 Found 622 hits with Last Name = 'durham' and Initial = 'tb'
Found 622 hits with Last Name = 'durham' and Initial = 'tb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50062937

(CHEMBL3397908)Show SMILES OC(=O)\C=C\C(O)=O.CNC(=O)c1ccc2ccn(C3CCN(CCc4c(OC)ccc5C(=O)CC(C)(C)Oc45)CC3)c2c1 Show InChI InChI=1S/C29H35N3O4/c1-29(2)18-25(33)22-7-8-26(35-4)23(27(22)36-29)12-15-31-13-10-21(11-14-31)32-16-9-19-5-6-20(17-24(19)32)28(34)30-3/h5-9,16-17,21H,10-15,18H2,1-4H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPPF from 5HT1A receptor in Sprague-Dawley rat hippocampal membrane fraction incubated for 60 mins by scintillation counting meth... |
Bioorg Med Chem Lett 25: 998-1008 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.076
BindingDB Entry DOI: 10.7270/Q28S4RMQ |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50607111
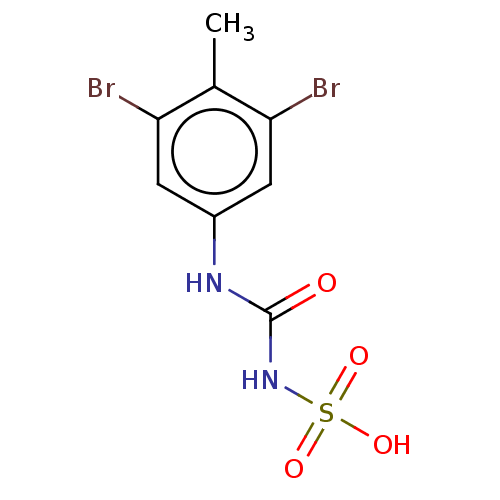
(CHEMBL5218807) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01143
BindingDB Entry DOI: 10.7270/Q2BK1HGF |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396
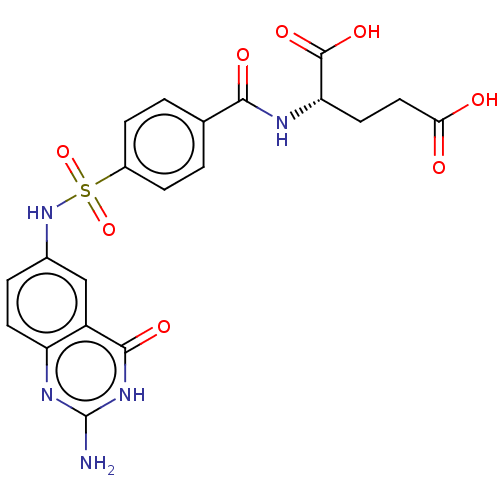
(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human AICARFT |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103373
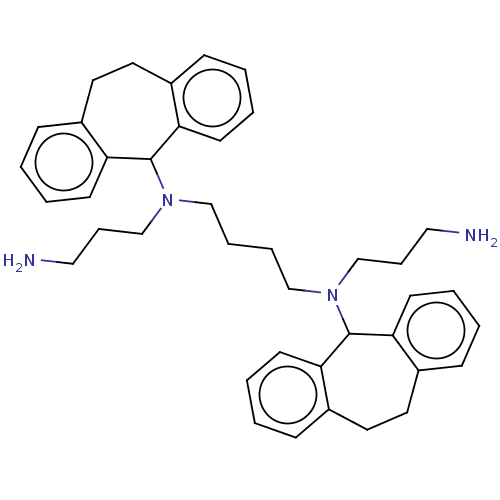
(CHEMBL3398189)Show SMILES NCCCN(CCCCN(CCCN)C1c2ccccc2CCc2ccccc12)C1c2ccccc2CCc2ccccc12 Show InChI InChI=1S/C40H50N4/c41-25-11-29-43(39-35-17-5-1-13-31(35)21-22-32-14-2-6-18-36(32)39)27-9-10-28-44(30-12-26-42)40-37-19-7-3-15-33(37)23-24-34-16-4-8-20-38(34)40/h1-8,13-20,39-40H,9-12,21-30,41-42H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103372
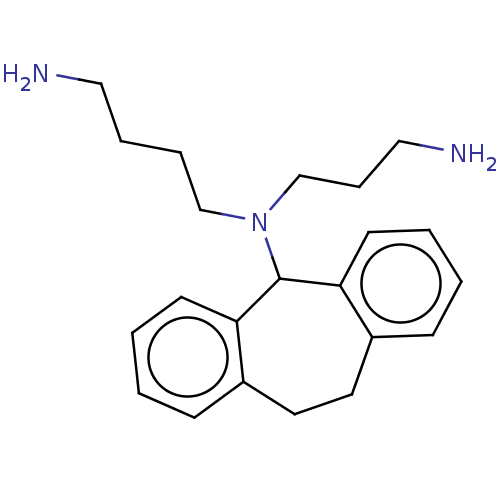
(CHEMBL378650)Show InChI InChI=1S/C22H31N3/c23-14-5-6-16-25(17-7-15-24)22-20-10-3-1-8-18(20)12-13-19-9-2-4-11-21(19)22/h1-4,8-11,22H,5-7,12-17,23-24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM77970
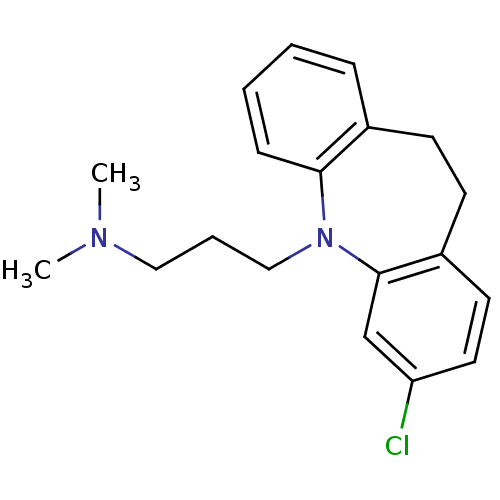
(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103371

(CHEMBL3398188)Show InChI InChI=1S/C21H29N3/c22-13-5-15-24(16-6-14-23)21-19-9-3-1-7-17(19)11-12-18-8-2-4-10-20(18)21/h1-4,7-10,21H,5-6,11-16,22-23H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103370

(CHEMBL3398187)Show InChI InChI=1S/C18H24N4/c19-9-12-22(13-10-20)18-16-6-2-1-4-14(16)7-8-15-5-3-11-21-17(15)18/h1-6,11,18H,7-10,12-13,19-20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103369
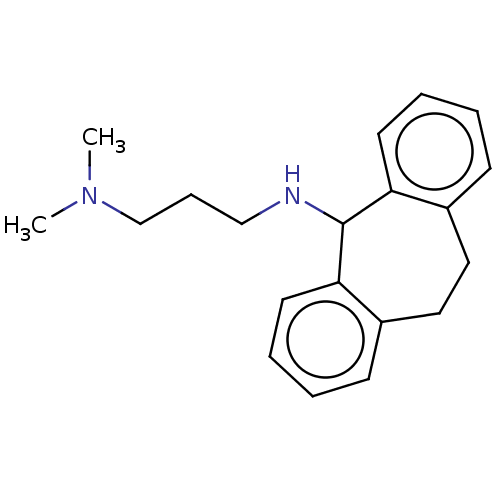
(CHEMBL3398186)Show InChI InChI=1S/C20H26N2/c1-22(2)15-7-14-21-20-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)20/h3-6,8-11,20-21H,7,12-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50103368
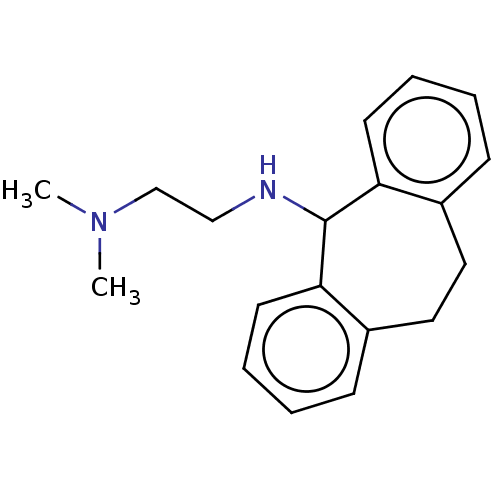
(CHEMBL332939)Show InChI InChI=1S/C19H24N2/c1-21(2)14-13-20-19-17-9-5-3-7-15(17)11-12-16-8-4-6-10-18(16)19/h3-10,19-20H,11-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically |
Bioorg Med Chem 23: 996-1010 (2015)
Article DOI: 10.1016/j.bmc.2015.01.018
BindingDB Entry DOI: 10.7270/Q27P9166 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50062936
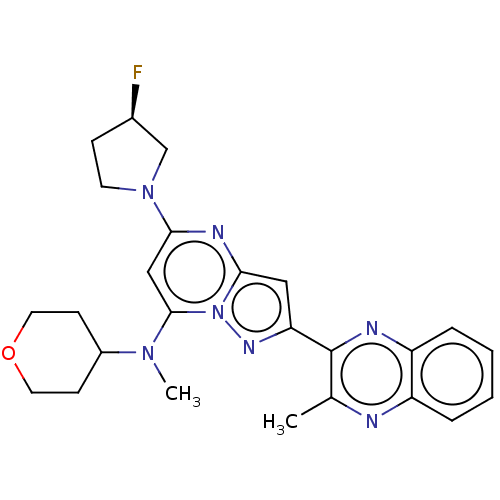
(CHEMBL3397907)Show SMILES Cc1nc2ccccc2nc1-c1cc2nc(cc(N([11CH3])C3CCOCC3)n2n1)N1CC[C@@H](F)C1 |r| Show InChI InChI=1S/C25H28FN7O/c1-16-25(28-20-6-4-3-5-19(20)27-16)21-13-23-29-22(32-10-7-17(26)15-32)14-24(33(23)30-21)31(2)18-8-11-34-12-9-18/h3-6,13-14,17-18H,7-12,15H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A |
Bioorg Med Chem Lett 25: 998-1008 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.076
BindingDB Entry DOI: 10.7270/Q28S4RMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50062942
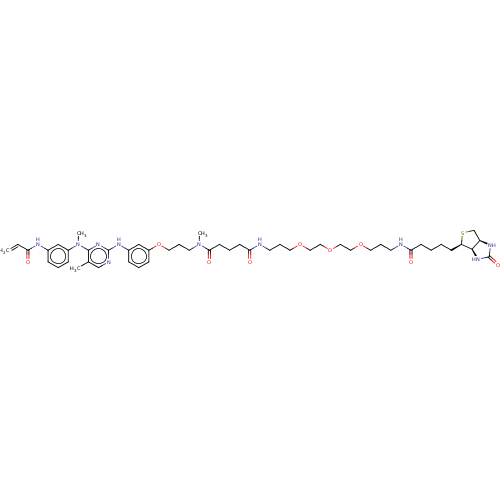
(CHEMBL3397911)Show SMILES [H][C@@]12CS[C@H](CCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCC(=O)N(C)CCCOc3cccc(Nc4ncc(C)c(n4)N(C)c4cccc(NC(=O)C=C)c4)c3)[C@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C50H72N10O9S/c1-5-43(61)54-37-14-8-16-39(32-37)60(4)48-36(2)34-53-49(58-48)55-38-15-9-17-40(33-38)69-27-13-24-59(3)46(64)21-10-20-45(63)52-23-12-26-67-29-31-68-30-28-66-25-11-22-51-44(62)19-7-6-18-42-47-41(35-70-42)56-50(65)57-47/h5,8-9,14-17,32-34,41-42,47H,1,6-7,10-13,18-31,35H2,2-4H3,(H,51,62)(H,52,63)(H,54,61)(H,53,55,58)(H2,56,57,65)/t41-,42-,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) using ATP and Y5 Sox15 substrate mix incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 25: 998-1008 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.076
BindingDB Entry DOI: 10.7270/Q28S4RMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50062940

(CHEMBL3397910)Show SMILES COCCOc1ccc(Nc2ncc(F)c(n2)N(C)c2cccc(NC(=O)C=C)c2)cc1 Show InChI InChI=1S/C23H24FN5O3/c1-4-21(30)26-17-6-5-7-18(14-17)29(2)22-20(24)15-25-23(28-22)27-16-8-10-19(11-9-16)32-13-12-31-3/h4-11,14-15H,1,12-13H2,2-3H3,(H,26,30)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) using ATP and Y5 Sox15 substrate mix incubated for 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 25: 998-1008 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.076
BindingDB Entry DOI: 10.7270/Q28S4RMQ |
More data for this
Ligand-Target Pair | |
ADAM metallopeptidase with thrombospondin type 1 motif 4
(Canis lupus familiaris (Dog)) | BDBM194639
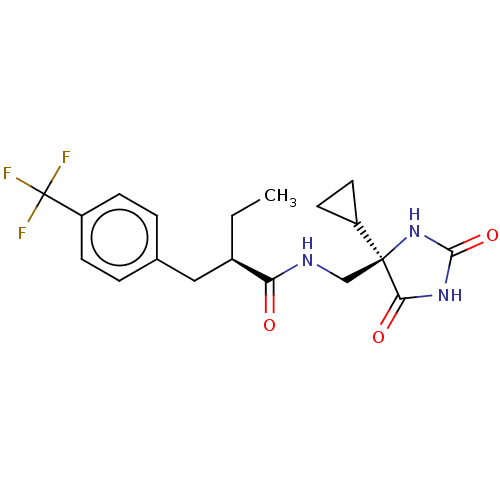
(US9206139, 2)Show SMILES CC[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C19H22F3N3O3/c1-2-12(9-11-3-5-14(6-4-11)19(20,21)22)15(26)23-10-18(13-7-8-13)16(27)24-17(28)25-18/h3-6,12-13H,2,7-10H2,1H3,(H,23,26)(H2,24,25,27,28)/t12-,18+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs |
J Med Chem 57: 10476-85 (2014)
Article DOI: 10.1021/jm501522n
BindingDB Entry DOI: 10.7270/Q2K35W85 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS-4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay |
J Med Chem 57: 10476-85 (2014)
Article DOI: 10.1021/jm501522n
BindingDB Entry DOI: 10.7270/Q2K35W85 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS-5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay |
J Med Chem 57: 10476-85 (2014)
Article DOI: 10.1021/jm501522n
BindingDB Entry DOI: 10.7270/Q2K35W85 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194638

(US9206139, 1)Show SMILES C[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194638

(US9206139, 1)Show SMILES C[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive inhibition against rat cytoplasmic thymidine kinase |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194638

(US9206139, 1)Show SMILES C[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
ADAM metallopeptidase with thrombospondin type 1 motif 4
(Canis lupus familiaris (Dog)) | BDBM194638

(US9206139, 1)Show SMILES C[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
Isoform A of Ketohexokinase (Peripheral)
(Homo sapiens (Human)) | BDBM518444

((2S,3R)-2-Methyl-1-[4-[1-(1-methylazetidin-3-yl)py...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(n1)C(F)(F)F)-c1cnn(c1)C1CN(C)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CR5XGZ |
More data for this
Ligand-Target Pair | |
ADAM metallopeptidase with thrombospondin type 1 motif 4
(Canis lupus familiaris (Dog)) | BDBM194644
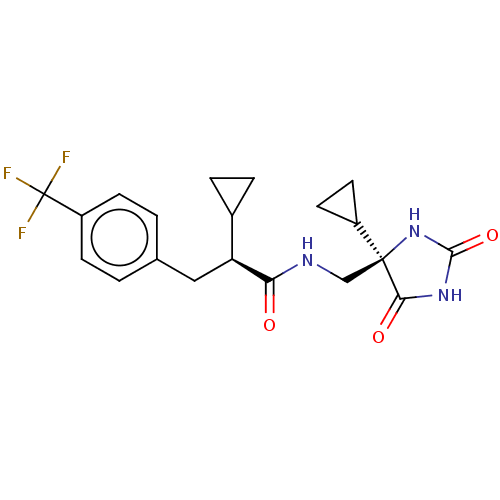
(US9206139, 3)Show SMILES FC(F)(F)c1ccc(C[C@@H](C2CC2)C(=O)NC[C@]2(NC(=O)NC2=O)C2CC2)cc1 |r| Show InChI InChI=1S/C20H22F3N3O3/c21-20(22,23)14-5-1-11(2-6-14)9-15(12-3-4-12)16(27)24-10-19(13-7-8-13)17(28)25-18(29)26-19/h1-2,5-6,12-13,15H,3-4,7-10H2,(H,24,27)(H2,25,26,28,29)/t15-,19-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194639

(US9206139, 2)Show SMILES CC[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C19H22F3N3O3/c1-2-12(9-11-3-5-14(6-4-11)19(20,21)22)15(26)23-10-18(13-7-8-13)16(27)24-17(28)25-18/h3-6,12-13H,2,7-10H2,1H3,(H,23,26)(H2,24,25,27,28)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194644

(US9206139, 3)Show SMILES FC(F)(F)c1ccc(C[C@@H](C2CC2)C(=O)NC[C@]2(NC(=O)NC2=O)C2CC2)cc1 |r| Show InChI InChI=1S/C20H22F3N3O3/c21-20(22,23)14-5-1-11(2-6-14)9-15(12-3-4-12)16(27)24-10-19(13-7-8-13)17(28)25-18(29)26-19/h1-2,5-6,12-13,15H,3-4,7-10H2,(H,24,27)(H2,25,26,28,29)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194639

(US9206139, 2)Show SMILES CC[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C19H22F3N3O3/c1-2-12(9-11-3-5-14(6-4-11)19(20,21)22)15(26)23-10-18(13-7-8-13)16(27)24-17(28)25-18/h3-6,12-13H,2,7-10H2,1H3,(H,23,26)(H2,24,25,27,28)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194644

(US9206139, 3)Show SMILES FC(F)(F)c1ccc(C[C@@H](C2CC2)C(=O)NC[C@]2(NC(=O)NC2=O)C2CC2)cc1 |r| Show InChI InChI=1S/C20H22F3N3O3/c21-20(22,23)14-5-1-11(2-6-14)9-15(12-3-4-12)16(27)24-10-19(13-7-8-13)17(28)25-18(29)26-19/h1-2,5-6,12-13,15H,3-4,7-10H2,(H,24,27)(H2,25,26,28,29)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194644

(US9206139, 3)Show SMILES FC(F)(F)c1ccc(C[C@@H](C2CC2)C(=O)NC[C@]2(NC(=O)NC2=O)C2CC2)cc1 |r| Show InChI InChI=1S/C20H22F3N3O3/c21-20(22,23)14-5-1-11(2-6-14)9-15(12-3-4-12)16(27)24-10-19(13-7-8-13)17(28)25-18(29)26-19/h1-2,5-6,12-13,15H,3-4,7-10H2,(H,24,27)(H2,25,26,28,29)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194638

(US9206139, 1)Show SMILES C[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194639

(US9206139, 2)Show SMILES CC[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C19H22F3N3O3/c1-2-12(9-11-3-5-14(6-4-11)19(20,21)22)15(26)23-10-18(13-7-8-13)16(27)24-17(28)25-18/h3-6,12-13H,2,7-10H2,1H3,(H,23,26)(H2,24,25,27,28)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM194644

(US9206139, 3)Show SMILES FC(F)(F)c1ccc(C[C@@H](C2CC2)C(=O)NC[C@]2(NC(=O)NC2=O)C2CC2)cc1 |r| Show InChI InChI=1S/C20H22F3N3O3/c21-20(22,23)14-5-1-11(2-6-14)9-15(12-3-4-12)16(27)24-10-19(13-7-8-13)17(28)25-18(29)26-19/h1-2,5-6,12-13,15H,3-4,7-10H2,(H,24,27)(H2,25,26,28,29)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM194639

(US9206139, 2)Show SMILES CC[C@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C19H22F3N3O3/c1-2-12(9-11-3-5-14(6-4-11)19(20,21)22)15(26)23-10-18(13-7-8-13)16(27)24-17(28)25-18/h3-6,12-13H,2,7-10H2,1H3,(H,23,26)(H2,24,25,27,28)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Eli Lilly and Company
US Patent
| Assay Description
The compounds of the present invention can be evaluated by using an aggrecanase ADAMTS-4 and ADAMTS-5 AlphaScreen assay (Miller J. A., et al. Anal. B... |
US Patent US9206139 (2015)
BindingDB Entry DOI: 10.7270/Q25X27R0 |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM518444

((2S,3R)-2-Methyl-1-[4-[1-(1-methylazetidin-3-yl)py...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(n1)C(F)(F)F)-c1cnn(c1)C1CN(C)C1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CR5XGZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs |
J Med Chem 57: 10476-85 (2014)
Article DOI: 10.1021/jm501522n
BindingDB Entry DOI: 10.7270/Q2K35W85 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806
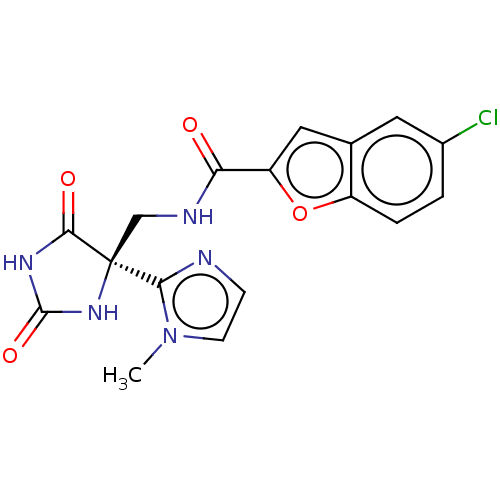
(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313
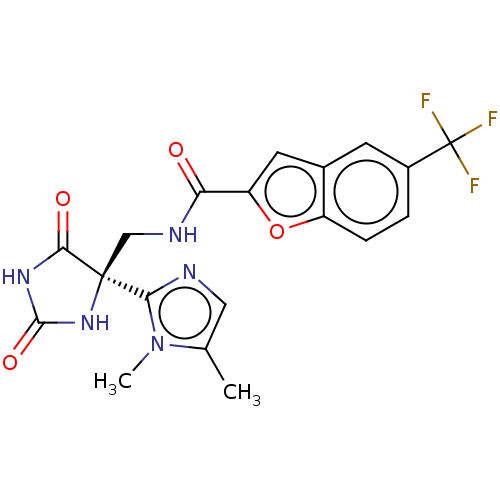
(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Mus musculus) | BDBM50607111

(CHEMBL5218807) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01143
BindingDB Entry DOI: 10.7270/Q2BK1HGF |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Rattus norvegicus) | BDBM50607111

(CHEMBL5218807) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01143
BindingDB Entry DOI: 10.7270/Q2BK1HGF |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Isoform A of Ketohexokinase (Peripheral)
(Homo sapiens (Human)) | BDBM518415
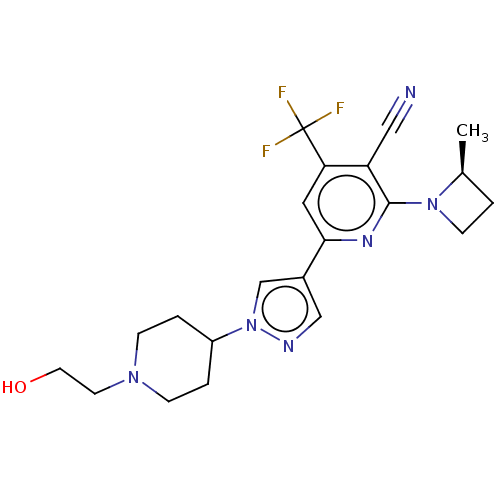
(6-[1-[1-(2-Hydroxyethyl)-4-piperidyl]pyrazol-4-yl]...)Show SMILES C[C@H]1CCN1c1nc(cc(c1C#N)C(F)(F)F)-c1cnn(c1)C1CCN(CCO)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CR5XGZ |
More data for this
Ligand-Target Pair | |
Isoform A of Ketohexokinase (Peripheral)
(Homo sapiens (Human)) | BDBM518442

(4-[1-(Azetidin-3-yl)pyrazol-4-yl]-2-[(2S)-2-methyl...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)-c1cnn(c1)C1CNC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CR5XGZ |
More data for this
Ligand-Target Pair | |
Isoform A of Ketohexokinase (Peripheral)
(Homo sapiens (Human)) | BDBM518418

(2-[(2S)-2-Methylazetidin-1-yl]-6-[1-(4-piperidyl)p...)Show SMILES C[C@H]1CCN1c1nc(cc(c1C#N)C(F)(F)F)-c1cnn(c1)C1CCNCC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The intrinsic potency for inhibition of KHK C or A activity may be measured using an enzymatic assay which measures the production of FIP. Compounds ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CR5XGZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data