

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00525 BindingDB Entry DOI: 10.7270/Q29W0K29 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00528 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against Dihydrofolate reductase derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008293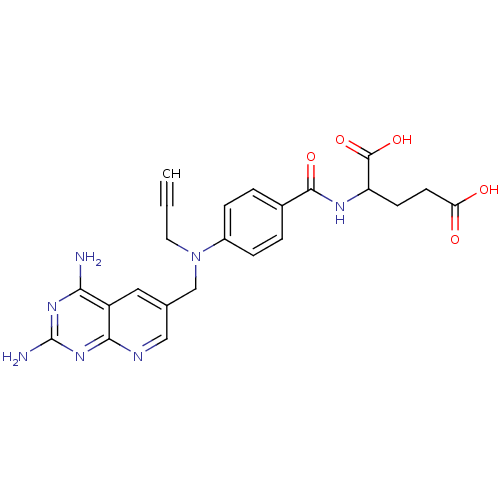 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against dihydrofolate reductase(DHFR) derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008292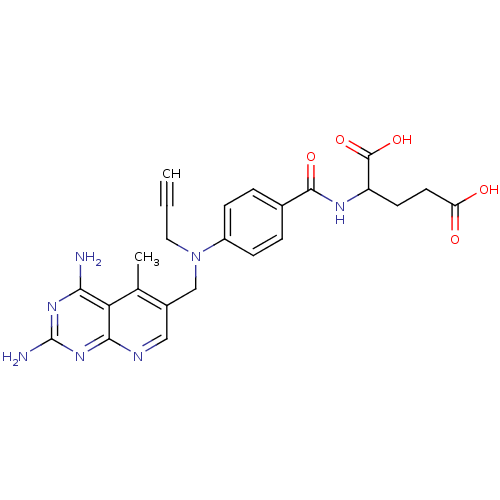 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against dihydrofolate reductase(DHFR) derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089452 (CHEMBL3578201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined | Bioorg Med Chem Lett 12: 1511-5 (2002) BindingDB Entry DOI: 10.7270/Q2P84B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of wild-type HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089451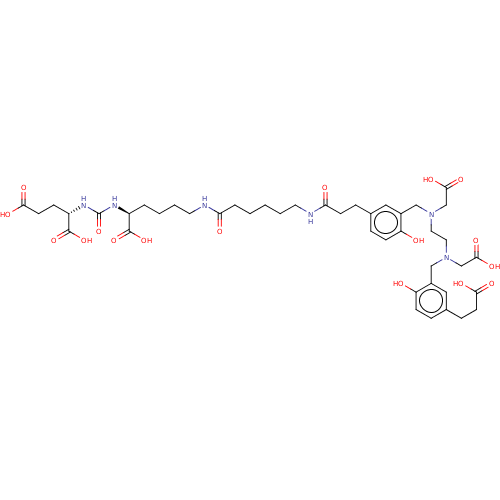 (CHEMBL3578202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50194731 (Boc-Phe-psi[S-CH(OH)CH2]Phe-Gln-Phe-NH2 | CHEMBL26...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 49: 5777-84 (2006) Article DOI: 10.1021/jm0605583 BindingDB Entry DOI: 10.7270/Q2X63MK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease M46I/A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pyruvate decarboxylase (Zymomonas mobilis subsp. mobilis (strain ATCC 3182...) | BDBM50589544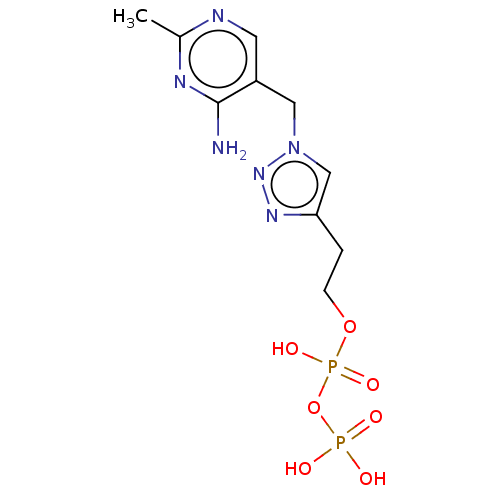 (CHEMBL3559521) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00085g BindingDB Entry DOI: 10.7270/Q2M90DMB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50581791 (CHEMBL5073221) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV-1 protease assessed as initial inhibition constant by spectrophotometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02177 BindingDB Entry DOI: 10.7270/Q208695M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease M46I/A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021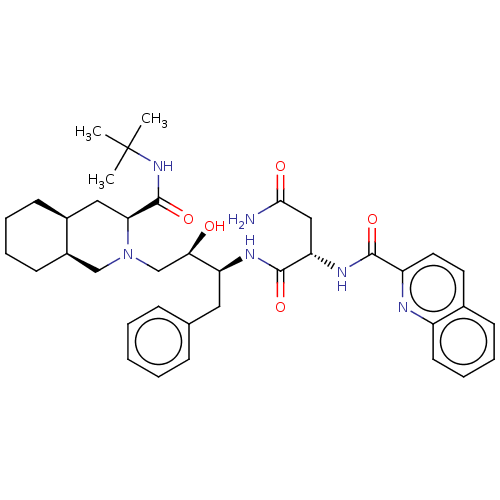 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046832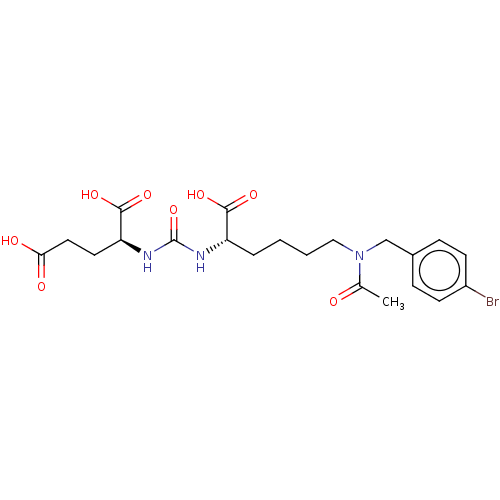 (CHEMBL3309678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046832 (CHEMBL3309678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089454 (CHEMBL3578199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy Curated by PDSP Ki Database | Synapse 35: 79-95 (2000) Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X BindingDB Entry DOI: 10.7270/Q2XK8D4N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy Curated by PDSP Ki Database | Synapse 35: 79-95 (2000) Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X BindingDB Entry DOI: 10.7270/Q2XK8D4N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy Curated by PDSP Ki Database | Synapse 35: 79-95 (2000) Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X BindingDB Entry DOI: 10.7270/Q2XK8D4N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089455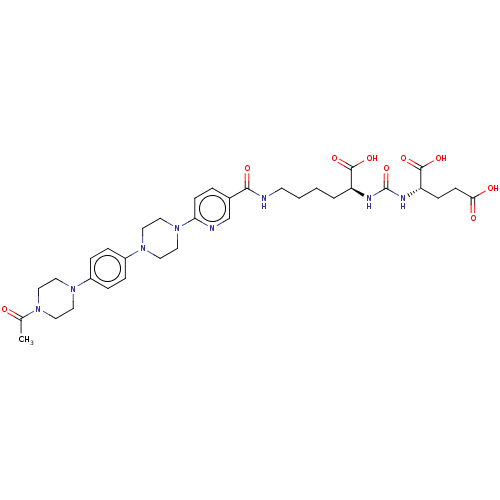 (CHEMBL3578197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50143837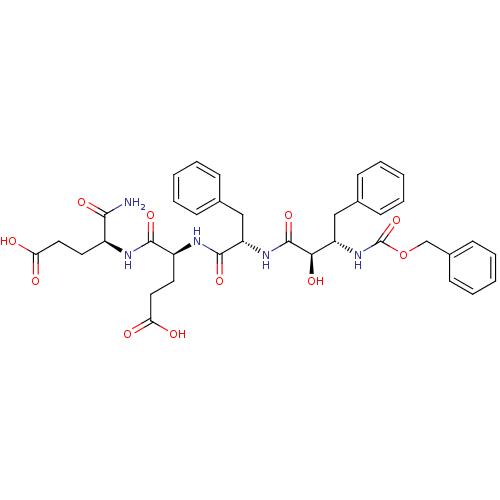 ((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of Val82Ala mutant of HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089453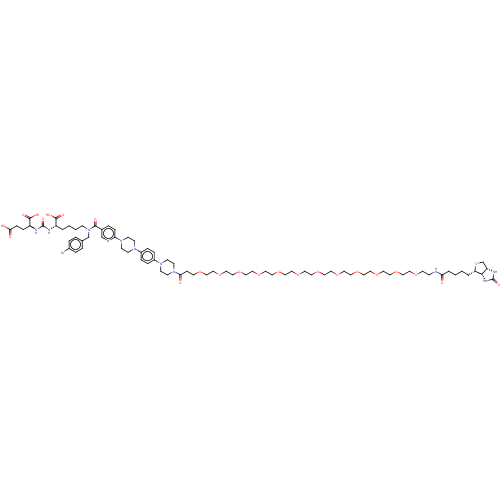 (CHEMBL3578200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593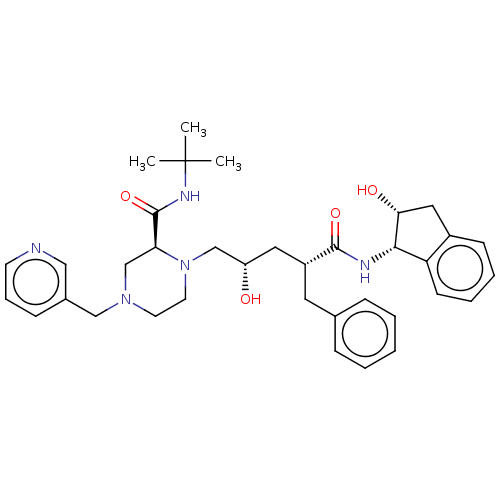 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier, Centre de Recherches de Croissy Curated by PDSP Ki Database | Synapse 35: 79-95 (2000) Article DOI: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X BindingDB Entry DOI: 10.7270/Q2XK8D4N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of D25N/V82A mutant of HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM17350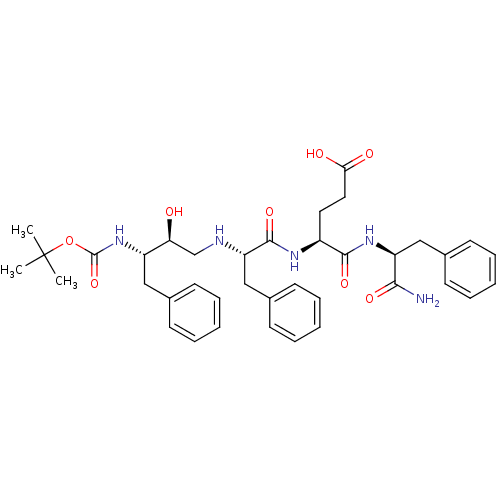 ((4S)-4-[(2S)-2-{[(2S,3S)-3-{[(tert-butoxy)carbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 49: 5777-84 (2006) Article DOI: 10.1021/jm0605583 BindingDB Entry DOI: 10.7270/Q2X63MK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM17350 ((4S)-4-[(2S)-2-{[(2S,3S)-3-{[(tert-butoxy)carbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Academy of Sciences of the Czech Republic | Assay Description Inhibition constants were determined by a spectrophotometric assay with the chromogenic peptide substrate. | J Med Chem 46: 1636-44 (2003) Article DOI: 10.1021/jm021079g BindingDB Entry DOI: 10.7270/Q29Z932M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease V32I/I47A mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046850 (CHEMBL3311268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046850 (CHEMBL3311268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50143837 ((S)-4-[(S)-2-((2R,3S)-3-Benzyloxycarbonylamino-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of wild-type HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50113598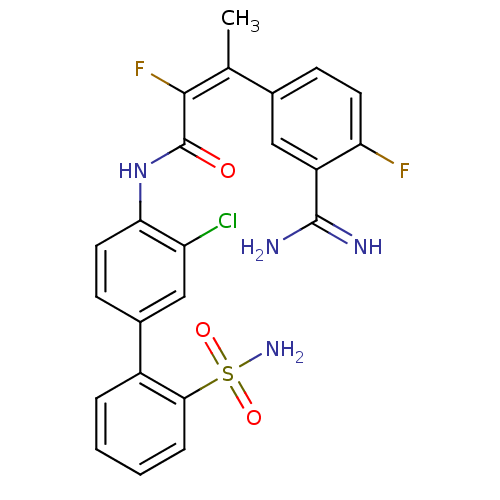 ((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM | Bioorg Med Chem Lett 12: 1511-5 (2002) BindingDB Entry DOI: 10.7270/Q2P84B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50213021 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease V32I/I47A mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046831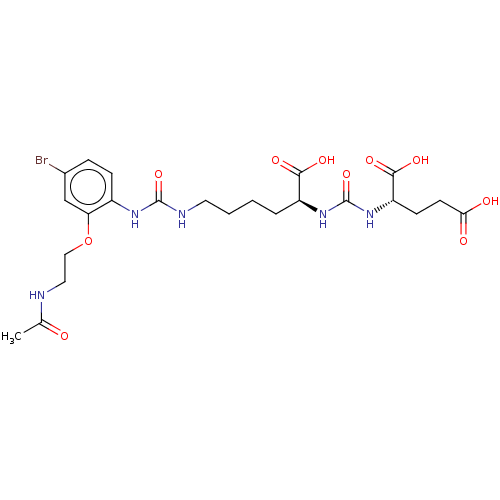 (CHEMBL3309680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 10513 total ) | Next | Last >> |