 Found 9780 hits with Last Name = 'ala' and Initial = 'p'
Found 9780 hits with Last Name = 'ala' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457611
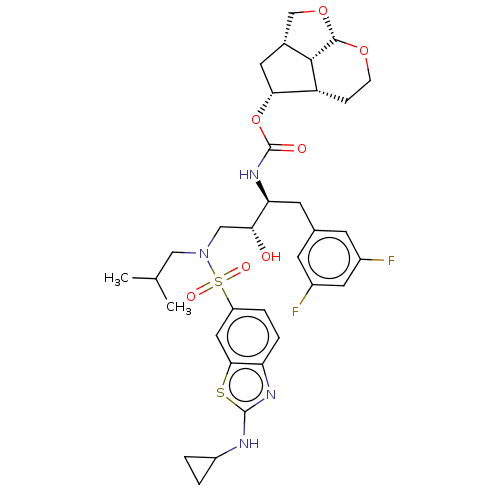
(CHEMBL4214453)Show SMILES [H][C@]12CO[C@]3([H])OCC[C@]([H])([C@@H](C1)OC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc4nc(NC5CC5)sc4c1)[C@]23[H] |r| Show InChI InChI=1S/C34H42F2N4O7S2/c1-18(2)15-40(49(43,44)24-5-6-26-30(14-24)48-33(38-26)37-23-3-4-23)16-28(41)27(11-19-9-21(35)13-22(36)10-19)39-34(42)47-29-12-20-17-46-32-31(20)25(29)7-8-45-32/h5-6,9-10,13-14,18,20,23,25,27-29,31-32,41H,3-4,7-8,11-12,15-17H2,1-2H3,(H,37,38)(H,39,42)/t20-,25-,27+,28-,29-,31+,32+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457604

(CHEMBL4213229)Show SMILES [H][C@@]12CO[C@@]3([H])OC[C@@]([H])([C@H](C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1)[C@@]23[H] |r| Show InChI InChI=1S/C30H40N2O8S/c1-19(2)15-32(41(35,36)23-11-9-22(37-3)10-12-23)16-26(33)25(13-20-7-5-4-6-8-20)31-30(34)40-27-14-21-17-38-29-28(21)24(27)18-39-29/h4-12,19,21,24-29,33H,13-18H2,1-3H3,(H,31,34)/t21-,24-,25-,26+,27-,28+,29-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50489369
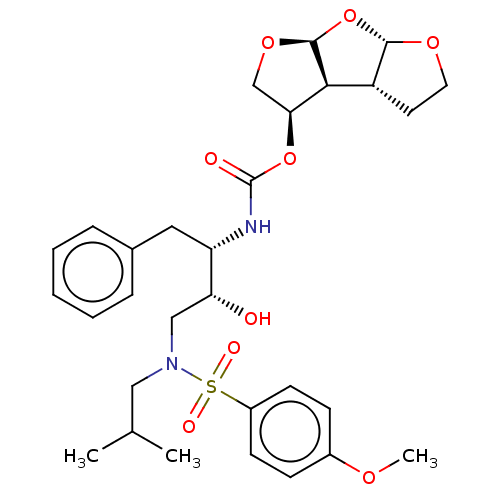
(CHEMBL1232930 | GRL-0519)Show SMILES [H][C@@]12OCC[C@]1([H])[C@@]1([H])[C@H](CO[C@@]1([H])O2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H40N2O9S/c1-19(2)16-32(42(35,36)22-11-9-21(37-3)10-12-22)17-25(33)24(15-20-7-5-4-6-8-20)31-30(34)40-26-18-39-29-27(26)23-13-14-38-28(23)41-29/h4-12,19,23-29,33H,13-18H2,1-3H3,(H,31,34)/t23-,24+,25-,26+,27+,28+,29+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 56: 6792-802 (2013)
Article DOI: 10.1021/jm400768f
BindingDB Entry DOI: 10.7270/Q2K07764 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457612

(CHEMBL4218164)Show SMILES [H][C@]12CO[C@]3([H])OCC[C@]([H])([C@@H](C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1)[C@]23[H] |r| Show InChI InChI=1S/C31H42N2O8S/c1-20(2)17-33(42(36,37)24-11-9-23(38-3)10-12-24)18-27(34)26(15-21-7-5-4-6-8-21)32-31(35)41-28-16-22-19-40-30-29(22)25(28)13-14-39-30/h4-12,20,22,25-30,34H,13-19H2,1-3H3,(H,32,35)/t22-,25-,26+,27-,28-,29+,30+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457608

(CHEMBL4211505)Show SMILES [H][C@]12OCC3C[C@H](OC(=O)N[C@@H](Cc4ccccc4)[C@H](O)CN(CC(C)C)S(=O)(=O)c4ccc5nc(NC6CC6)sc5c4)[C@]([H])(CCO1)[C@]23[H] |r| Show InChI InChI=1S/C34H44N4O7S2/c1-20(2)17-38(47(41,42)24-10-11-26-30(16-24)46-33(36-26)35-23-8-9-23)18-28(39)27(14-21-6-4-3-5-7-21)37-34(40)45-29-15-22-19-44-32-31(22)25(29)12-13-43-32/h3-7,10-11,16,20,22-23,25,27-29,31-32,39H,8-9,12-15,17-19H2,1-2H3,(H,35,36)(H,37,40)/t22?,25-,27-,28+,29-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108
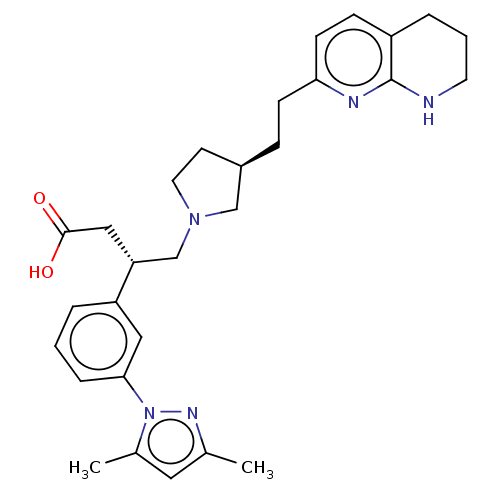
(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50493304

(CHEMBL2426453)Show SMILES [H][C@@]12CCC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(OC)cc3)[C@]1([H])[C@@]1([H])CCO[C@@]1([H])O2 |r| Show InChI InChI=1S/C32H44N2O8S/c1-21(2)19-34(43(37,38)24-14-12-23(39-3)13-15-24)20-27(35)26(18-22-8-5-4-6-9-22)33-32(36)42-29-11-7-10-28-30(29)25-16-17-40-31(25)41-28/h4-6,8-9,12-15,21,25-31,35H,7,10-11,16-20H2,1-3H3,(H,33,36)/t25-,26+,27-,28-,29+,30-,31+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 LAI protease by fluorescence assay |
J Med Chem 56: 6792-802 (2013)
Article DOI: 10.1021/jm400768f
BindingDB Entry DOI: 10.7270/Q2K07764 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457610

(CHEMBL4207145)Show SMILES [H][C@]12OCC3C[C@H](OC(=O)N[C@@H](Cc4cc(F)cc(F)c4)[C@H](O)CN(CC(C)C)S(=O)(=O)c4ccc5nc(NC6CC6)sc5c4)[C@]([H])(CCO1)[C@]23[H] |r| Show InChI InChI=1S/C34H42F2N4O7S2/c1-18(2)15-40(49(43,44)24-5-6-26-30(14-24)48-33(38-26)37-23-3-4-23)16-28(41)27(11-19-9-21(35)13-22(36)10-19)39-34(42)47-29-12-20-17-46-32-31(20)25(29)7-8-45-32/h5-6,9-10,13-14,18,20,23,25,27-29,31-32,41H,3-4,7-8,11-12,15-17H2,1-2H3,(H,37,38)(H,39,42)/t20?,25-,27-,28+,29-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM160
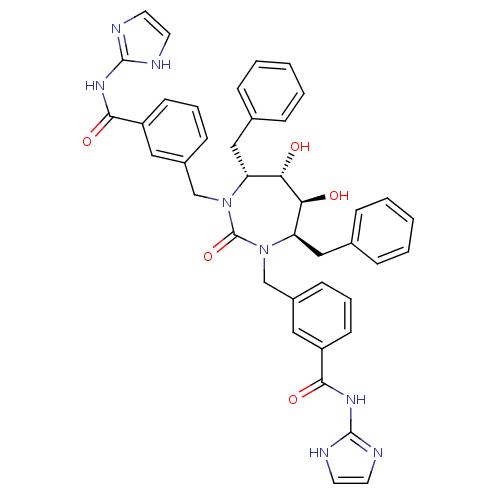
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H40N8O5/c50-35-33(23-27-9-3-1-4-10-27)48(25-29-13-7-15-31(21-29)37(52)46-39-42-17-18-43-39)41(54)49(34(36(35)51)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(53)47-40-44-19-20-45-40/h1-22,33-36,50-51H,23-26H2,(H2,42,43,46,52)(H2,44,45,47,53)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055590
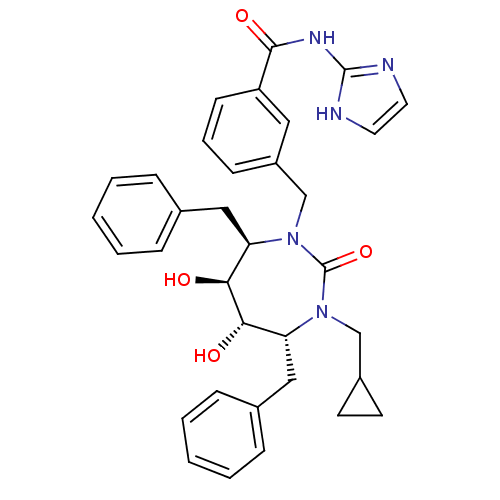
(3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-cyclopropylmethyl-...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(CC2CC2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C34H37N5O4/c40-30-28(19-23-8-3-1-4-9-23)38(21-25-14-15-25)34(43)39(29(31(30)41)20-24-10-5-2-6-11-24)22-26-12-7-13-27(18-26)32(42)37-33-35-16-17-36-33/h1-13,16-18,25,28-31,40-41H,14-15,19-22H2,(H2,35,36,37,42)/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464104
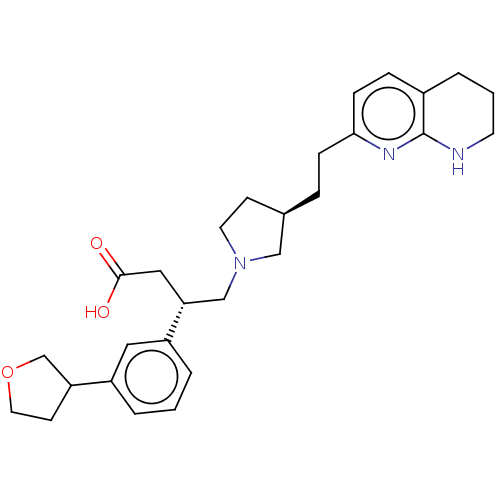
(CHEMBL4244784)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)C1CCOC1 |r| Show InChI InChI=1S/C28H37N3O3/c32-27(33)16-25(23-4-1-3-22(15-23)24-11-14-34-19-24)18-31-13-10-20(17-31)6-8-26-9-7-21-5-2-12-29-28(21)30-26/h1,3-4,7,9,15,20,24-25H,2,5-6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,24?,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50493301
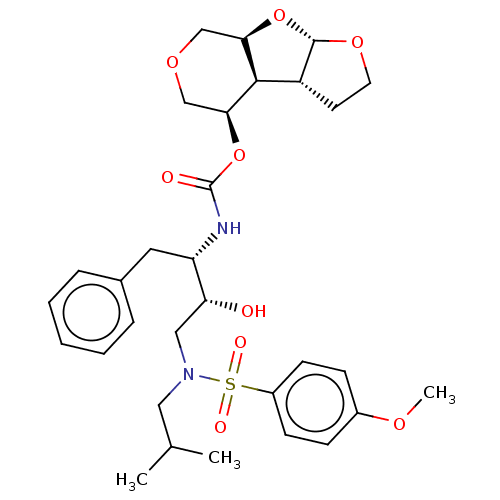
(CHEMBL2426458)Show SMILES [H][C@@]12COC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(OC)cc3)[C@]1([H])[C@@]1([H])CCO[C@@]1([H])O2 |r| Show InChI InChI=1S/C31H42N2O9S/c1-20(2)16-33(43(36,37)23-11-9-22(38-3)10-12-23)17-26(34)25(15-21-7-5-4-6-8-21)32-31(35)42-28-19-39-18-27-29(28)24-13-14-40-30(24)41-27/h4-12,20,24-30,34H,13-19H2,1-3H3,(H,32,35)/t24-,25+,26-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 LAI protease by fluorescence assay |
J Med Chem 56: 6792-802 (2013)
Article DOI: 10.1021/jm400768f
BindingDB Entry DOI: 10.7270/Q2K07764 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM155
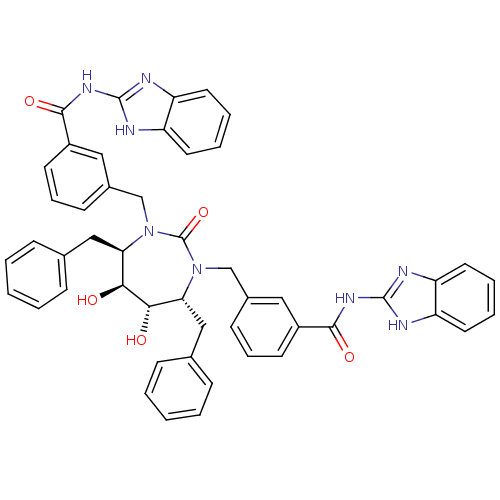
(CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C49H44N8O5/c58-43-41(27-31-13-3-1-4-14-31)56(29-33-17-11-19-35(25-33)45(60)54-47-50-37-21-7-8-22-38(37)51-47)49(62)57(42(44(43)59)28-32-15-5-2-6-16-32)30-34-18-12-20-36(26-34)46(61)55-48-52-39-23-9-10-24-40(39)53-48/h1-26,41-44,58-59H,27-30H2,(H2,50,51,54,60)(H2,52,53,55,61)/t41-,42-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055587
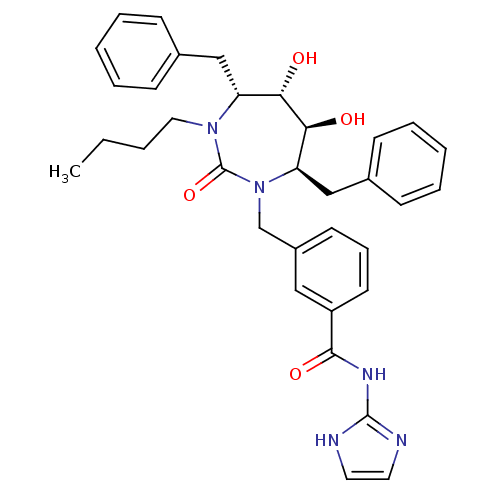
(3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-butyl-5,6-dihydrox...)Show SMILES CCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C1=O Show InChI InChI=1S/C34H39N5O4/c1-2-3-19-38-28(21-24-11-6-4-7-12-24)30(40)31(41)29(22-25-13-8-5-9-14-25)39(34(38)43)23-26-15-10-16-27(20-26)32(42)37-33-35-17-18-36-33/h4-18,20,28-31,40-41H,2-3,19,21-23H2,1H3,(H2,35,36,37,42)/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464110
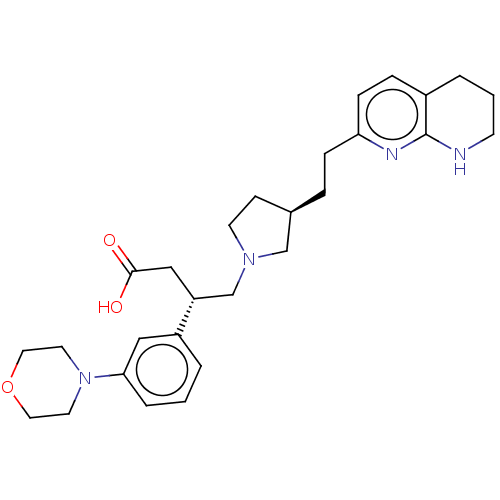
(CHEMBL4238909)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H38N4O3/c33-27(34)18-24(23-3-1-5-26(17-23)32-13-15-35-16-14-32)20-31-12-10-21(19-31)6-8-25-9-7-22-4-2-11-29-28(22)30-25/h1,3,5,7,9,17,21,24H,2,4,6,8,10-16,18-20H2,(H,29,30)(H,33,34)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM154

(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nccs2)C(=O)N(Cc2cccc(c2)C(=O)Nc2nccs2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H38N6O5S2/c48-35-33(23-27-9-3-1-4-10-27)46(25-29-13-7-15-31(21-29)37(50)44-39-42-17-19-53-39)41(52)47(34(36(35)49)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(51)45-40-43-18-20-54-40/h1-22,33-36,48-49H,23-26H2,(H,42,44,50)(H,43,45,51)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464123

(CHEMBL4242263)Show SMILES Cc1cc(C)n(n1)-c1cc(cc(c1)N1CCOCC1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C33H44N6O3/c1-23-16-24(2)39(36-23)31-18-27(17-30(20-31)38-12-14-42-15-13-38)28(19-32(40)41)22-37-11-9-25(21-37)5-7-29-8-6-26-4-3-10-34-33(26)35-29/h6,8,16-18,20,25,28H,3-5,7,9-15,19,21-22H2,1-2H3,(H,34,35)(H,40,41)/t25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055593
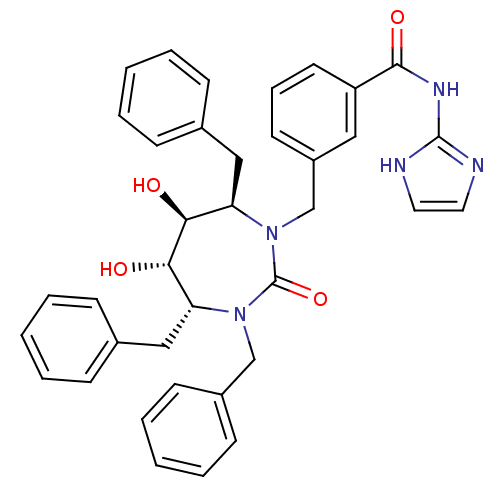
(CHEMBL292905 | N-(1H-Imidazol-2-yl)-3-((4R,5S,6S,7...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(Cc2ccccc2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H37N5O4/c43-33-31(22-26-11-4-1-5-12-26)41(24-28-15-8-3-9-16-28)37(46)42(32(34(33)44)23-27-13-6-2-7-14-27)25-29-17-10-18-30(21-29)35(45)40-36-38-19-20-39-36/h1-21,31-34,43-44H,22-25H2,(H2,38,39,40,45)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055588
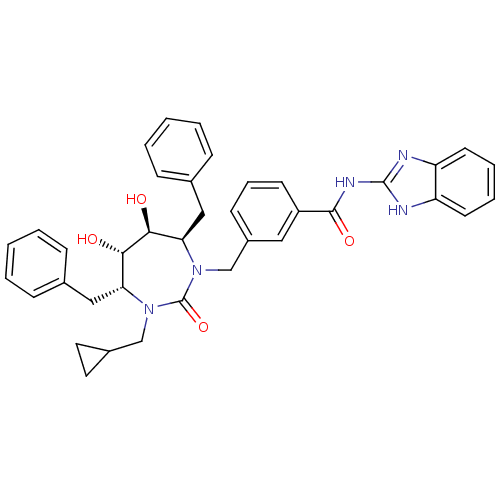
(CHEMBL301219 | N-(1H-Benzoimidazol-2-yl)-3-((4R,5S...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)C(=O)N(CC2CC2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C38H39N5O4/c44-34-32(21-25-10-3-1-4-11-25)42(23-27-18-19-27)38(47)43(33(35(34)45)22-26-12-5-2-6-13-26)24-28-14-9-15-29(20-28)36(46)41-37-39-30-16-7-8-17-31(30)40-37/h1-17,20,27,32-35,44-45H,18-19,21-24H2,(H2,39,40,41,46)/t32-,33-,34+,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464119
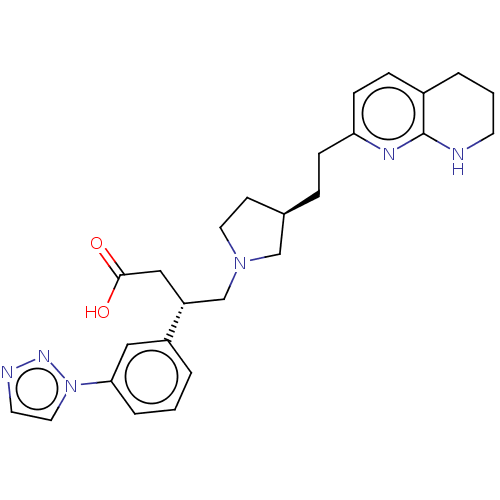
(CHEMBL4241584)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)-n1ccnn1 |r| Show InChI InChI=1S/C26H32N6O2/c33-25(34)16-22(21-3-1-5-24(15-21)32-14-12-28-30-32)18-31-13-10-19(17-31)6-8-23-9-7-20-4-2-11-27-26(20)29-23/h1,3,5,7,9,12,14-15,19,22H,2,4,6,8,10-11,13,16-18H2,(H,27,29)(H,33,34)/t19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464118
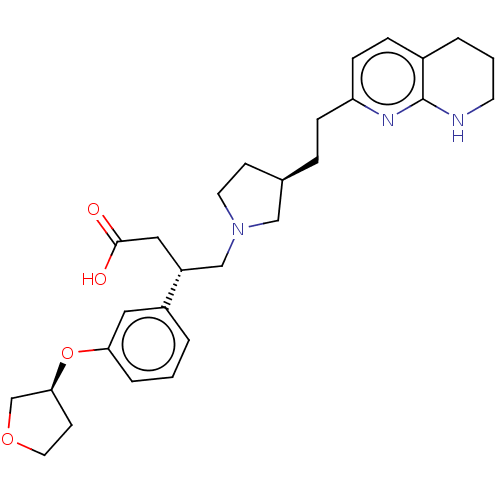
(CHEMBL4249172)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(O[C@H]2CCOC2)c1 |r| Show InChI InChI=1S/C28H37N3O4/c32-27(33)16-23(22-3-1-5-25(15-22)35-26-11-14-34-19-26)18-31-13-10-20(17-31)6-8-24-9-7-21-4-2-12-29-28(21)30-24/h1,3,5,7,9,15,20,23,26H,2,4,6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,23-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464124
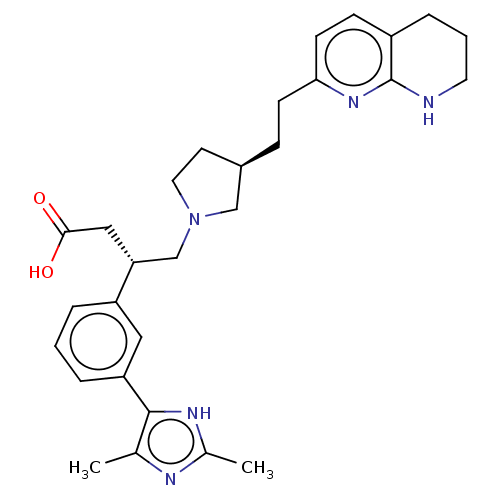
(CHEMBL4249629)Show SMILES Cc1nc(C)c([nH]1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-19-28(32-20(2)31-19)24-6-3-5-23(15-24)25(16-27(35)36)18-34-14-12-21(17-34)8-10-26-11-9-22-7-4-13-30-29(22)33-26/h3,5-6,9,11,15,21,25H,4,7-8,10,12-14,16-18H2,1-2H3,(H,30,33)(H,31,32)(H,35,36)/t21-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108

(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311157
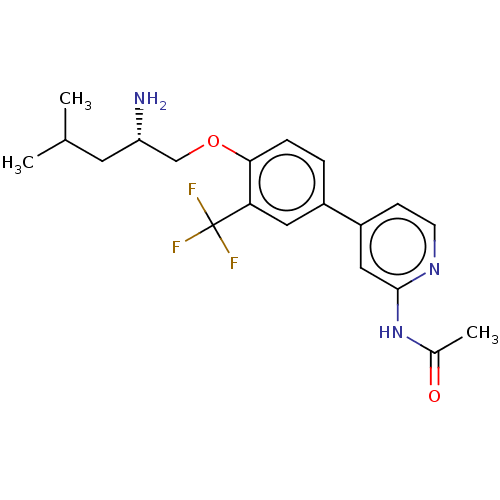
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-12(2)8-16(24)11-28-18-5-4-14(9-17(18)20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311155
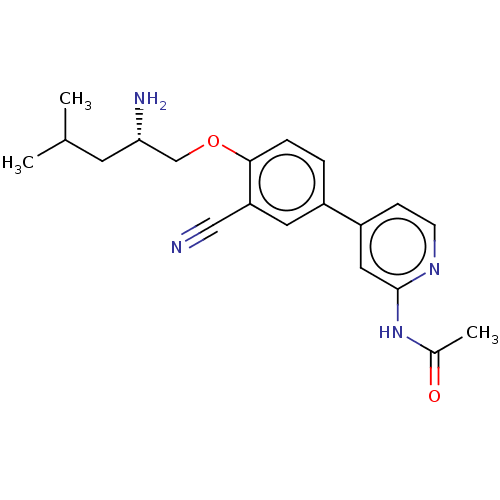
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-cyanop...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24N4O2/c1-13(2)8-18(22)12-26-19-5-4-15(9-17(19)11-21)16-6-7-23-20(10-16)24-14(3)25/h4-7,9-10,13,18H,8,12,22H2,1-3H3,(H,23,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311182

((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C21H26F3N3O4/c1-13(2)11-20(3,25)12-30-16-6-5-14(9-17(16)31-21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311180
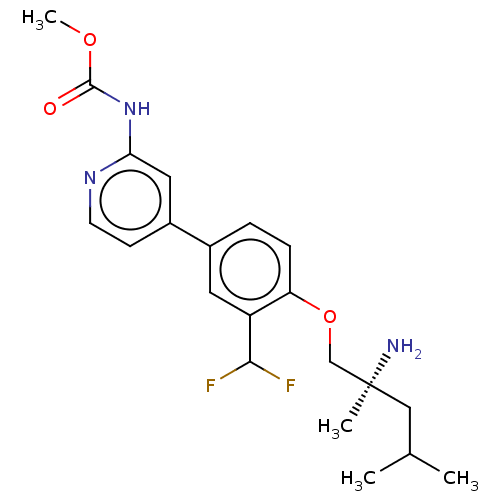
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)F |r| Show InChI InChI=1S/C21H27F2N3O3/c1-13(2)11-21(3,24)12-29-17-6-5-14(9-16(17)19(22)23)15-7-8-25-18(10-15)26-20(27)28-4/h5-10,13,19H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311170
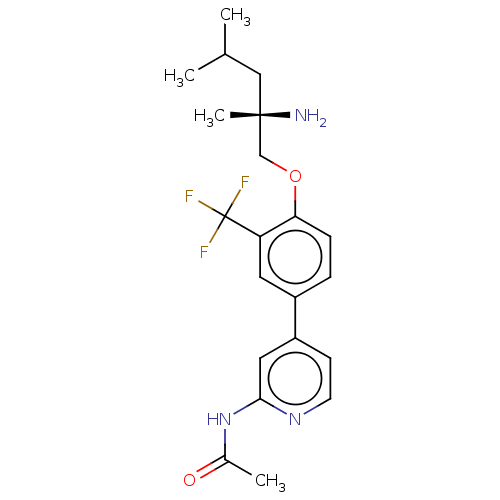
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O2/c1-13(2)11-20(4,25)12-29-18-6-5-15(9-17(18)21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311158
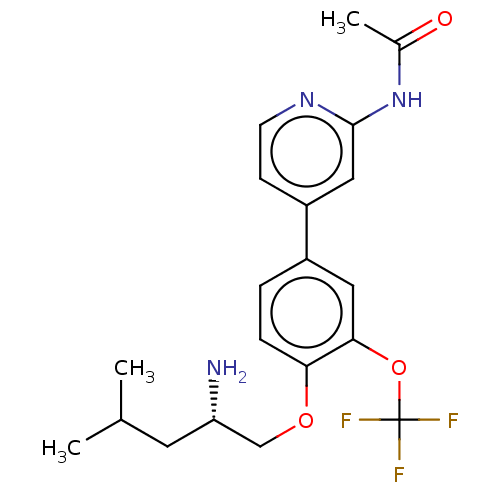
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O3/c1-12(2)8-16(24)11-28-17-5-4-14(9-18(17)29-20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457613
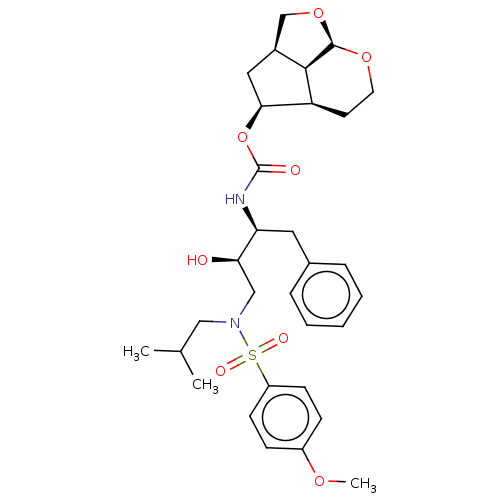
(CHEMBL4205056)Show SMILES [H][C@@]12CO[C@@]3([H])OCC[C@@]([H])([C@H](C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1)[C@@]23[H] |r| Show InChI InChI=1S/C31H42N2O8S/c1-20(2)17-33(42(36,37)24-11-9-23(38-3)10-12-24)18-27(34)26(15-21-7-5-4-6-8-21)32-31(35)41-28-16-22-19-40-30-29(22)25(28)13-14-39-30/h4-12,20,22,25-30,34H,13-19H2,1-3H3,(H,32,35)/t22-,25-,26-,27+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311179
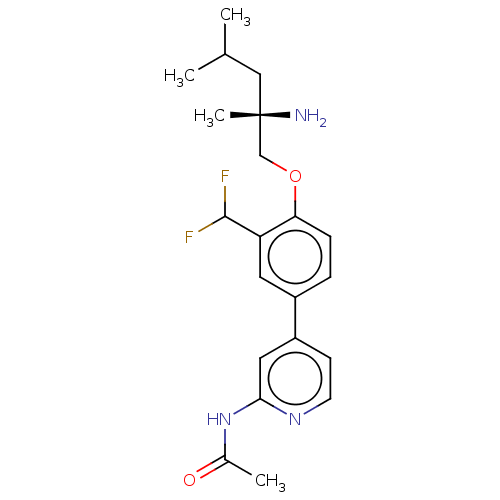
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(d...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H27F2N3O2/c1-13(2)11-21(4,24)12-28-18-6-5-15(9-17(18)20(22)23)16-7-8-25-19(10-16)26-14(3)27/h5-10,13,20H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311189
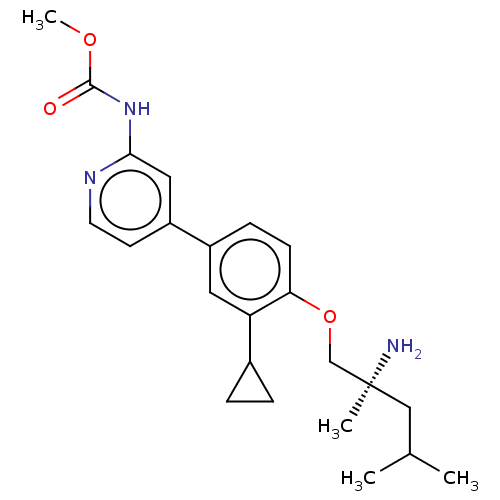
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C1CC1 |r| Show InChI InChI=1S/C23H31N3O3/c1-15(2)13-23(3,24)14-29-20-8-7-17(11-19(20)16-5-6-16)18-9-10-25-21(12-18)26-22(27)28-4/h7-12,15-16H,5-6,13-14,24H2,1-4H3,(H,25,26,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457603
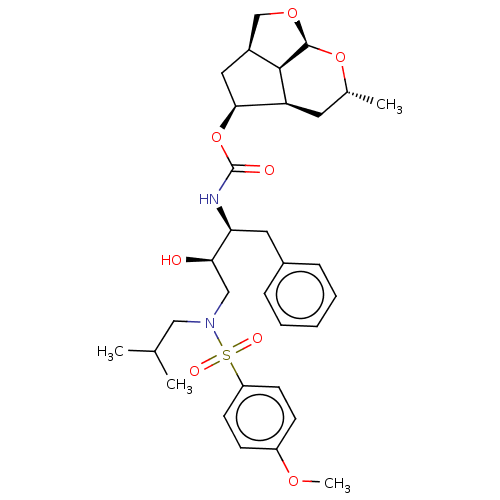
(CHEMBL4217920)Show SMILES [H][C@@]12CO[C@@]3([H])O[C@H](C)C[C@@]([H])([C@H](C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1)[C@@]23[H] |r| Show InChI InChI=1S/C32H44N2O8S/c1-20(2)17-34(43(37,38)25-12-10-24(39-4)11-13-25)18-28(35)27(15-22-8-6-5-7-9-22)33-32(36)42-29-16-23-19-40-31-30(23)26(29)14-21(3)41-31/h5-13,20-21,23,26-31,35H,14-19H2,1-4H3,(H,33,36)/t21-,23+,26+,27+,28-,29+,30-,31+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50457605
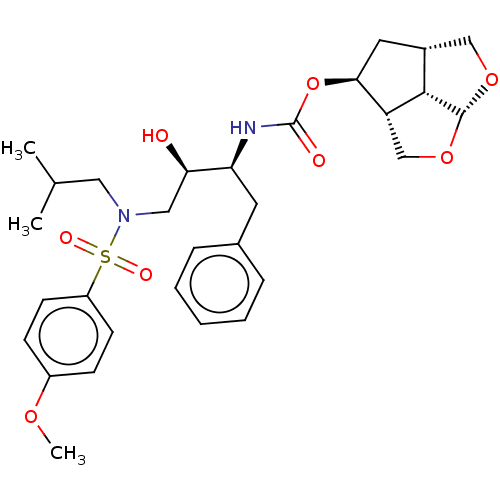
(CHEMBL4210992)Show SMILES [H][C@]12CO[C@]3([H])OC[C@]([H])([C@H](C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1)[C@]23[H] |r| Show InChI InChI=1S/C30H40N2O8S/c1-19(2)15-32(41(35,36)23-11-9-22(37-3)10-12-23)16-26(33)25(13-20-7-5-4-6-8-20)31-30(34)40-27-14-21-17-38-29-28(21)24(27)18-39-29/h4-12,19,21,24-29,33H,13-18H2,1-3H3,(H,31,34)/t21-,24-,25+,26-,27+,28+,29-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... |
J Med Chem 61: 4561-4577 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00298
BindingDB Entry DOI: 10.7270/Q2445Q35 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311176
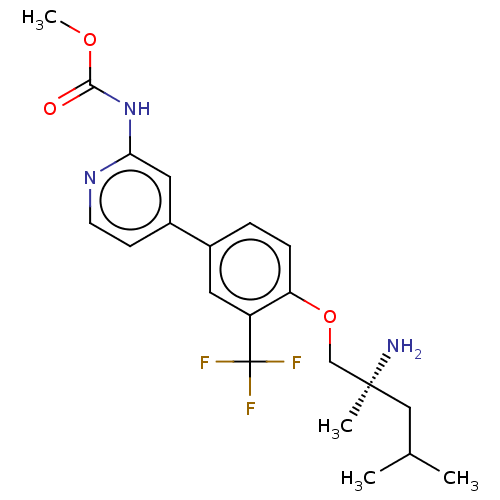
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(3,25)12-30-17-6-5-14(9-16(17)21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176723
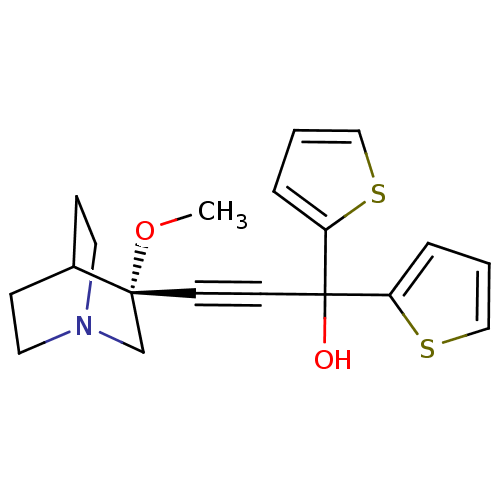
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311268
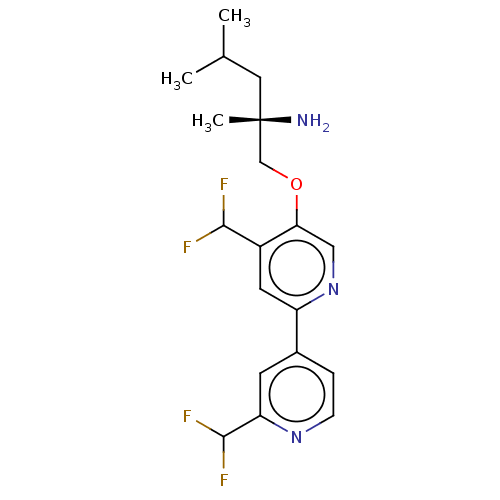
((S)-1-((2′,4-bis(difluoromethyl)-[2,4′...)Show SMILES CC(C)C[C@](C)(N)COc1cnc(cc1C(F)F)-c1ccnc(c1)C(F)F |r| Show InChI InChI=1S/C19H23F4N3O/c1-11(2)8-19(3,24)10-27-16-9-26-14(7-13(16)17(20)21)12-4-5-25-15(6-12)18(22)23/h4-7,9,11,17-18H,8,10,24H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50419334
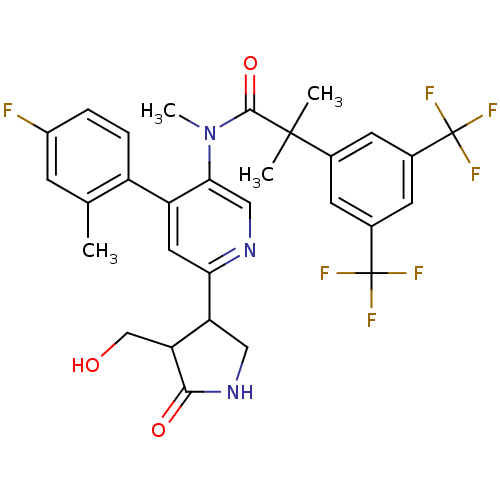
(CHEMBL1911965 | CHEMBL1911967)Show SMILES CN(C(=O)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cnc(cc1-c1ccc(F)cc1C)C1CNC(=O)C1CO Show InChI InChI=1S/C30H28F7N3O3/c1-15-7-19(31)5-6-20(15)21-11-24(22-12-39-26(42)23(22)14-41)38-13-25(21)40(4)27(43)28(2,3)16-8-17(29(32,33)34)10-18(9-16)30(35,36)37/h5-11,13,22-23,41H,12,14H2,1-4H3,(H,39,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... |
Bioorg Med Chem Lett 21: 6899-904 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.116
BindingDB Entry DOI: 10.7270/Q24X592P |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464120
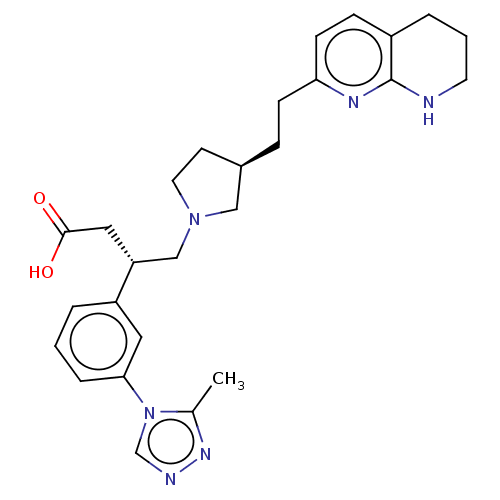
(CHEMBL4237919)Show SMILES Cc1nncn1-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C27H34N6O2/c1-19-31-29-18-33(19)25-6-2-4-22(14-25)23(15-26(34)35)17-32-13-11-20(16-32)7-9-24-10-8-21-5-3-12-28-27(21)30-24/h2,4,6,8,10,14,18,20,23H,3,5,7,9,11-13,15-17H2,1H3,(H,28,30)(H,34,35)/t20-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055589
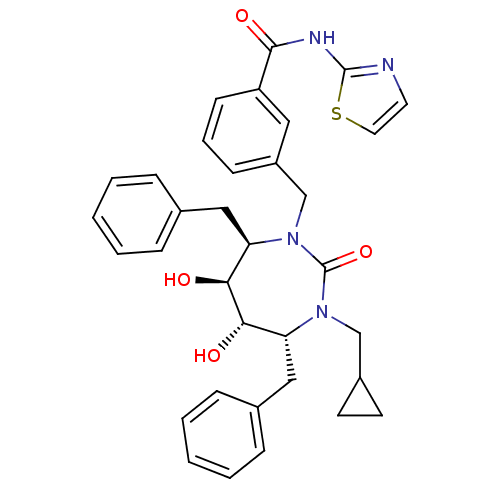
(3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-cyclopropylmethyl-...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nccs2)C(=O)N(CC2CC2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C34H36N4O4S/c39-30-28(19-23-8-3-1-4-9-23)37(21-25-14-15-25)34(42)38(29(31(30)40)20-24-10-5-2-6-11-24)22-26-12-7-13-27(18-26)32(41)36-33-35-16-17-43-33/h1-13,16-18,25,28-31,39-40H,14-15,19-22H2,(H,35,36,41)/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311160
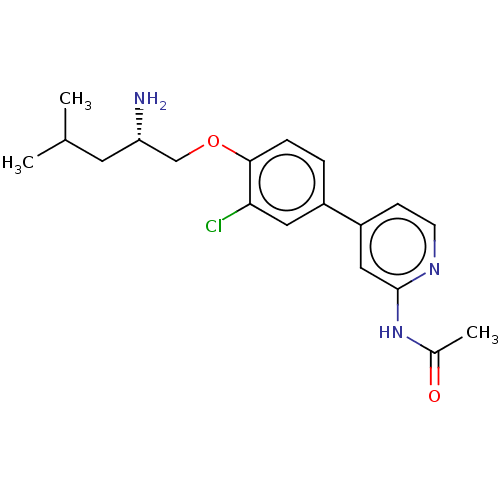
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-chloro...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1Cl)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-12(2)8-16(21)11-25-18-5-4-14(9-17(18)20)15-6-7-22-19(10-15)23-13(3)24/h4-7,9-10,12,16H,8,11,21H2,1-3H3,(H,22,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464113
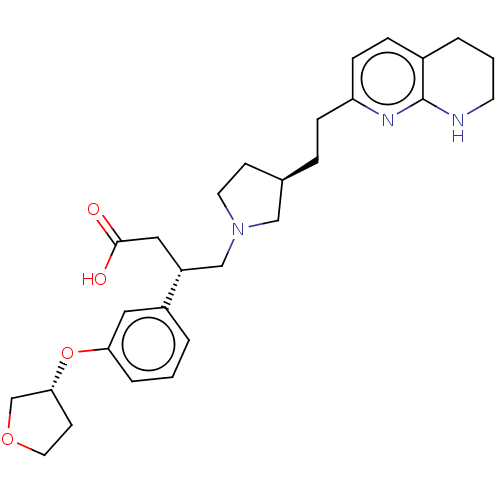
(CHEMBL4239085)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(O[C@@H]2CCOC2)c1 |r| Show InChI InChI=1S/C28H37N3O4/c32-27(33)16-23(22-3-1-5-25(15-22)35-26-11-14-34-19-26)18-31-13-10-20(17-31)6-8-24-9-7-21-4-2-12-29-28(21)30-24/h1,3,5,7,9,15,20,23,26H,2,4,6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464110

(CHEMBL4238909)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H38N4O3/c33-27(34)18-24(23-3-1-5-26(17-23)32-13-15-35-16-14-32)20-31-12-10-21(19-31)6-8-25-9-7-22-4-2-11-29-28(22)30-25/h1,3,5,7,9,17,21,24H,2,4,6,8,10-16,18-20H2,(H,29,30)(H,33,34)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464097
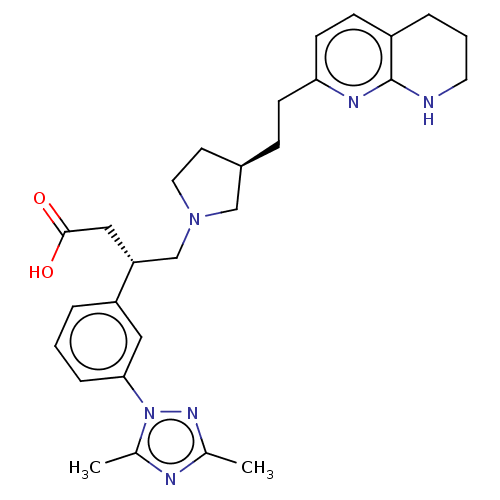
(CHEMBL4237868)Show SMILES Cc1nc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C28H36N6O2/c1-19-30-20(2)34(32-19)26-7-3-5-23(15-26)24(16-27(35)36)18-33-14-12-21(17-33)8-10-25-11-9-22-6-4-13-29-28(22)31-25/h3,5,7,9,11,15,21,24H,4,6,8,10,12-14,16-18H2,1-2H3,(H,29,31)(H,35,36)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464100
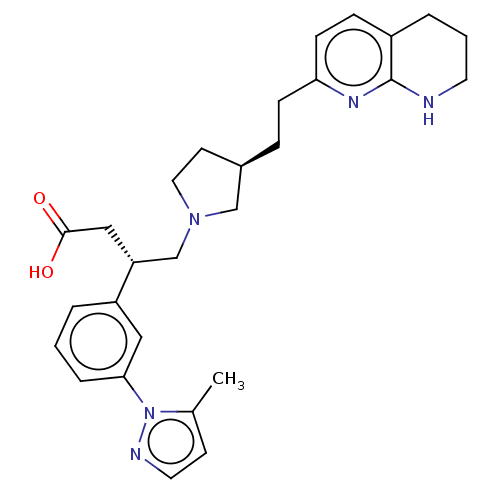
(CHEMBL4243367)Show SMILES Cc1ccnn1-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C28H35N5O2/c1-20-11-14-30-33(20)26-6-2-4-23(16-26)24(17-27(34)35)19-32-15-12-21(18-32)7-9-25-10-8-22-5-3-13-29-28(22)31-25/h2,4,6,8,10-11,14,16,21,24H,3,5,7,9,12-13,15,17-19H2,1H3,(H,29,31)(H,34,35)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50419337
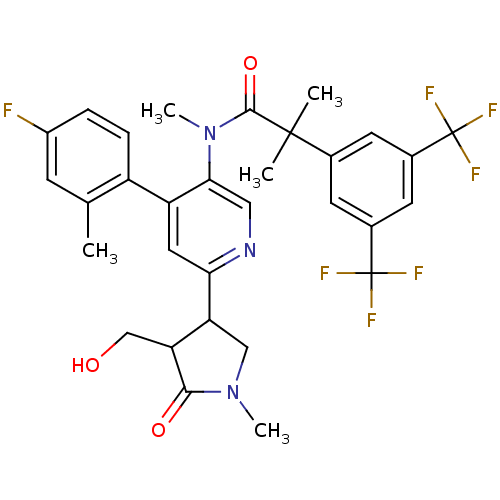
(CHEMBL1911968)Show SMILES CN(C(=O)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cnc(cc1-c1ccc(F)cc1C)C1CN(C)C(=O)C1CO Show InChI InChI=1S/C31H30F7N3O3/c1-16-8-20(32)6-7-21(16)22-12-25(23-14-40(4)27(43)24(23)15-42)39-13-26(22)41(5)28(44)29(2,3)17-9-18(30(33,34)35)11-19(10-17)31(36,37)38/h6-13,23-24,42H,14-15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... |
Bioorg Med Chem Lett 21: 6899-904 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.116
BindingDB Entry DOI: 10.7270/Q24X592P |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311264
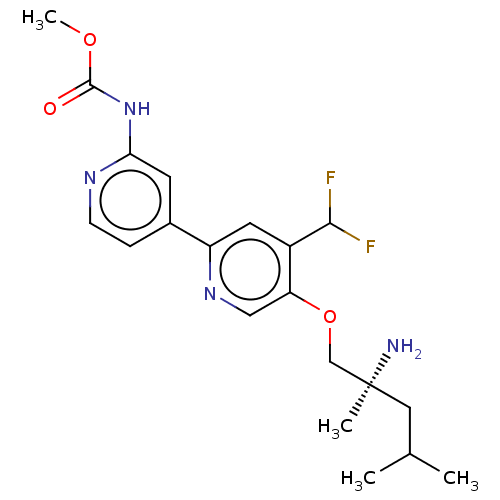
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(C(F)F)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)9-20(3,23)11-29-16-10-25-15(8-14(16)18(21)22)13-5-6-24-17(7-13)26-19(27)28-4/h5-8,10,12,18H,9,11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311178
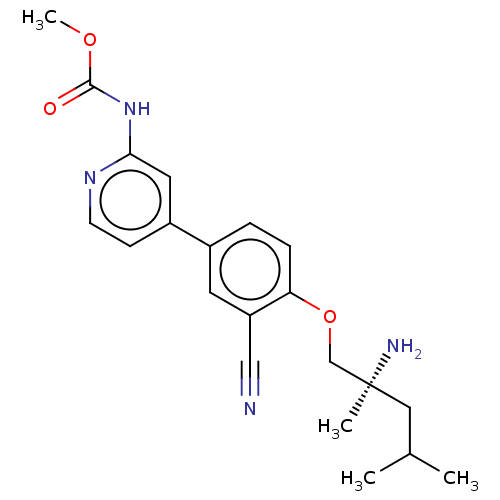
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C#N |r| Show InChI InChI=1S/C21H26N4O3/c1-14(2)11-21(3,23)13-28-18-6-5-15(9-17(18)12-22)16-7-8-24-19(10-16)25-20(26)27-4/h5-10,14H,11,13,23H2,1-4H3,(H,24,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311181
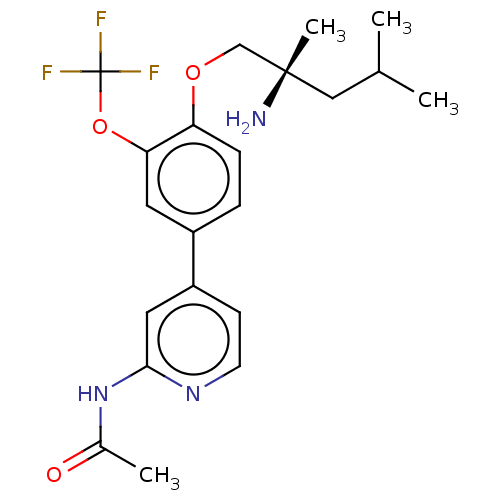
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(4,25)12-29-17-6-5-15(9-18(17)30-21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data