 Found 18326 hits with Last Name = 'yang' and Initial = 'y'
Found 18326 hits with Last Name = 'yang' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Endothelin A receptor |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 |
J Med Chem 45: 3829-35 (2002)
BindingDB Entry DOI: 10.7270/Q2Z60NDM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253328
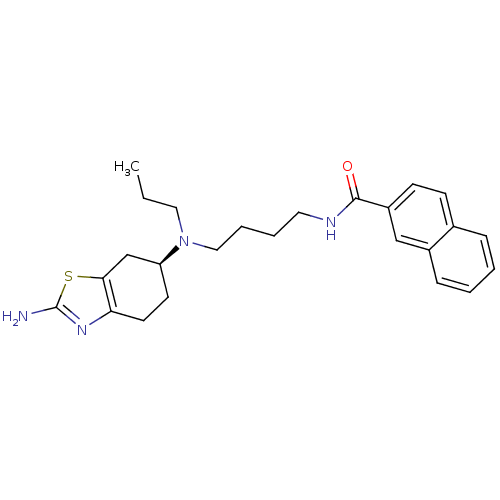
((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...)Show SMILES CCCN(CCCCNC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r| Show InChI InChI=1S/C25H32N4OS/c1-2-14-29(21-11-12-22-23(17-21)31-25(26)28-22)15-6-5-13-27-24(30)20-10-9-18-7-3-4-8-19(18)16-20/h3-4,7-10,16,21H,2,5-6,11-15,17H2,1H3,(H2,26,28)(H,27,30)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513202
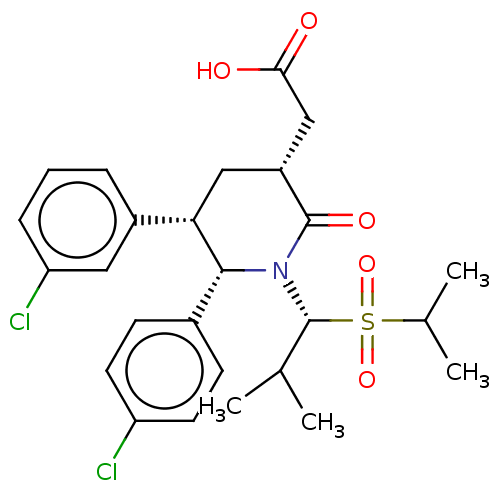
(CHEMBL4463050)Show SMILES CC(C)[C@H](N1[C@@H]([C@@H](C[C@H](CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C(C)C |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-15(2)26(35(33,34)16(3)4)29-24(17-8-10-20(27)11-9-17)22(18-6-5-7-21(28)12-18)13-19(25(29)32)14-23(30)31/h5-12,15-16,19,22,24,26H,13-14H2,1-4H3,(H,30,31)/t19-,22+,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 in human SJSA1 cells |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
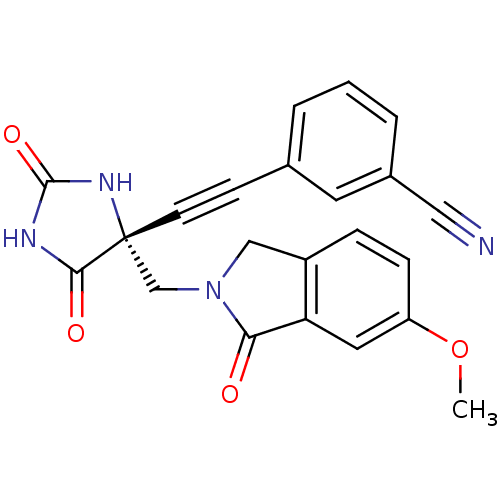
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
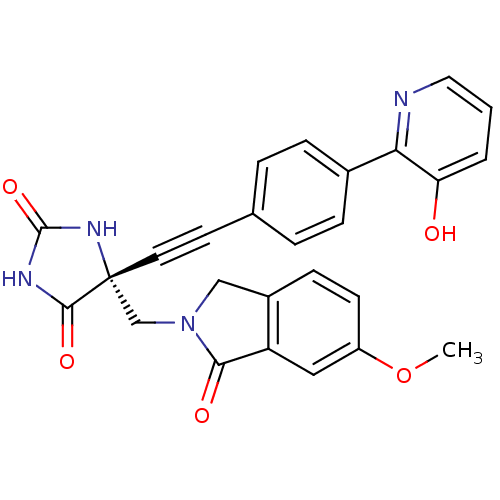
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325003
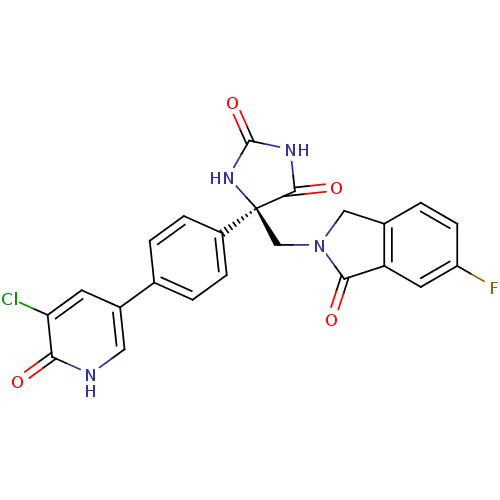
((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3c[nH]c(=O)c(Cl)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H16ClFN4O4/c24-18-7-14(9-26-19(18)30)12-1-4-15(5-2-12)23(21(32)27-22(33)28-23)11-29-10-13-3-6-16(25)8-17(13)20(29)31/h1-9H,10-11H2,(H,26,30)(H2,27,28,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669
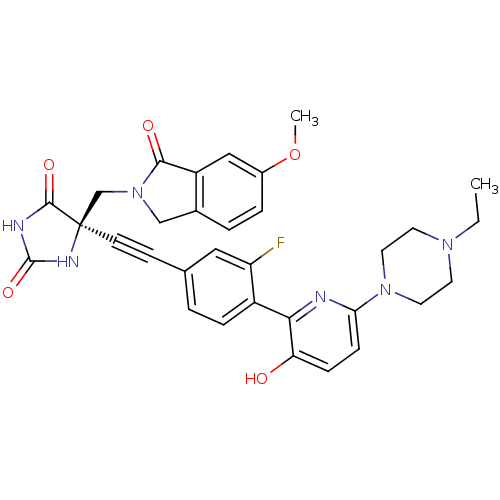
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50035105

(1'-{4-[1-(4-fluorophenyl)-1H-3-indolyl]butyl}spiro...)Show SMILES Fc1ccc(cc1)-n1cc(CCCCN2CCC3(CC2)OCc2ccccc32)c2ccccc12 Show InChI InChI=1S/C30H31FN2O/c31-25-12-14-26(15-13-25)33-21-23(27-9-2-4-11-29(27)33)7-5-6-18-32-19-16-30(17-20-32)28-10-3-1-8-24(28)22-34-30/h1-4,8-15,21H,5-7,16-20,22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangdong Medical University
Curated by ChEMBL
| Assay Description
Binding affinity to sigma-2 receptor (unknown origin) |
Eur J Med Chem 147: 227-237 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.016
BindingDB Entry DOI: 10.7270/Q2SQ92XB |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
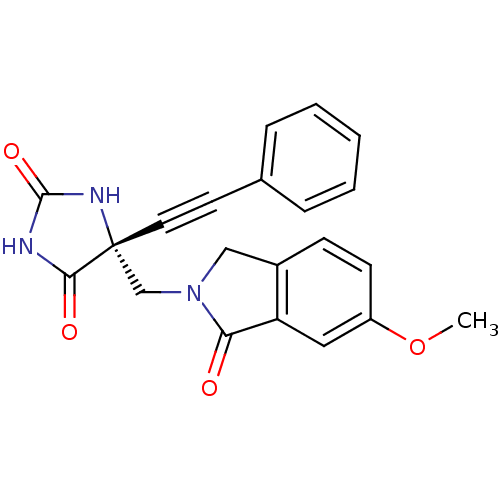
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332289

((R)-5-((4-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C28H30N6O5/c1-3-32-12-14-33(15-13-32)24(31-38)20-6-4-19(5-7-20)10-11-28(26(36)29-27(37)30-28)18-34-17-21-8-9-22(39-2)16-23(21)25(34)35/h4-9,16,38H,3,12-15,17-18H2,1-2H3,(H2,29,30,36,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332288
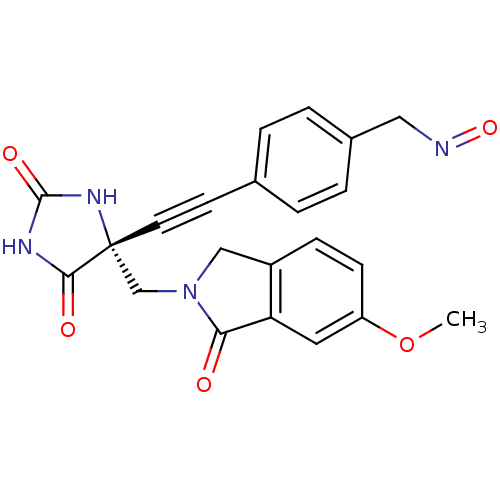
((R)-4-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(CN=O)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C22H18N4O5/c1-31-17-7-6-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)9-8-14-2-4-15(5-3-14)11-23-30/h2-7,10H,11-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332278
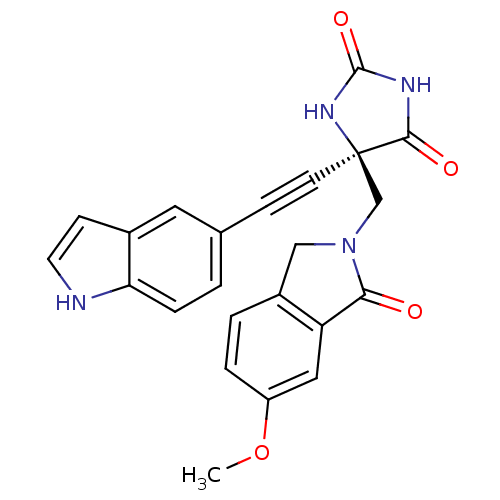
((R)-5-((1H-indol-5-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4[nH]ccc4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-4-3-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-5-19-15(10-14)7-9-24-19/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332290
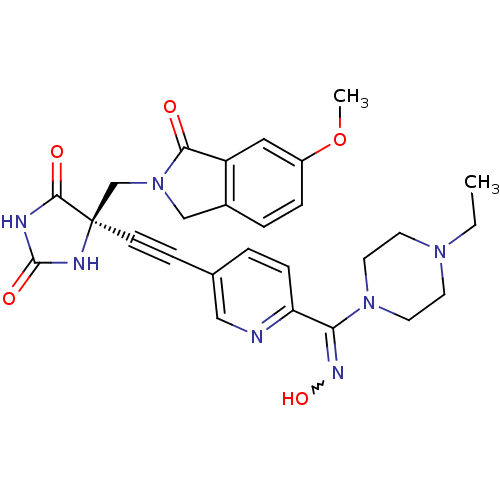
((R)-5-((6-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cn1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C27H29N7O5/c1-3-32-10-12-33(13-11-32)23(31-38)22-7-4-18(15-28-22)8-9-27(25(36)29-26(37)30-27)17-34-16-19-5-6-20(39-2)14-21(19)24(34)35/h4-7,14-15,38H,3,10-13,16-17H2,1-2H3,(H2,29,30,36,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50459819
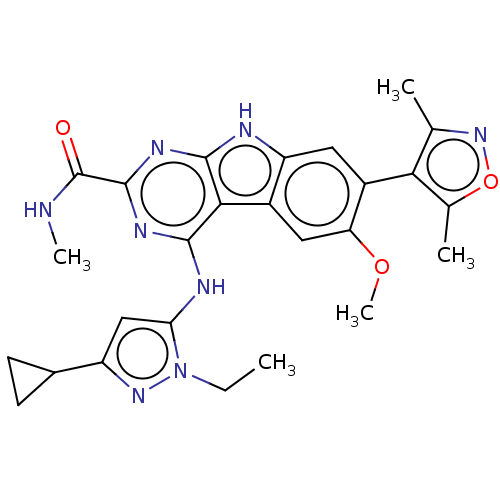
(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 1 (24 to 144 residues) (unknown origin) expressed in ... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332279
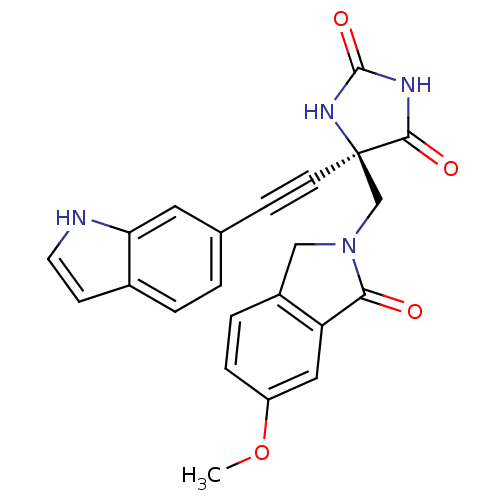
((R)-5-((1H-indol-6-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4cc[nH]c4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-5-4-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-3-15-7-9-24-19(15)10-14/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332264
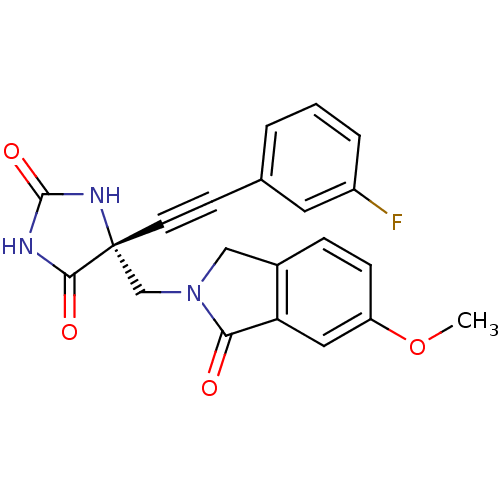
((R)-5-((3-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(F)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-16-6-5-14-11-25(18(26)17(14)10-16)12-21(19(27)23-20(28)24-21)8-7-13-3-2-4-15(22)9-13/h2-6,9-10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325002
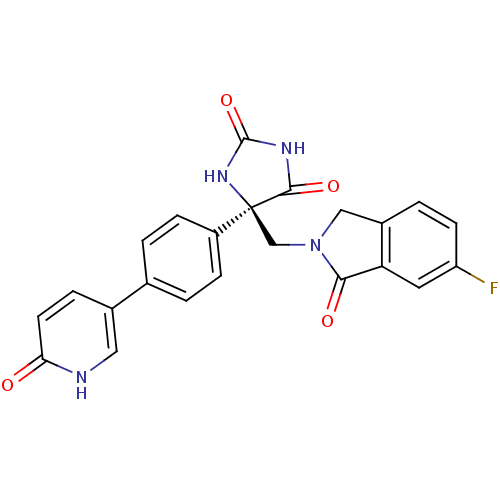
((R)-5-((6-fluoro-1-oxoisoindolin-2-yl)methyl)-5-(4...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3ccc(=O)[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H17FN4O4/c24-17-7-3-15-11-28(20(30)18(15)9-17)12-23(21(31)26-22(32)27-23)16-5-1-13(2-6-16)14-4-8-19(29)25-10-14/h1-10H,11-12H2,(H,25,29)(H2,26,27,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50596723

(CHEMBL5205903 | US20230348421, Compound 59)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cn1)C(=O)NCc1ccc(OCC(F)(F)F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114246
BindingDB Entry DOI: 10.7270/Q20P142F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329148
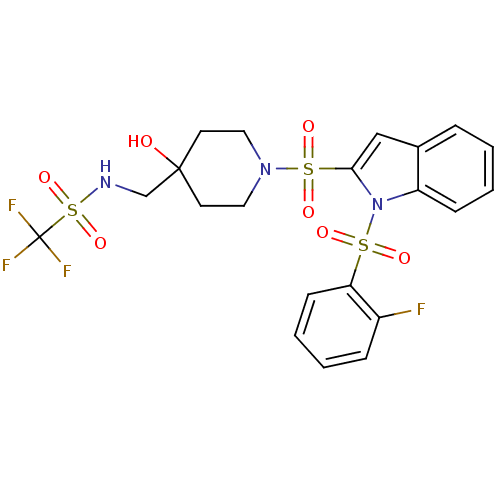
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES OC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H21F4N3O7S3/c22-16-6-2-4-8-18(16)36(30,31)28-17-7-3-1-5-15(17)13-19(28)37(32,33)27-11-9-20(29,10-12-27)14-26-38(34,35)21(23,24)25/h1-8,13,26,29H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50459819

(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 2 (306 to 417residues) (unknown origin) expressed in ... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332291
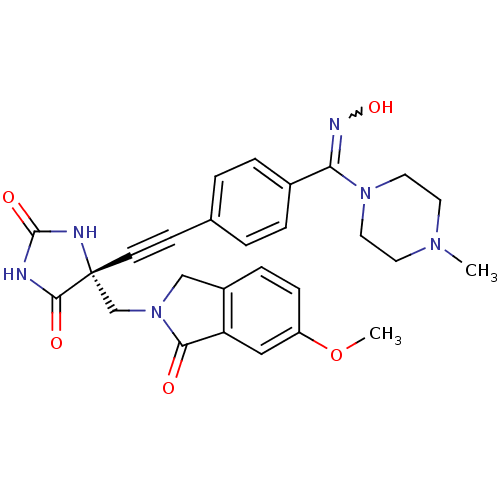
((R)-5-((4-((hydroxyimino)(4-methylpiperazin-1-yl)m...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(=NO)N3CCN(C)CC3)C(=O)c2c1 |r,w:25.27| Show InChI InChI=1S/C27H28N6O5/c1-31-11-13-32(14-12-31)23(30-37)19-5-3-18(4-6-19)9-10-27(25(35)28-26(36)29-27)17-33-16-20-7-8-21(38-2)15-22(20)24(33)34/h3-8,15,37H,11-14,16-17H2,1-2H3,(H2,28,29,35,36)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50255183
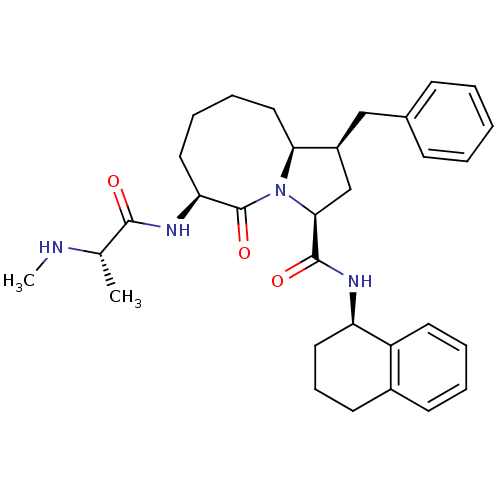
((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCCC[C@H]2[C@@H](Cc3ccccc3)C[C@H](N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r| Show InChI InChI=1S/C32H42N4O3/c1-21(33-2)30(37)35-27-16-8-9-18-28-24(19-22-11-4-3-5-12-22)20-29(36(28)32(27)39)31(38)34-26-17-10-14-23-13-6-7-15-25(23)26/h3-7,11-13,15,21,24,26-29,33H,8-10,14,16-20H2,1-2H3,(H,34,38)(H,35,37)/t21-,24-,26+,27-,28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescent SM5F peptide from His-tagged human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... |
J Med Chem 52: 593-6 (2009)
Article DOI: 10.1021/jm801101z
BindingDB Entry DOI: 10.7270/Q2Z03816 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50216056

((N-[4-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-...)Show SMILES COc1cc2CCN(CCCCNC(=O)c3cc(I)cc(OC)c3OCCF)Cc2cc1OC Show InChI InChI=1S/C25H32FIN2O5/c1-31-21-12-17-6-10-29(16-18(17)13-22(21)32-2)9-5-4-8-28-25(30)20-14-19(27)15-23(33-3)24(20)34-11-7-26/h12-15H,4-11,16H2,1-3H3,(H,28,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangdong Medical University
Curated by ChEMBL
| Assay Description
Binding affinity to sigma-2 receptor (unknown origin) |
Eur J Med Chem 147: 227-237 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.016
BindingDB Entry DOI: 10.7270/Q2SQ92XB |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332263
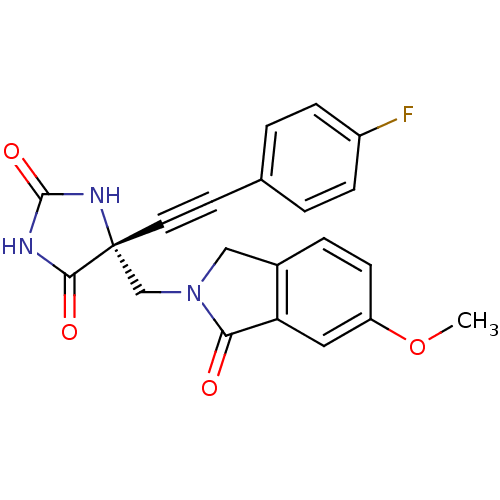
((R)-5-((4-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(F)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-16-7-4-14-11-25(18(26)17(14)10-16)12-21(19(27)23-20(28)24-21)9-8-13-2-5-15(22)6-3-13/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50459819

(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD2 bromodomain 2 (349 to 460 residues) (unknown origin) expressed in... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... |
Science 374: 1-13 (2021)
BindingDB Entry DOI: 10.7270/Q23T9MCM |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332285
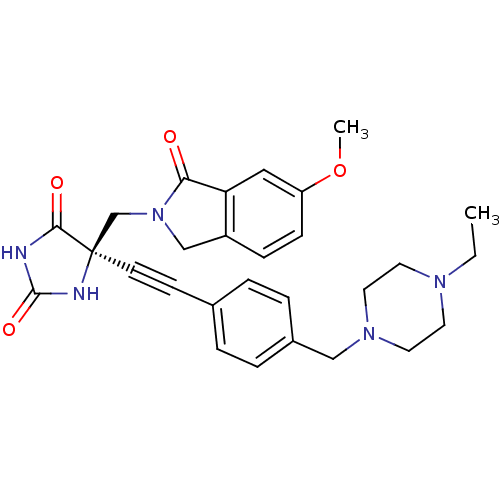
((R)-5-((4-((4-ethylpiperazin-1-yl)methyl)phenyl)et...)Show SMILES CCN1CCN(Cc2ccc(cc2)C#C[C@]2(CN3Cc4ccc(OC)cc4C3=O)NC(=O)NC2=O)CC1 |r| Show InChI InChI=1S/C28H31N5O4/c1-3-31-12-14-32(15-13-31)17-21-6-4-20(5-7-21)10-11-28(26(35)29-27(36)30-28)19-33-18-22-8-9-23(37-2)16-24(22)25(33)34/h4-9,16H,3,12-15,17-19H2,1-2H3,(H2,29,30,35,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50020310
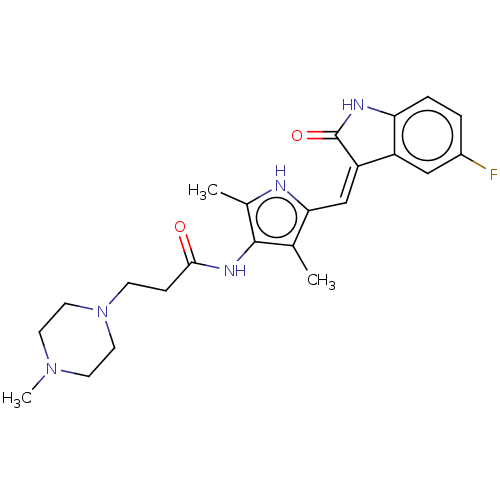
(CHEMBL3288854)Show SMILES CN1CCN(CCC(=O)Nc2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H28FN5O2/c1-14-20(13-18-17-12-16(24)4-5-19(17)26-23(18)31)25-15(2)22(14)27-21(30)6-7-29-10-8-28(3)9-11-29/h4-5,12-13,25H,6-11H2,1-3H3,(H,26,31)(H,27,30)/b18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to FLT3 (unknown origin) |
Eur J Med Chem 82: 139-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.051
BindingDB Entry DOI: 10.7270/Q2F1918D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50020310

(CHEMBL3288854)Show SMILES CN1CCN(CCC(=O)Nc2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H28FN5O2/c1-14-20(13-18-17-12-16(24)4-5-19(17)26-23(18)31)25-15(2)22(14)27-21(30)6-7-29-10-8-28(3)9-11-29/h4-5,12-13,25H,6-11H2,1-3H3,(H,26,31)(H,27,30)/b18-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to VEGFR3 (unknown origin) |
Eur J Med Chem 82: 139-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.051
BindingDB Entry DOI: 10.7270/Q2F1918D |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangdong Medical University
Curated by ChEMBL
| Assay Description
Binding affinity to sigma-2 receptor (unknown origin) |
Eur J Med Chem 147: 227-237 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.016
BindingDB Entry DOI: 10.7270/Q2SQ92XB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329147
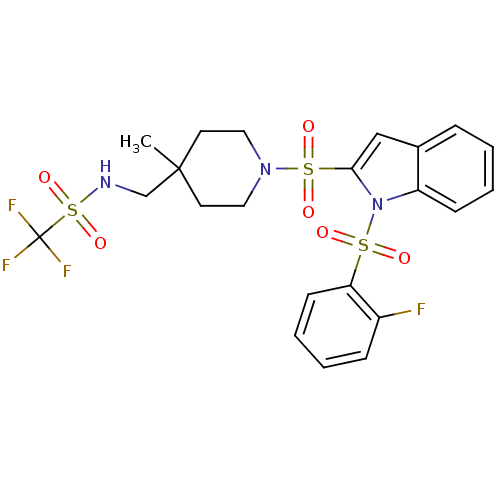
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES CC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-21(15-27-38(34,35)22(24,25)26)10-12-28(13-11-21)37(32,33)20-14-16-6-2-4-8-18(16)29(20)36(30,31)19-9-5-3-7-17(19)23/h2-9,14,27H,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50255183

((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCCC[C@H]2[C@@H](Cc3ccccc3)C[C@H](N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r| Show InChI InChI=1S/C32H42N4O3/c1-21(33-2)30(37)35-27-16-8-9-18-28-24(19-22-11-4-3-5-12-22)20-29(36(28)32(27)39)31(38)34-26-17-10-14-23-13-6-7-15-25(23)26/h3-7,11-13,15,21,24,26-29,33H,8-10,14,16-20H2,1-2H3,(H,34,38)(H,35,37)/t21-,24-,26+,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... |
J Med Chem 52: 593-6 (2009)
Article DOI: 10.1021/jm801101z
BindingDB Entry DOI: 10.7270/Q2Z03816 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329151

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES Fc1ccccc1S(=O)(=O)n1c(cc2ccccc12)S(=O)(=O)N1CCC(CNS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C21H21F4N3O6S3/c22-17-6-2-4-8-19(17)35(29,30)28-18-7-3-1-5-16(18)13-20(28)36(31,32)27-11-9-15(10-12-27)14-26-37(33,34)21(23,24)25/h1-8,13,15,26H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50313839
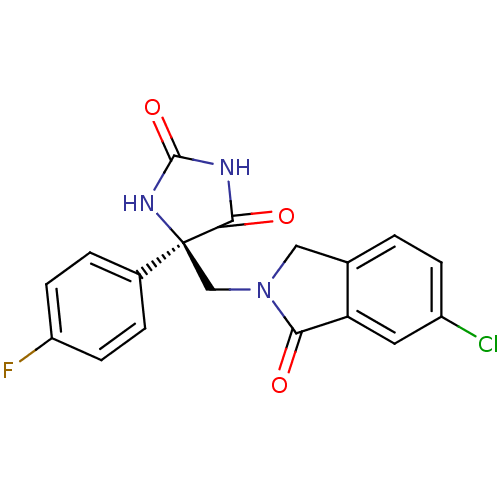
((R)-5-((6-chloro-1-oxoisoindolin-2-yl)methyl)-5-(4...)Show SMILES Fc1ccc(cc1)[C@]1(CN2Cc3ccc(Cl)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H13ClFN3O3/c19-12-4-1-10-8-23(15(24)14(10)7-12)9-18(16(25)21-17(26)22-18)11-2-5-13(20)6-3-11/h1-7H,8-9H2,(H2,21,22,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE by FRET assay |
Bioorg Med Chem Lett 20: 1877-80 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.148
BindingDB Entry DOI: 10.7270/Q2F76CQ9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329129
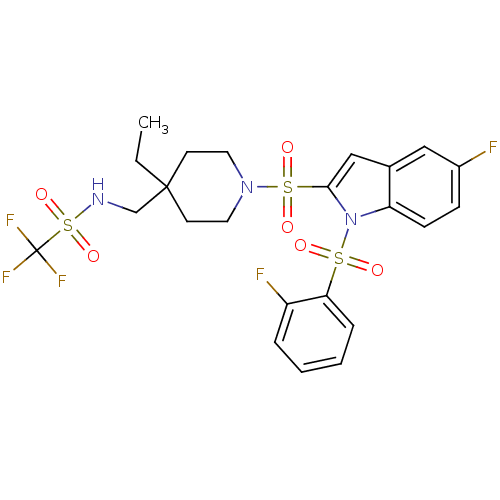
(CHEMBL1271093 | N-((4-ethyl-1-(5-fluoro-1-(2-fluor...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2cc(F)ccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C23H24F5N3O6S3/c1-2-22(15-29-40(36,37)23(26,27)28)9-11-30(12-10-22)39(34,35)21-14-16-13-17(24)7-8-19(16)31(21)38(32,33)20-6-4-3-5-18(20)25/h3-8,13-14,29H,2,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329130
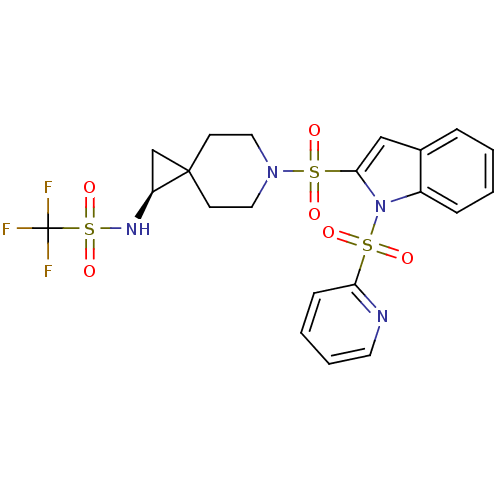
((S)-1,1,1-trifluoro-N-(6-(1-(pyridin-2-ylsulfonyl)...)Show SMILES FC(F)(F)S(=O)(=O)N[C@H]1CC11CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C21H21F3N4O6S3/c22-21(23,24)37(33,34)26-17-14-20(17)8-11-27(12-9-20)36(31,32)19-13-15-5-1-2-6-16(15)28(19)35(29,30)18-7-3-4-10-25-18/h1-7,10,13,17,26H,8-9,11-12,14H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329150

(1,1,1-trifluoro-N-(1-(1-(1-(2-fluorophenylsulfonyl...)Show SMILES CC(NS(=O)(=O)C(F)(F)F)C1CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-15(27-38(34,35)22(24,25)26)16-10-12-28(13-11-16)37(32,33)21-14-17-6-2-4-8-19(17)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,14-16,27H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50383200
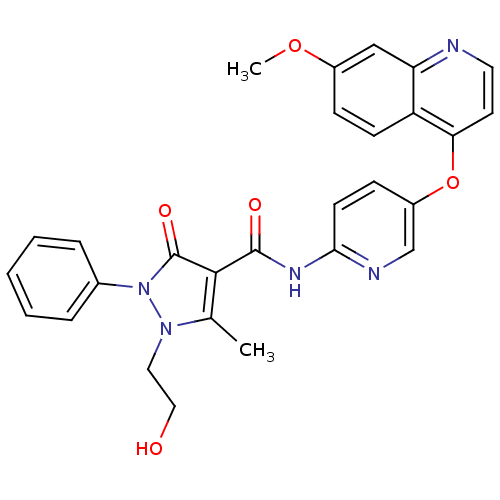
(CHEMBL2032026)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CCO)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C28H25N5O5/c1-18-26(28(36)33(32(18)14-15-34)19-6-4-3-5-7-19)27(35)31-25-11-9-21(17-30-25)38-24-12-13-29-23-16-20(37-2)8-10-22(23)24/h3-13,16-17,34H,14-15H2,1-2H3,(H,30,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of c-Met by HTRF assay |
J Med Chem 55: 1868-97 (2012)
Article DOI: 10.1021/jm201331s
BindingDB Entry DOI: 10.7270/Q23B616C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172146
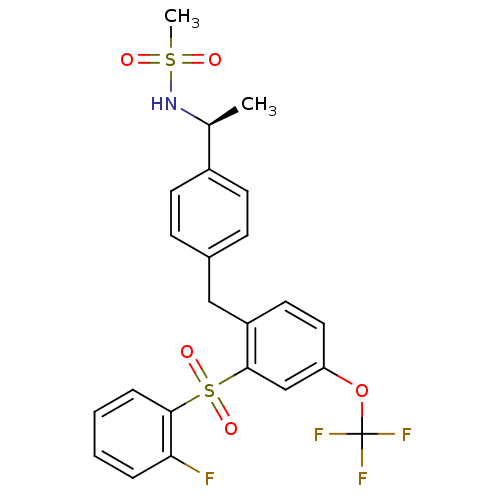
(CHEMBL381669 | N-((S)-1-{4-[2-(2-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(Cc2ccc(OC(F)(F)F)cc2S(=O)(=O)c2ccccc2F)cc1 Show InChI InChI=1S/C23H21F4NO5S2/c1-15(28-34(2,29)30)17-9-7-16(8-10-17)13-18-11-12-19(33-23(25,26)27)14-22(18)35(31,32)21-6-4-3-5-20(21)24/h3-12,14-15,28H,13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160446
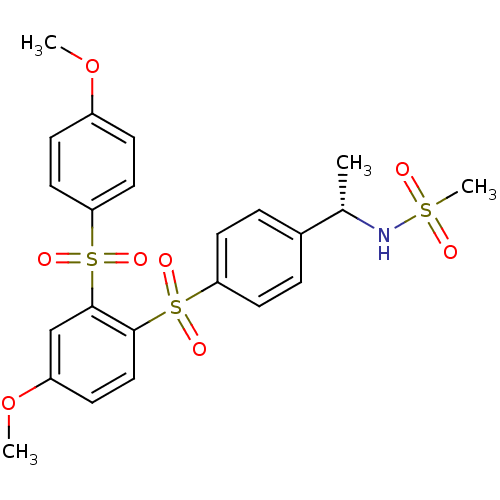
(CHEMBL180465 | N-((S)-1-{4-[4-Methoxy-2-(4-methoxy...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(OC)ccc1S(=O)(=O)c1ccc(cc1)[C@H](C)NS(C)(=O)=O Show InChI InChI=1S/C23H25NO8S3/c1-16(24-33(4,25)26)17-5-10-20(11-6-17)34(27,28)22-14-9-19(32-3)15-23(22)35(29,30)21-12-7-18(31-2)8-13-21/h5-16,24H,1-4H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50393632
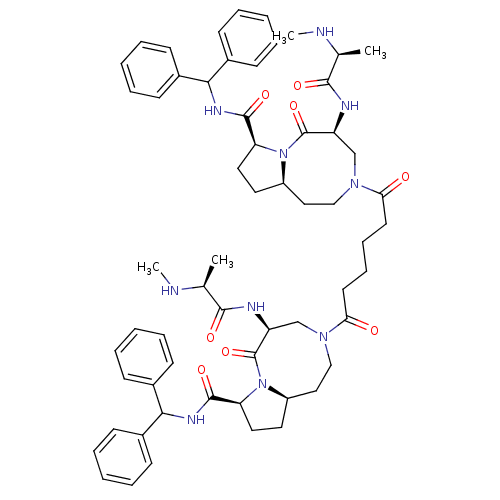
(CHEMBL2158601)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(CC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1)C(=O)CCCCC(=O)N1CC[C@H]2CC[C@H](N2C(=O)[C@H](C1)NC(=O)[C@H](C)NC)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C60H76N10O8/c1-39(61-3)55(73)63-47-37-67(35-33-45-29-31-49(69(45)59(47)77)57(75)65-53(41-19-9-5-10-20-41)42-21-11-6-12-22-42)51(71)27-17-18-28-52(72)68-36-34-46-30-32-50(70(46)60(78)48(38-68)64-56(74)40(2)62-4)58(76)66-54(43-23-13-7-14-24-43)44-25-15-8-16-26-44/h5-16,19-26,39-40,45-50,53-54,61-62H,17-18,27-38H2,1-4H3,(H,63,73)(H,64,74)(H,65,75)(H,66,76)/t39-,40-,45+,46+,47-,48-,49-,50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to BIR3 domain of cIAP1 by fluorescence polarization assay |
J Med Chem 55: 106-14 (2012)
Article DOI: 10.1021/jm201072x
BindingDB Entry DOI: 10.7270/Q2BG2Q3B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253393

(CHEMBL495327 | Naphthalene-2-carboxylic acid (4-{2...)Show SMILES CCCN(CC[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r,wU:6.5,wD:25.27,9.12,(-.71,-29.3,;-.71,-27.76,;-2.04,-26.99,;-2.04,-25.45,;-.71,-24.68,;.63,-25.45,;1.96,-24.68,;1.95,-23.14,;3.3,-22.38,;4.63,-23.15,;4.62,-24.69,;3.29,-25.46,;5.96,-22.39,;7.29,-23.16,;7.29,-24.7,;8.63,-22.39,;9.96,-23.16,;11.29,-22.4,;11.29,-20.86,;12.62,-20.09,;12.62,-18.55,;11.28,-17.78,;9.95,-18.55,;9.95,-20.08,;8.62,-20.85,;-3.37,-24.67,;-3.37,-23.13,;-4.72,-22.35,;-6.05,-23.12,;-7.53,-22.64,;-8.45,-23.9,;-9.99,-23.9,;-7.53,-25.16,;-6.05,-24.67,;-4.72,-25.44,)| Show InChI InChI=1S/C29H38N4OS/c1-2-16-33(25-13-14-26-27(19-25)35-29(30)32-26)17-15-20-7-11-24(12-8-20)31-28(34)23-10-9-21-5-3-4-6-22(21)18-23/h3-6,9-10,18,20,24-25H,2,7-8,11-17,19H2,1H3,(H2,30,32)(H,31,34)/t20-,24-,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253473

(CHEMBL492883 | N-(4-{2-[((S)-2-Amino-4,5,6,7-tetra...)Show SMILES CCCN(CC[C@H]1CC[C@@H](CC1)NC(=O)\C=C\c1ccccc1)[C@H]1CCc2nc(N)sc2C1 |r,wU:6.5,wD:9.12,23.24,(-.41,-16.74,;-.41,-15.2,;-1.74,-14.43,;-1.74,-12.89,;-.41,-12.12,;.93,-12.89,;2.26,-12.12,;2.25,-10.58,;3.59,-9.82,;4.93,-10.59,;4.92,-12.14,;3.59,-12.9,;6.26,-9.83,;7.59,-10.6,;7.59,-12.14,;8.93,-9.83,;10.26,-10.6,;11.6,-9.83,;12.92,-10.61,;14.26,-9.85,;14.26,-8.3,;12.92,-7.53,;11.59,-8.3,;-3.07,-12.11,;-3.07,-10.57,;-4.42,-9.79,;-5.75,-10.56,;-7.23,-10.08,;-8.15,-11.34,;-9.69,-11.34,;-7.23,-12.6,;-5.75,-12.11,;-4.42,-12.88,)| Show InChI InChI=1S/C27H38N4OS/c1-2-17-31(23-13-14-24-25(19-23)33-27(28)30-24)18-16-21-8-11-22(12-9-21)29-26(32)15-10-20-6-4-3-5-7-20/h3-7,10,15,21-23H,2,8-9,11-14,16-19H2,1H3,(H2,28,30)(H,29,32)/b15-10+/t21-,22-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332266
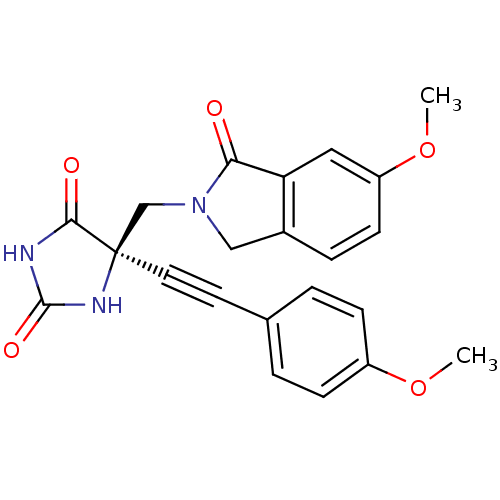
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C22H19N3O5/c1-29-16-6-3-14(4-7-16)9-10-22(20(27)23-21(28)24-22)13-25-12-15-5-8-17(30-2)11-18(15)19(25)26/h3-8,11H,12-13H2,1-2H3,(H2,23,24,27,28)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102890
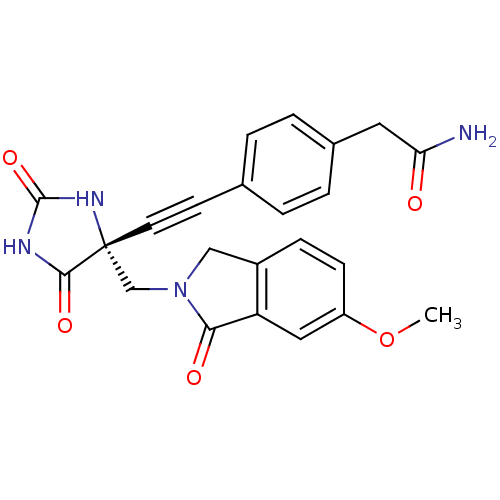
(CHEMBL1288211 | US8541572, 2283)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(CC(N)=O)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H20N4O5/c1-32-17-7-6-16-12-27(20(29)18(16)11-17)13-23(21(30)25-22(31)26-23)9-8-14-2-4-15(5-3-14)10-19(24)28/h2-7,11H,10,12-13H2,1H3,(H2,24,28)(H2,25,26,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 (unknown origin) by western blot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513204

(CHEMBL4537250)Show SMILES CC(C)(C)C[C@H]1NC([C@H](c2cccc(Cl)c2F)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:33.38,17.29,31.34,8.8,wD:5.4,(55.7,-19.72,;54.25,-19.21,;53.96,-17.7,;55.33,-18.11,;53.08,-20.22,;51.63,-19.71,;51.18,-18.23,;49.64,-18.2,;49.13,-19.66,;47.62,-19.35,;47.13,-17.89,;45.63,-17.58,;44.6,-18.74,;45.1,-20.2,;44.09,-21.36,;46.61,-20.5,;45.83,-21.82,;50.36,-20.59,;51.61,-21.5,;53.08,-21.03,;51.13,-22.97,;49.59,-22.97,;48.57,-24.1,;47.07,-23.79,;46.05,-24.94,;46.59,-22.32,;47.62,-21.19,;49.12,-21.51,;49.2,-16.72,;47.7,-16.36,;50.26,-15.6,;51.76,-15.96,;53.06,-15.15,;53.86,-16.46,;54.95,-15.36,;55.36,-16.85,;52.56,-17.27,)| Show InChI InChI=1S/C28H32Cl2FN3O3/c1-26(2,3)13-20-28(17-9-8-14(29)10-19(17)33-25(28)36)21(16-6-5-7-18(30)22(16)31)23(34-20)24(35)32-15-11-27(4,37)12-15/h5-10,15,20-21,23,34,37H,11-13H2,1-4H3,(H,32,35)(H,33,36)/t15-,20-,21+,23?,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type p53 protein binding to MDM2 in human HCT116 cells by immunoblot analysis |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data