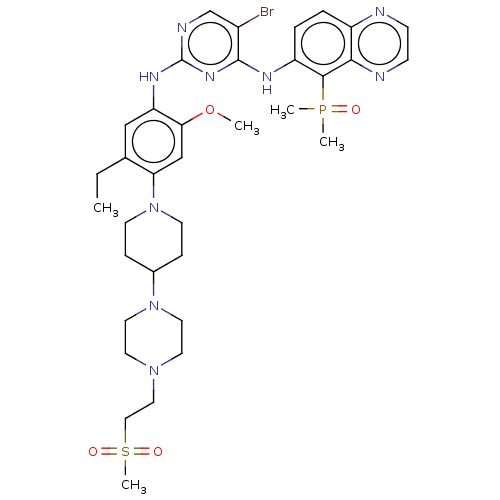
(CHEMBL5206715)Show SMILES CCc1cc(Nc2ncc(Br)c(Nc3ccc4nccnc4c3P(C)(C)=O)n2)c(OC)cc1N1CCC(CC1)N1CCN(CCS(C)(=O)=O)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128729
BindingDB Entry DOI: 10.7270/Q2930Z4T |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185287
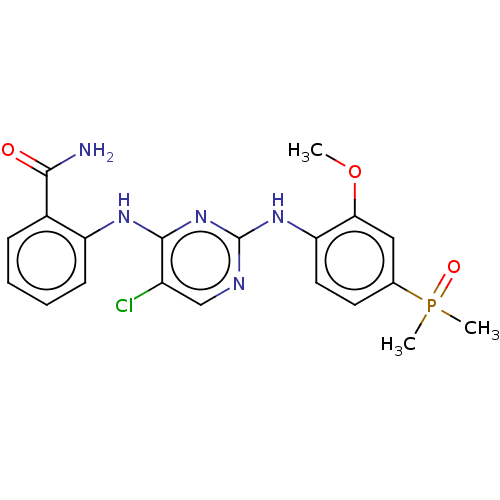
(CHEMBL3823268)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H21ClN5O3P/c1-29-17-10-12(30(2,3)28)8-9-16(17)25-20-23-11-14(21)19(26-20)24-15-7-5-4-6-13(15)18(22)27/h4-11H,1-3H3,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185237

(CHEMBL3824308)Show SMILES CCP(=O)(CC)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C31H43ClN7O2P/c1-5-42(40,6-2)29-10-8-7-9-27(29)34-30-25(32)22-33-31(36-30)35-26-12-11-24(21-28(26)41-4)38-15-13-23(14-16-38)39-19-17-37(3)18-20-39/h7-12,21-23H,5-6,13-20H2,1-4H3,(H2,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM482158
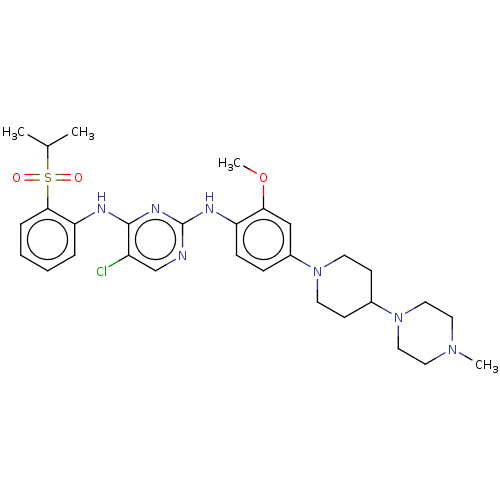
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185280

(CHEMBL3822611)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H30ClN6O2P/c1-30-11-13-31(14-12-30)17-9-10-19(21(15-17)33-2)28-24-26-16-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)32/h5-10,15-16H,11-14H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185275
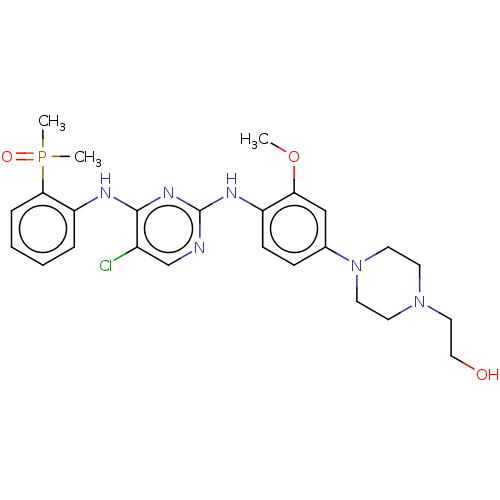
(CHEMBL3823235)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C25H32ClN6O3P/c1-35-22-16-18(32-12-10-31(11-13-32)14-15-33)8-9-20(22)29-25-27-17-19(26)24(30-25)28-21-6-4-5-7-23(21)36(2,3)34/h4-9,16-17,33H,10-15H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RET using KKKSPGEYVNIEFG by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50591813

(CHEMBL5206715)Show SMILES CCc1cc(Nc2ncc(Br)c(Nc3ccc4nccnc4c3P(C)(C)=O)n2)c(OC)cc1N1CCC(CC1)N1CCN(CCS(C)(=O)=O)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128729
BindingDB Entry DOI: 10.7270/Q2930Z4T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50591813

(CHEMBL5206715)Show SMILES CCc1cc(Nc2ncc(Br)c(Nc3ccc4nccnc4c3P(C)(C)=O)n2)c(OC)cc1N1CCC(CC1)N1CCN(CCS(C)(=O)=O)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128729
BindingDB Entry DOI: 10.7270/Q2930Z4T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ARIAD Pharmaceuticals Inc
| Assay Description
Inhibition of wild-type Abl and Abl T315I kinase activity was measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FRET... |
Chem Biol Drug Des 70: 171-81 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00556.x
BindingDB Entry DOI: 10.7270/Q228063T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185284
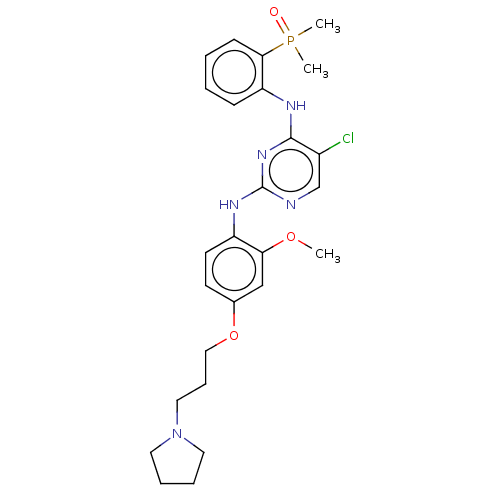
(CHEMBL3823549)Show SMILES COc1cc(OCCCN2CCCC2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C26H33ClN5O3P/c1-34-23-17-19(35-16-8-15-32-13-6-7-14-32)11-12-21(23)30-26-28-18-20(27)25(31-26)29-22-9-4-5-10-24(22)36(2,3)33/h4-5,9-12,17-18H,6-8,13-16H2,1-3H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185281
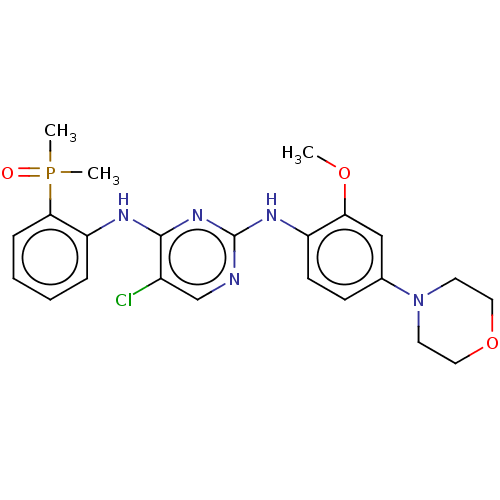
(CHEMBL3823416)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCOCC1 Show InChI InChI=1S/C23H27ClN5O3P/c1-31-20-14-16(29-10-12-32-13-11-29)8-9-18(20)27-23-25-15-17(24)22(28-23)26-19-6-4-5-7-21(19)33(2,3)30/h4-9,14-15H,10-13H2,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185268

(CHEMBL3824327)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-13-11-21(12-14-35)36-15-17-37(18-16-36)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50591813

(CHEMBL5206715)Show SMILES CCc1cc(Nc2ncc(Br)c(Nc3ccc4nccnc4c3P(C)(C)=O)n2)c(OC)cc1N1CCC(CC1)N1CCN(CCS(C)(=O)=O)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128729
BindingDB Entry DOI: 10.7270/Q2930Z4T |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259645
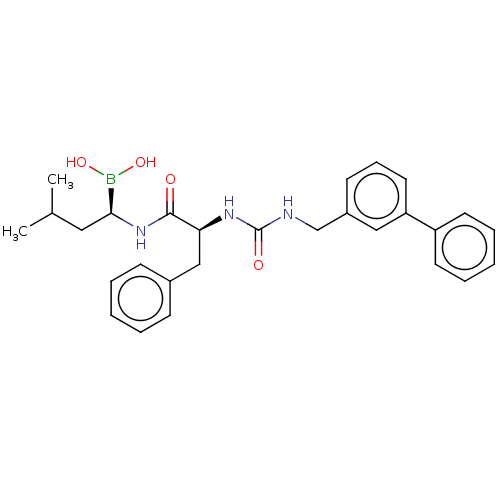
(CHEMBL4070336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C28H34BN3O4/c1-20(2)16-26(29(35)36)32-27(33)25(18-21-10-5-3-6-11-21)31-28(34)30-19-22-12-9-15-24(17-22)23-13-7-4-8-14-23/h3-15,17,20,25-26,35-36H,16,18-19H2,1-2H3,(H,32,33)(H2,30,31,34)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185290
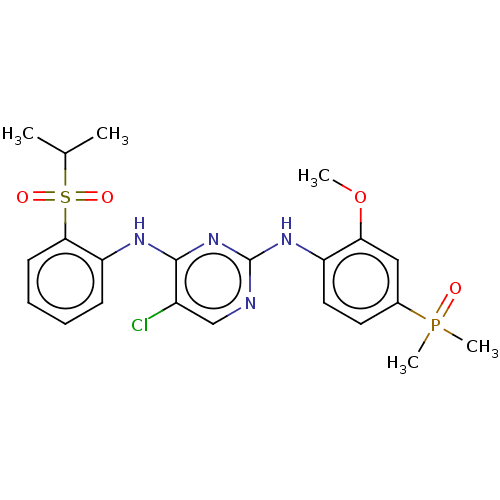
(CHEMBL3824304)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)P(C)(C)=O Show InChI InChI=1S/C22H26ClN4O4PS/c1-14(2)33(29,30)20-9-7-6-8-18(20)25-21-16(23)13-24-22(27-21)26-17-11-10-15(32(4,5)28)12-19(17)31-3/h6-14H,1-5H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50556595
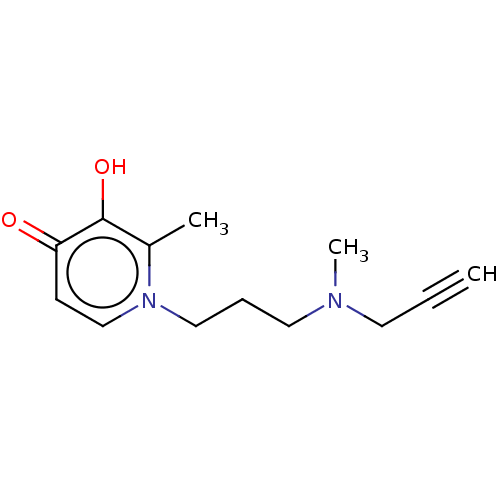
(CHEMBL4740154) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human H3 receptor expressed in HEK293 cells centrifuged for 3 mins followed by 60 mins incubation by LANCE Ultra cAMP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01480
BindingDB Entry DOI: 10.7270/Q28P646M |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185272
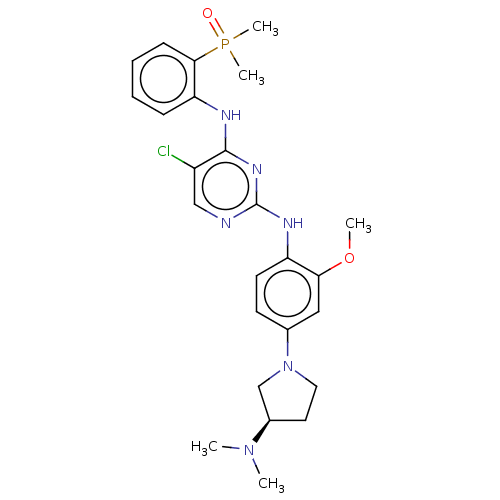
(CHEMBL3823107)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CC[C@H](C1)N(C)C |r| Show InChI InChI=1S/C25H32ClN6O2P/c1-31(2)18-12-13-32(16-18)17-10-11-20(22(14-17)34-3)29-25-27-15-19(26)24(30-25)28-21-8-6-7-9-23(21)35(4,5)33/h6-11,14-15,18H,12-13,16H2,1-5H3,(H2,27,28,29,30)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185283

(CHEMBL3823603)Show SMILES COc1cc(OC2CCN(C)C2)ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1 Show InChI InChI=1S/C24H29ClN5O3P/c1-30-12-11-17(15-30)33-16-9-10-19(21(13-16)32-2)28-24-26-14-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)31/h5-10,13-14,17H,11-12,15H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185286
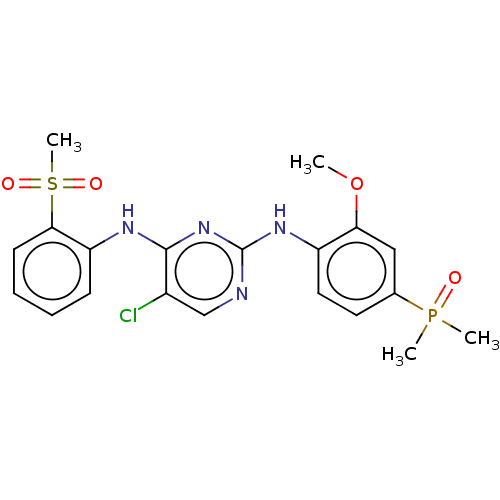
(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM482158

(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185278

(CHEMBL3823017)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(O)CC1 Show InChI InChI=1S/C24H29ClN5O3P/c1-33-21-14-16(30-12-10-17(31)11-13-30)8-9-19(21)28-24-26-15-18(25)23(29-24)27-20-6-4-5-7-22(20)34(2,3)32/h4-9,14-15,17,31H,10-13H2,1-3H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ABL autophosphorylation |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185140
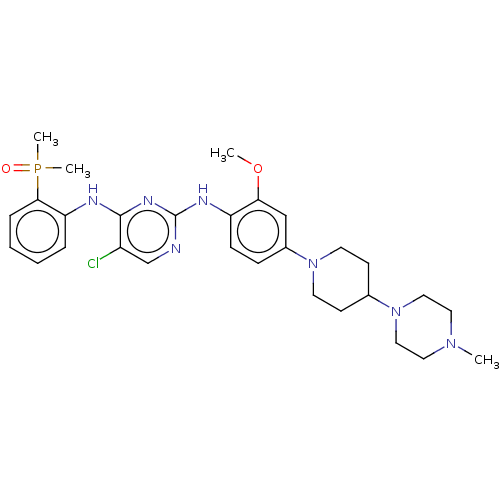
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185245

(CHEMBL3823190)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)C1CCN(C)CC1 Show InChI InChI=1S/C25H31ClN5O2P/c1-31-13-11-17(12-14-31)18-9-10-20(22(15-18)33-2)29-25-27-16-19(26)24(30-25)28-21-7-5-6-8-23(21)34(3,4)32/h5-10,15-17H,11-14H2,1-4H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185239
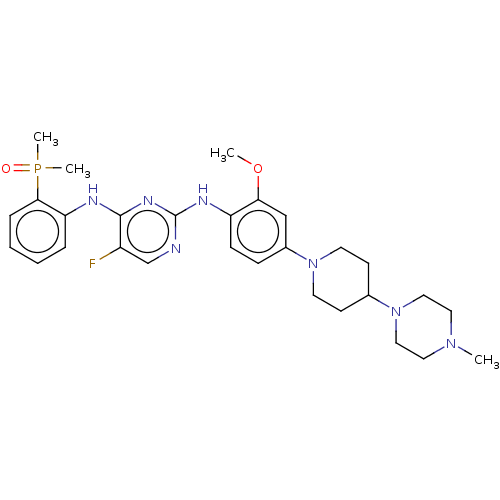
(CHEMBL3823296)Show SMILES COc1cc(ccc1Nc1ncc(F)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39FN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185286

(CHEMBL3823256)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(C)(=O)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H22ClN4O4PS/c1-29-17-11-13(30(2,3)26)9-10-15(17)24-20-22-12-14(21)19(25-20)23-16-7-5-6-8-18(16)31(4,27)28/h5-12H,1-4H3,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185238

(CHEMBL3823577)Show SMILES COc1cc(ccc1Nc1ncc(C)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H42N7O2P/c1-22-21-31-30(34-29(22)32-26-8-6-7-9-28(26)40(4,5)38)33-25-11-10-24(20-27(25)39-3)36-14-12-23(13-15-36)37-18-16-35(2)17-19-37/h6-11,20-21,23H,12-19H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
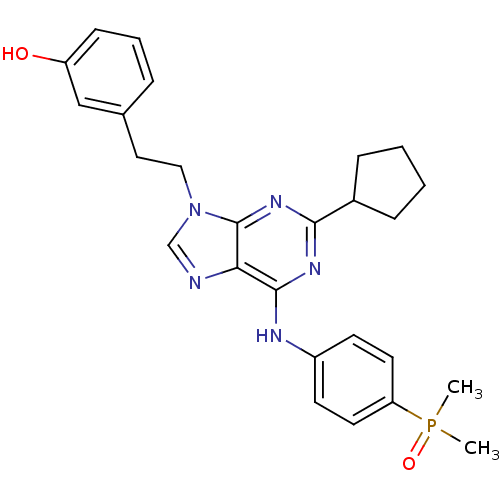
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 6x-His-tagged human Src kinase domain (T250 to L536 residues) expressed in Sf9 cells incubated for 2 hrs in presence of biotinylated cd... |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185288

(CHEMBL3824326)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(C)=O)n1)P(C)(C)=O Show InChI InChI=1S/C21H22ClN4O3P/c1-13(27)15-7-5-6-8-17(15)24-20-16(22)12-23-21(26-20)25-18-10-9-14(30(3,4)28)11-19(18)29-2/h5-12H,1-4H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185267
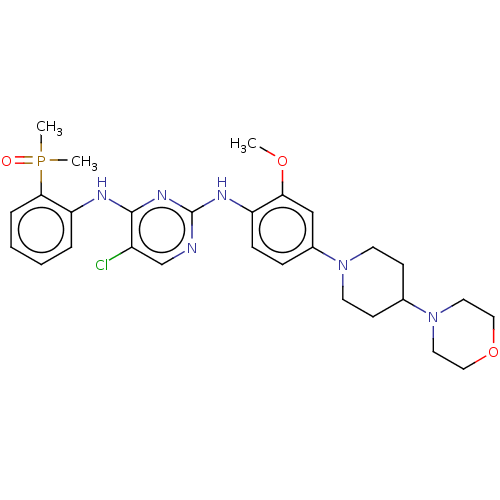
(CHEMBL3822557)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C28H36ClN6O3P/c1-37-25-18-21(34-12-10-20(11-13-34)35-14-16-38-17-15-35)8-9-23(25)32-28-30-19-22(29)27(33-28)31-24-6-4-5-7-26(24)39(2,3)36/h4-9,18-20H,10-17H2,1-3H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185265
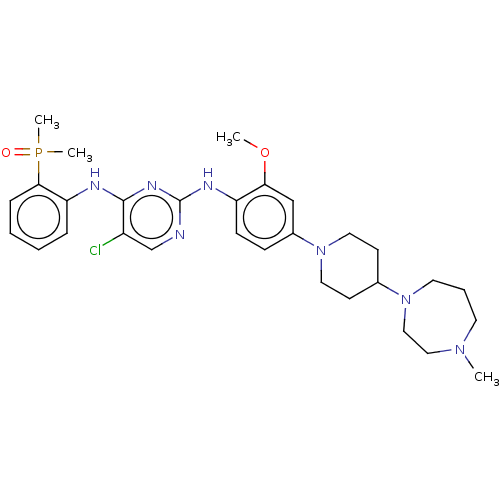
(CHEMBL3824290)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCCN(C)CC1 Show InChI InChI=1S/C30H41ClN7O2P/c1-36-14-7-15-37(19-18-36)22-12-16-38(17-13-22)23-10-11-25(27(20-23)40-2)34-30-32-21-24(31)29(35-30)33-26-8-5-6-9-28(26)41(3,4)39/h5-6,8-11,20-22H,7,12-19H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244569
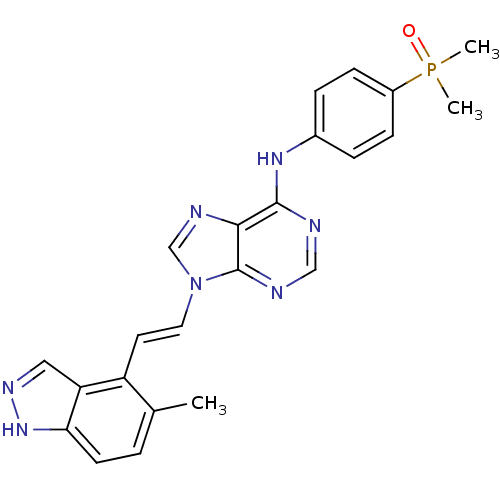
(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM82130
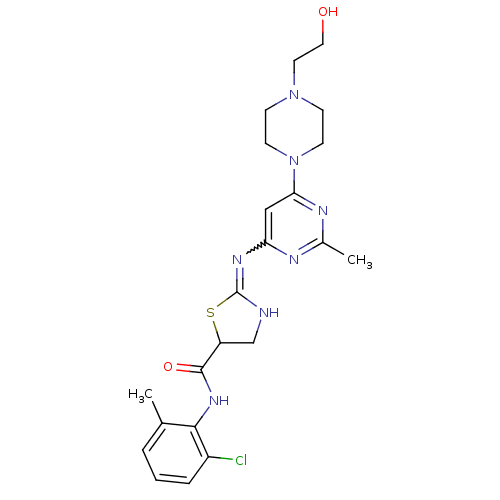
(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Isoform 1 of Fibronectin (1)
(Homo sapiens (Human)) | BDBM50244569

(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data