

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110122 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110120 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM36609 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110126 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493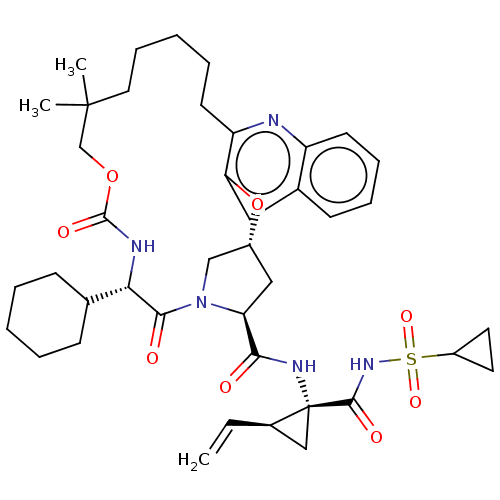 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432752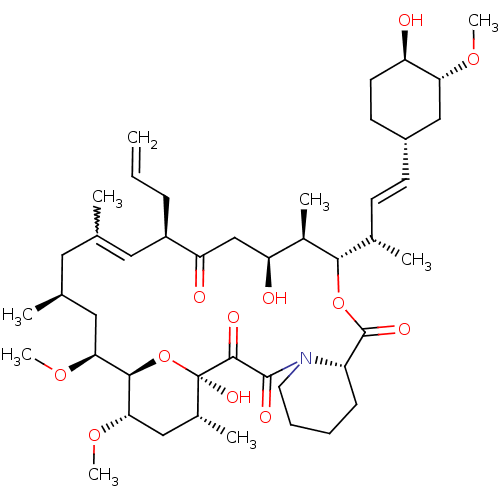 (FK-506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110123 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110124 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50388298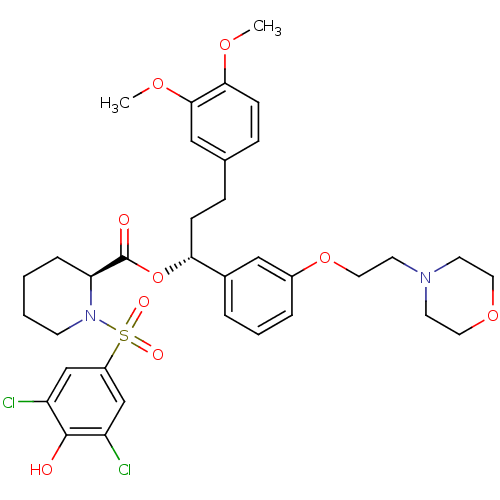 (CHEMBL2058794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432732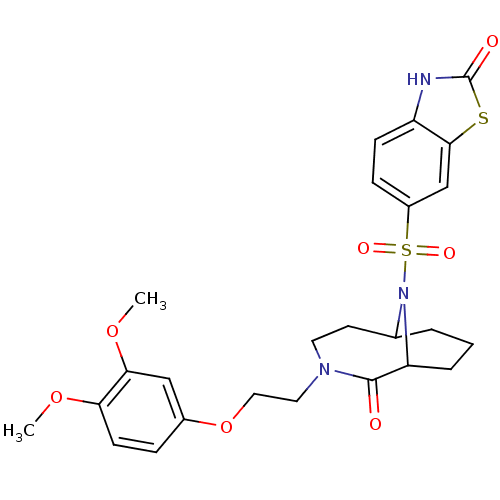 (CHEMBL2348594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50432739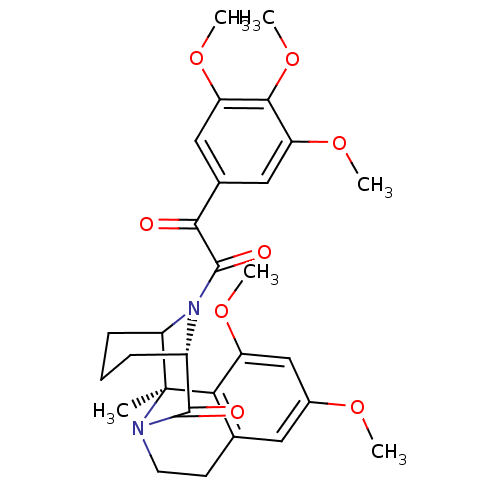 (CHEMBL2348604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110128 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme NS3 protease wild-type | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110127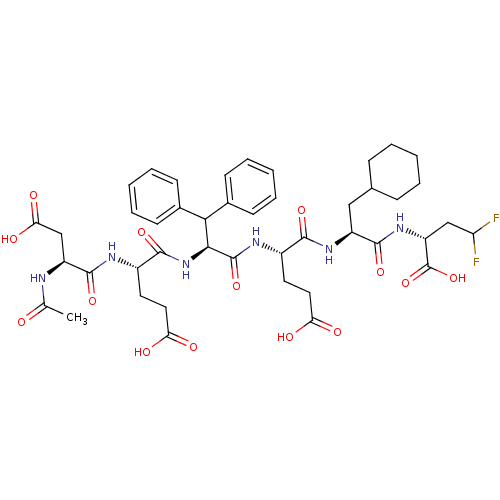 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110118 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50432736 (CHEMBL2348607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Binding affinity for enzyme K136R | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432740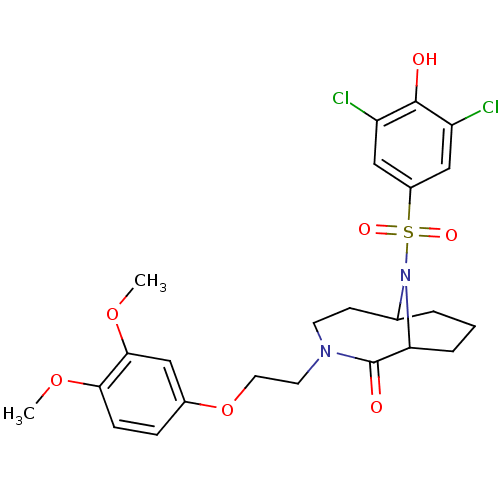 (CHEMBL2348599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432748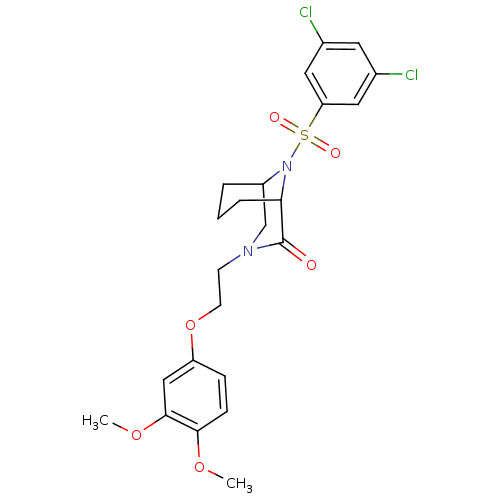 (CHEMBL2348587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme K136M | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110119 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122883 (3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for wild type NS3 protease | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50432735 (CHEMBL2348608) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1102 total ) | Next | Last >> |