 Found 16556 hits with Last Name = 'ay' and Initial = 'e'
Found 16556 hits with Last Name = 'ay' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50280415
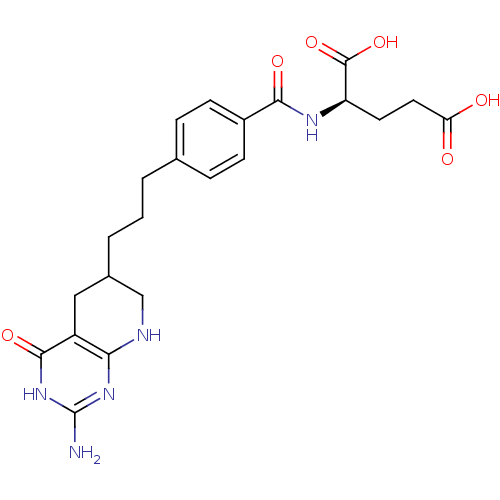
((R)-2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-p...)Show SMILES Nc1nc2NCC(CCCc3ccc(cc3)C(=O)N[C@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C22H27N5O6/c23-22-26-18-15(20(31)27-22)10-13(11-24-18)3-1-2-12-4-6-14(7-5-12)19(30)25-16(21(32)33)8-9-17(28)29/h4-7,13,16H,1-3,8-11H2,(H,25,30)(H,28,29)(H,32,33)(H4,23,24,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.0000190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells |
Bioorg Med Chem Lett 2: 339-342 (1992)
Article DOI: 10.1016/S0960-894X(01)80214-6
BindingDB Entry DOI: 10.7270/Q2RF5TXV |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM22590
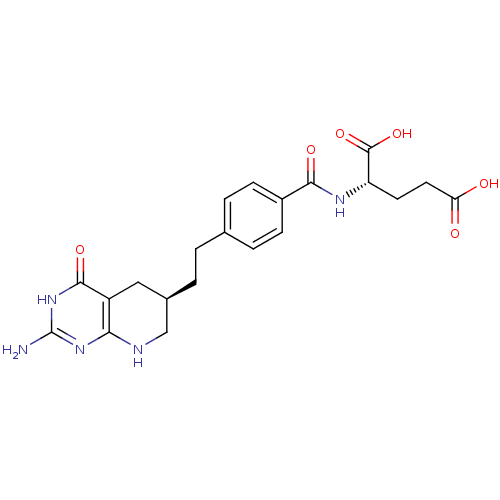
((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...)Show SMILES Nc1nc2NC[C@H](CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C21H25N5O6/c22-21-25-17-14(19(30)26-21)9-12(10-23-17)2-1-11-3-5-13(6-4-11)18(29)24-15(20(31)32)7-8-16(27)28/h3-6,12,15H,1-2,7-10H2,(H,24,29)(H,27,28)(H,31,32)(H4,22,23,25,26,30)/t12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.000120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells |
Bioorg Med Chem Lett 2: 339-342 (1992)
Article DOI: 10.1016/S0960-894X(01)80214-6
BindingDB Entry DOI: 10.7270/Q2RF5TXV |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50280414
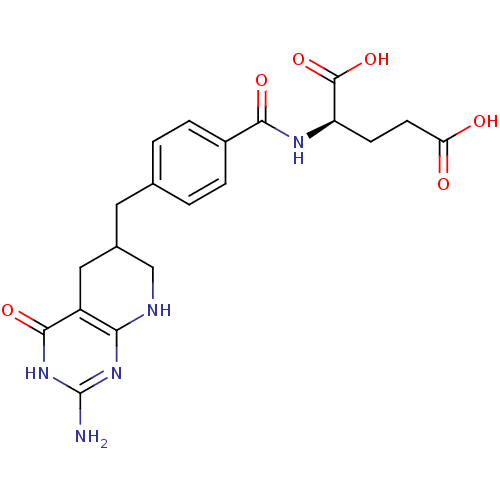
((R)-2-[4-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyri...)Show SMILES Nc1nc2NCC(Cc3ccc(cc3)C(=O)N[C@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C20H23N5O6/c21-20-24-16-13(18(29)25-20)8-11(9-22-16)7-10-1-3-12(4-2-10)17(28)23-14(19(30)31)5-6-15(26)27/h1-4,11,14H,5-9H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells |
Bioorg Med Chem Lett 2: 339-342 (1992)
Article DOI: 10.1016/S0960-894X(01)80214-6
BindingDB Entry DOI: 10.7270/Q2RF5TXV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82455
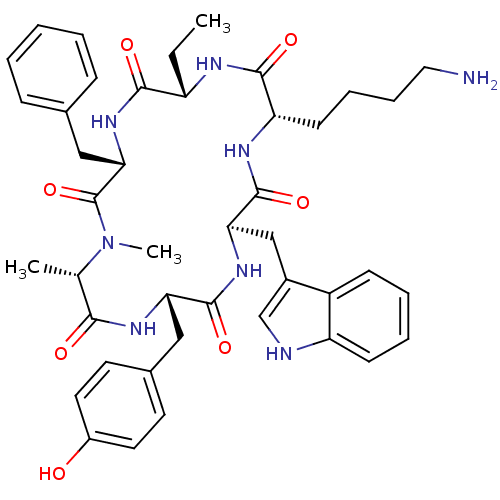
(BIM 23027 | BIM-23027 | N-Methylcyclo[L-Ala-L-Tyr-...)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C43H54N8O7/c1-4-32-39(54)50-37(23-27-12-6-5-7-13-27)43(58)51(3)26(2)38(53)48-35(22-28-17-19-30(52)20-18-28)41(56)49-36(24-29-25-45-33-15-9-8-14-31(29)33)42(57)47-34(40(55)46-32)16-10-11-21-44/h5-9,12-15,17-20,25-26,32,34-37,45,52H,4,10-11,16,21-24,44H2,1-3H3,(H,46,55)(H,47,57)(H,48,53)(H,49,56)(H,50,54)/t26-,32-,34-,35-,36+,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82465
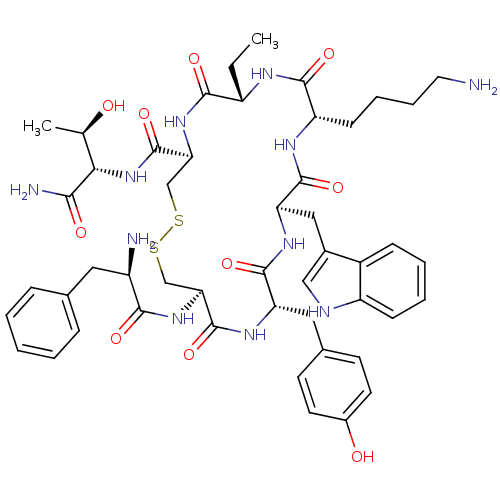
(BIM 23023 | BIM-23023 | D-Phe-L-Cys(1)-L-Tyr-D-Trp...)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C49H65N11O10S2/c1-3-34-44(65)59-40(49(70)60-41(27(2)61)42(52)63)26-72-71-25-39(58-43(64)33(51)21-28-11-5-4-6-12-28)48(69)56-37(22-29-16-18-31(62)19-17-29)46(67)57-38(23-30-24-53-35-14-8-7-13-32(30)35)47(68)55-36(45(66)54-34)15-9-10-20-50/h4-8,11-14,16-19,24,27,33-34,36-41,53,61-62H,3,9-10,15,20-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,66)(H,55,68)(H,56,69)(H,57,67)(H,58,64)(H,59,65)(H,60,70)/t27-,33-,34+,36+,37+,38-,39+,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM84623
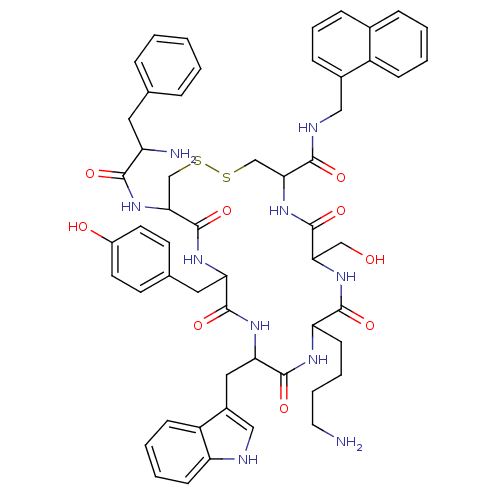
(NC4-28B)Show SMILES NCCCCC1NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C55H64N10O9S2/c56-24-9-8-19-43-51(70)63-46(30-66)54(73)65-47(50(69)59-28-36-15-10-14-35-13-4-5-16-39(35)36)31-75-76-32-48(64-49(68)41(57)25-33-11-2-1-3-12-33)55(74)61-44(26-34-20-22-38(67)23-21-34)52(71)62-45(53(72)60-43)27-37-29-58-42-18-7-6-17-40(37)42/h1-7,10-18,20-23,29,41,43-48,58,66-67H,8-9,19,24-28,30-32,56-57H2,(H,59,69)(H,60,72)(H,61,74)(H,62,71)(H,63,70)(H,64,68)(H,65,73) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM84626
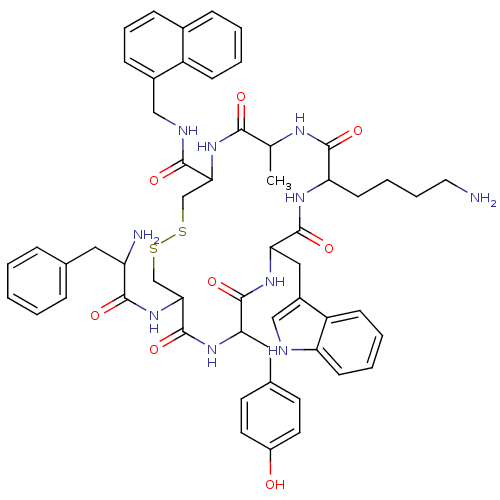
(BIM 23034)Show SMILES CC1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C55H64N10O8S2/c1-33-49(67)64-47(51(69)59-29-37-16-11-15-36-14-5-6-17-40(36)37)31-74-75-32-48(65-50(68)42(57)26-34-12-3-2-4-13-34)55(73)62-45(27-35-21-23-39(66)24-22-35)53(71)63-46(28-38-30-58-43-19-8-7-18-41(38)43)54(72)61-44(52(70)60-33)20-9-10-25-56/h2-8,11-19,21-24,30,33,42,44-48,58,66H,9-10,20,25-29,31-32,56-57H2,1H3,(H,59,69)(H,60,70)(H,61,72)(H,62,73)(H,63,71)(H,64,67)(H,65,68) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM50097783
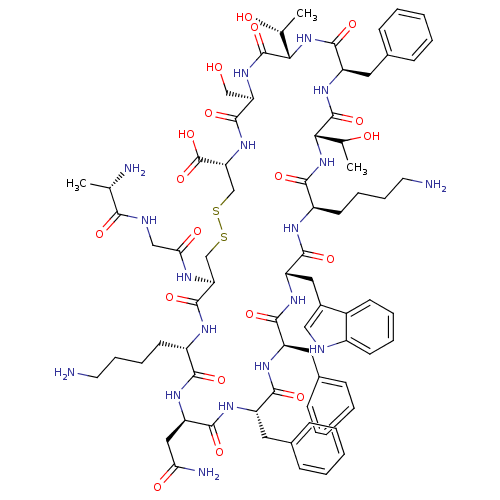
(CHEMBL438401 | D-Trp8 SST-14 | SOMATOSTATIN | SRIF...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@@H](NC(=O)[C@@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)C(C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42?,43+,50-,51+,52-,53+,54+,55-,56+,57+,58-,59+,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82469
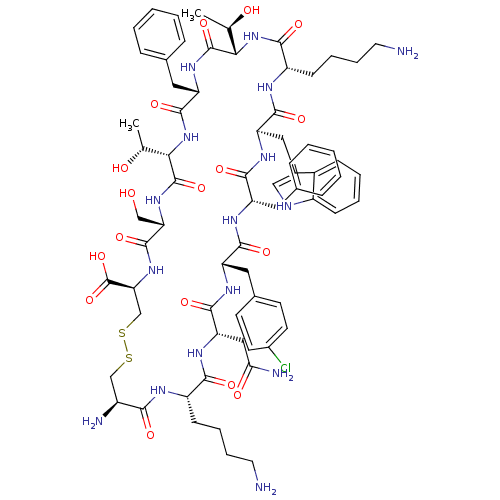
(BIM 23003 | BIM-23003 | EC5-21 | L-Cys(1)-L-Lys-L-...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H95ClN16O17S2/c1-38(90)58-69(102)84-52(30-41-17-7-4-8-18-41)67(100)88-59(39(2)91)70(103)85-55(35-89)68(101)86-56(71(104)105)37-107-106-36-46(75)60(93)78-48(21-11-13-27-73)61(94)83-54(33-57(76)92)66(99)81-51(31-42-23-25-44(72)26-24-42)63(96)80-50(29-40-15-5-3-6-16-40)64(97)82-53(32-43-34-77-47-20-10-9-19-45(43)47)65(98)79-49(62(95)87-58)22-12-14-28-74/h3-10,15-20,23-26,34,38-39,46,48-56,58-59,77,89-91H,11-14,21-22,27-33,35-37,73-75H2,1-2H3,(H2,76,92)(H,78,93)(H,79,98)(H,80,96)(H,81,99)(H,82,97)(H,83,94)(H,84,102)(H,85,103)(H,86,101)(H,87,95)(H,88,100)(H,104,105)/t38-,39-,46+,48+,49+,50+,51+,52+,53-,54+,55+,56+,58+,59+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82462
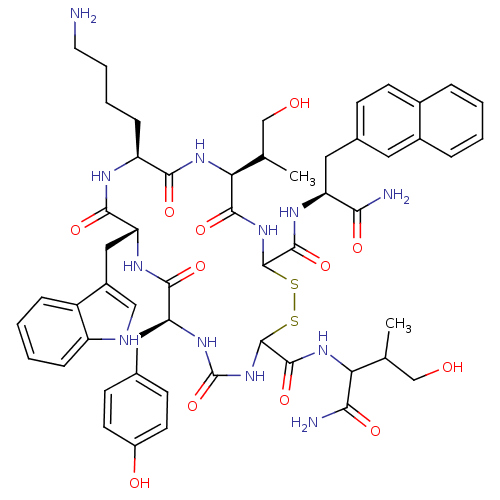
(BIM 23059 | D-Nal-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Thr...)Show SMILES CC(CO)C(NC(=O)C1NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)CO)C(=O)NC(SS1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H68N12O12S2/c1-28(26-67)42(45(57)71)63-51(77)53-66-54(78)62-40(22-30-15-18-35(69)19-16-30)47(73)61-41(24-34-25-58-37-12-6-5-11-36(34)37)48(74)59-38(13-7-8-20-55)46(72)64-43(29(2)27-68)49(75)65-52(79-80-53)50(76)60-39(44(56)70)23-31-14-17-32-9-3-4-10-33(32)21-31/h3-6,9-12,14-19,21,25,28-29,38-43,52-53,58,67-69H,7-8,13,20,22-24,26-27,55H2,1-2H3,(H2,56,70)(H2,57,71)(H,59,74)(H,60,76)(H,61,73)(H,63,77)(H,64,72)(H,65,75)(H2,62,66,78)/t28?,29?,38-,39-,40-,41+,42?,43-,52?,53?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
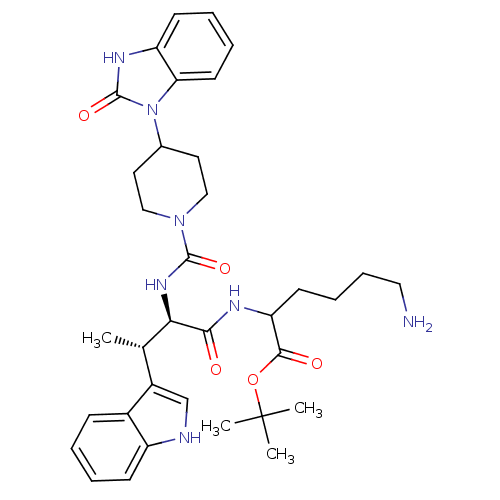
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM84630
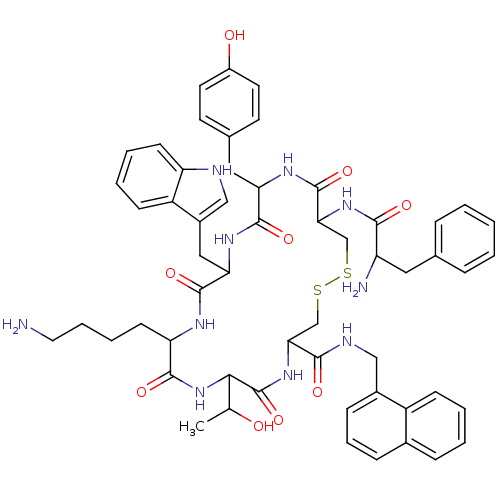
(BIM 23060)Show SMILES CC(O)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C56H66N10O9S2/c1-33(67)49-56(75)65-47(51(70)60-29-37-16-11-15-36-14-5-6-17-40(36)37)31-76-77-32-48(64-50(69)42(58)26-34-12-3-2-4-13-34)55(74)62-45(27-35-21-23-39(68)24-22-35)53(72)63-46(28-38-30-59-43-19-8-7-18-41(38)43)54(73)61-44(52(71)66-49)20-9-10-25-57/h2-8,11-19,21-24,30,33,42,44-49,59,67-68H,9-10,20,25-29,31-32,57-58H2,1H3,(H,60,70)(H,61,73)(H,62,74)(H,63,72)(H,64,69)(H,65,75)(H,66,71) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163726

(US9062059, 2-27)Show SMILES Cc1nnc(CNc2cc(OC[C@H]3C[C@@H]3c3ccc4ncccc4n3)nc(C)n2)s1 |r| Show InChI InChI=1S/C21H21N7OS/c1-12-24-19(23-10-21-28-27-13(2)30-21)9-20(25-12)29-11-14-8-15(14)16-5-6-17-18(26-16)4-3-7-22-17/h3-7,9,14-15H,8,10-11H2,1-2H3,(H,23,24,25)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM84629
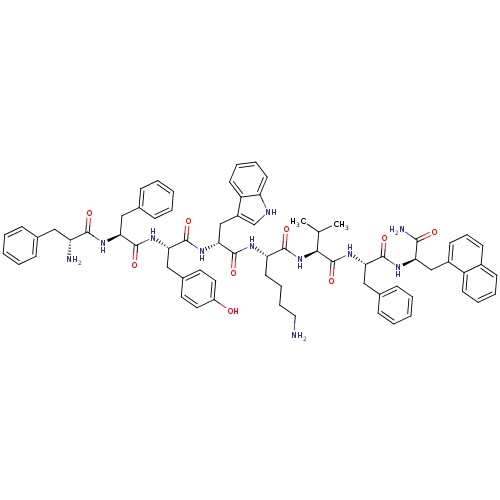
(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50230960
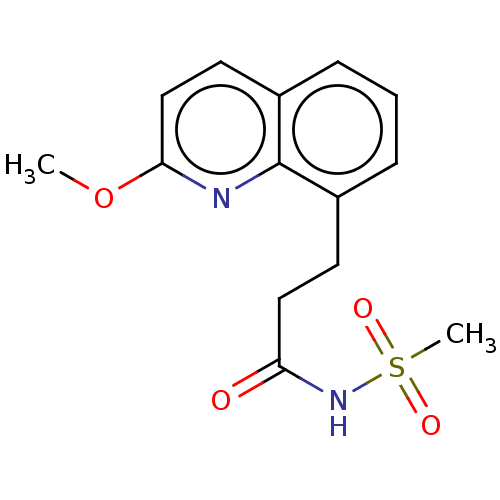
(CHEMBL4089965)Show InChI InChI=1S/C14H16N2O4S/c1-20-13-9-7-11-5-3-4-10(14(11)15-13)6-8-12(17)16-21(2,18)19/h3-5,7,9H,6,8H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in HEK or CHO cell membranes after 120 mins |
Eur J Med Chem 127: 621-631 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.013
BindingDB Entry DOI: 10.7270/Q24F1SZS |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163722
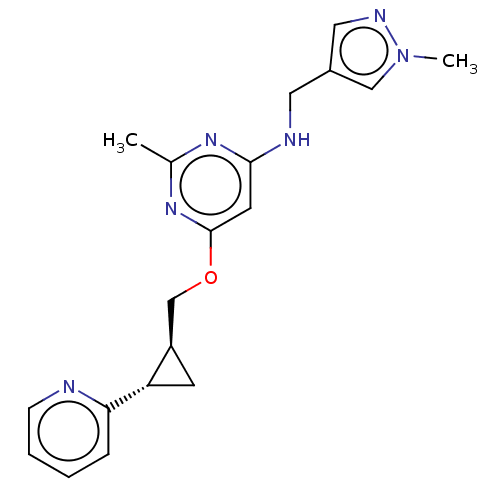
(US9062059, 1-2)Show SMILES Cc1nc(NCc2cnn(C)c2)cc(OC[C@H]2C[C@@H]2c2ccccn2)n1 |r| Show InChI InChI=1S/C19H22N6O/c1-13-23-18(21-9-14-10-22-25(2)11-14)8-19(24-13)26-12-15-7-16(15)17-5-3-4-6-20-17/h3-6,8,10-11,15-16H,7,9,12H2,1-2H3,(H,21,23,24)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085666

((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cnc[nH]3)nc12 Show InChI InChI=1S/C31H35N9O4/c1-2-33-29(43)26-24(41)25(42)30(44-26)40-18-37-23-27(38-31(39-28(23)40)34-14-13-21-15-32-17-36-21)35-16-22(19-9-5-3-6-10-19)20-11-7-4-8-12-20/h3-12,15,17-18,22,24-26,30,41-42H,2,13-14,16H2,1H3,(H,32,36)(H,33,43)(H2,34,35,38,39)/t24-,25+,26-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470670
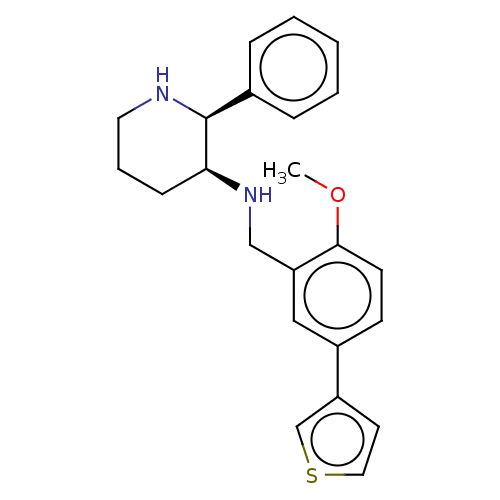
(CHEMBL149557)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccsc1 Show InChI InChI=1S/C23H26N2OS/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880
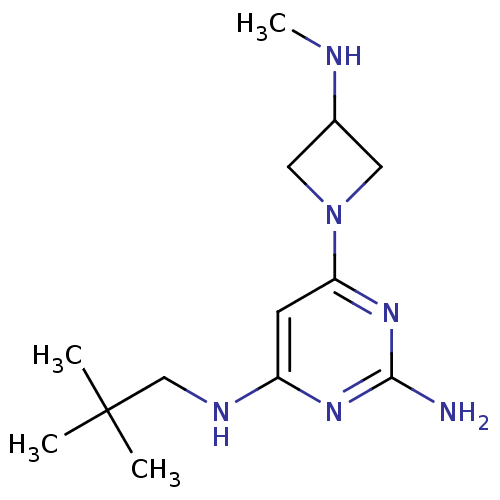
(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM82459
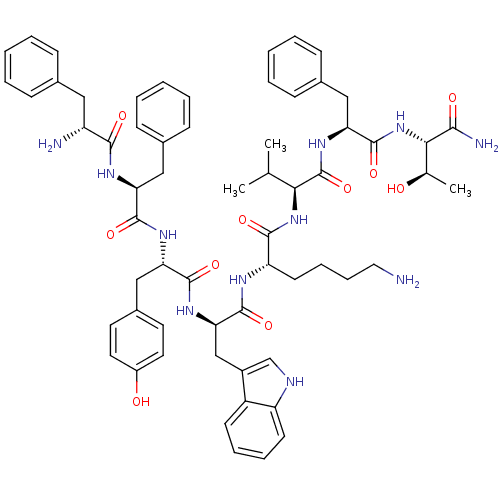
(BIM 23058 | BIM-23058 | D-Phe-L-Phe-L-Tyr-D-Trp-L-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C62H77N11O10/c1-37(2)53(62(83)71-51(33-41-21-11-6-12-22-41)61(82)73-54(38(3)74)55(65)76)72-57(78)48(25-15-16-30-63)67-60(81)52(35-43-36-66-47-24-14-13-23-45(43)47)70-59(80)50(34-42-26-28-44(75)29-27-42)69-58(79)49(32-40-19-9-5-10-20-40)68-56(77)46(64)31-39-17-7-4-8-18-39/h4-14,17-24,26-29,36-38,46,48-54,66,74-75H,15-16,25,30-35,63-64H2,1-3H3,(H2,65,76)(H,67,81)(H,68,77)(H,69,79)(H,70,80)(H,71,83)(H,72,78)(H,73,82)/t38-,46-,48+,49+,50+,51+,52-,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163741
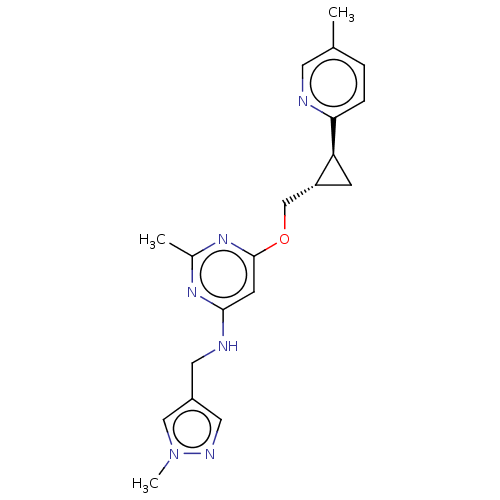
(US9062059, 2-3)Show SMILES Cc1ccc(nc1)[C@H]1C[C@@H]1COc1cc(NCc2cnn(C)c2)nc(C)n1 |r| Show InChI InChI=1S/C20H24N6O/c1-13-4-5-18(21-8-13)17-6-16(17)12-27-20-7-19(24-14(2)25-20)22-9-15-10-23-26(3)11-15/h4-5,7-8,10-11,16-17H,6,9,12H2,1-3H3,(H,22,24,25)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
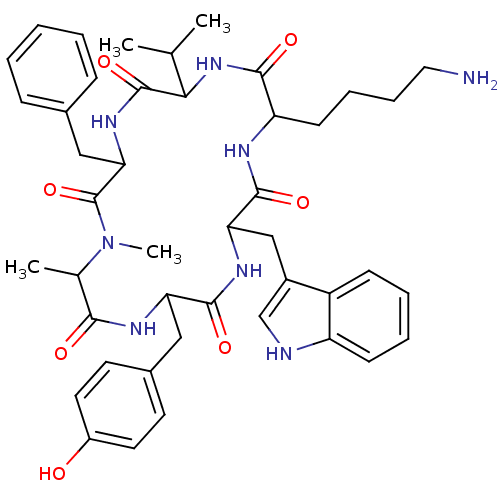
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50097783

(CHEMBL438401 | D-Trp8 SST-14 | SOMATOSTATIN | SRIF...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@@H](NC(=O)[C@@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)C(C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42?,43+,50-,51+,52-,53+,54+,55-,56+,57+,58-,59+,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163729
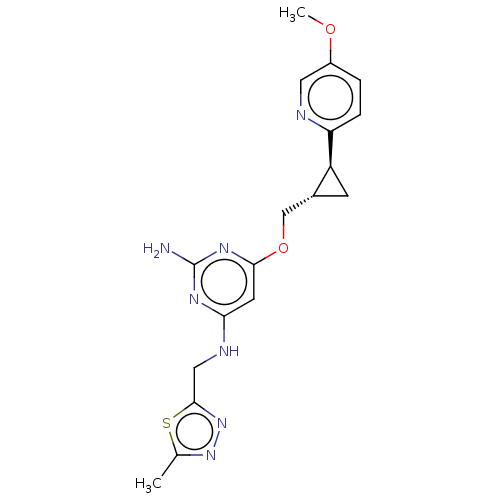
(US9062059, 2-53)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1cc(NCc2nnc(C)s2)nc(N)n1 |r| Show InChI InChI=1S/C18H21N7O2S/c1-10-24-25-17(28-10)8-21-15-6-16(23-18(19)22-15)27-9-11-5-13(11)14-4-3-12(26-2)7-20-14/h3-4,6-7,11,13H,5,8-9H2,1-2H3,(H3,19,21,22,23)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085674
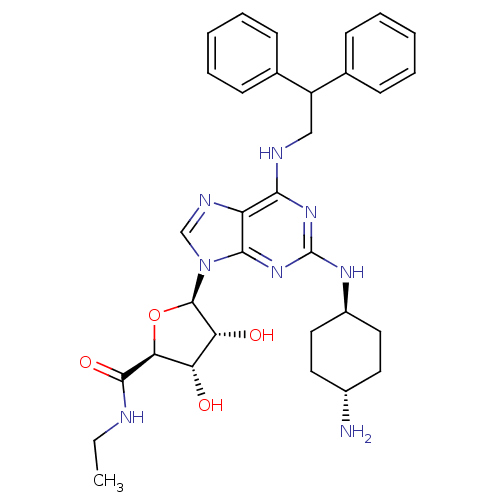
((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:7.12,5.4,35.37,wD:8.8,10.11,38.41,(-2.14,-10.42,;-1.05,-9.34,;-1.45,-7.85,;-.35,-6.77,;-.7,-5.26,;1.12,-7.24,;2.36,-6.34,;3.6,-7.24,;3.12,-8.72,;4.04,-9.95,;1.59,-8.72,;.69,-9.95,;3.38,-4.67,;4.3,-3.42,;3.38,-2.18,;1.92,-2.66,;.59,-1.89,;.57,-.35,;1.92,.42,;1.89,1.96,;3.23,2.73,;4.55,1.96,;5.89,2.73,;5.89,4.28,;4.53,5.03,;3.21,4.26,;.57,2.71,;.57,4.26,;-.77,5.01,;-2.1,4.24,;-2.09,2.7,;-.77,1.93,;-.75,-2.66,;-.75,-4.22,;-2.09,-4.98,;-2.87,-3.64,;-4.41,-3.66,;-5.2,-2.31,;-4.42,-.95,;-5.2,.37,;-2.87,-.95,;-2.09,-2.29,;.59,-4.98,;1.92,-4.2,)| Show InChI InChI=1S/C32H40N8O4/c1-2-34-30(43)27-25(41)26(42)31(44-27)40-18-36-24-28(38-32(39-29(24)40)37-22-15-13-21(33)14-16-22)35-17-23(19-9-5-3-6-10-19)20-11-7-4-8-12-20/h3-12,18,21-23,25-27,31,41-42H,2,13-17,33H2,1H3,(H,34,43)(H2,35,37,38,39)/t21-,22-,25-,26+,27-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470678
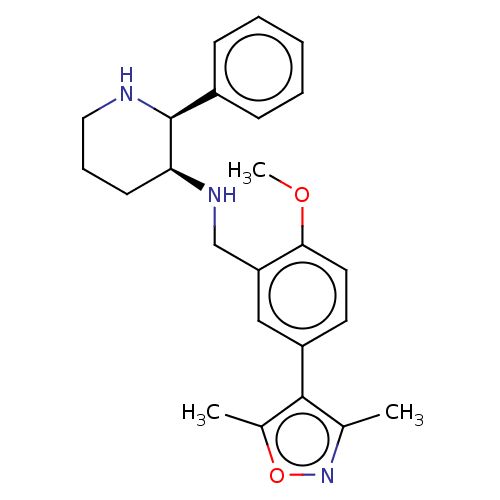
(CHEMBL146885)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1c(C)noc1C Show InChI InChI=1S/C24H29N3O2/c1-16-23(17(2)29-27-16)19-11-12-22(28-3)20(14-19)15-26-21-10-7-13-25-24(21)18-8-5-4-6-9-18/h4-6,8-9,11-12,14,21,24-26H,7,10,13,15H2,1-3H3/t21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50410193
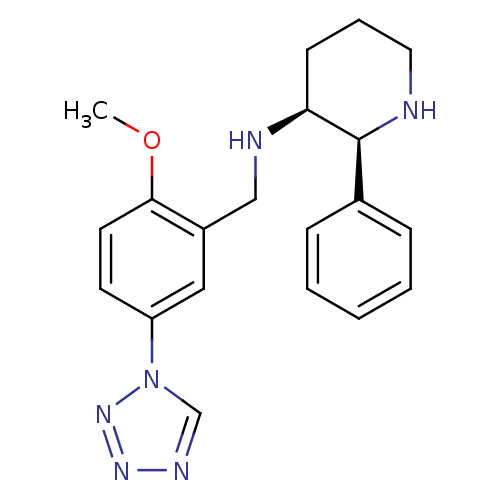
(CHEMBL356062 | GR-203040)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1cnnn1 Show InChI InChI=1S/C20H24N6O/c1-27-19-10-9-17(26-14-23-24-25-26)12-16(19)13-22-18-8-5-11-21-20(18)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,18,20-22H,5,8,11,13H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
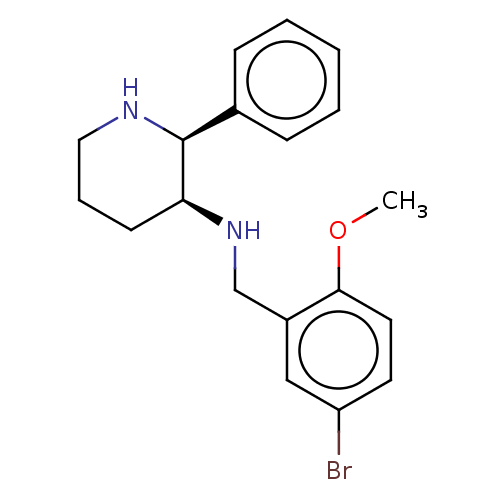
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470675
(CHEMBL344536)Show InChI InChI=1S/C19H23BrN2O/c1-23-18-10-9-16(20)12-15(18)13-22-17-8-5-11-21-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,21-22H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470669

(CHEMBL359188)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccncc1 Show InChI InChI=1S/C24H27N3O/c1-28-23-10-9-20(18-11-14-25-15-12-18)16-21(23)17-27-22-8-5-13-26-24(22)19-6-3-2-4-7-19/h2-4,6-7,9-12,14-16,22,24,26-27H,5,8,13,17H2,1H3/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
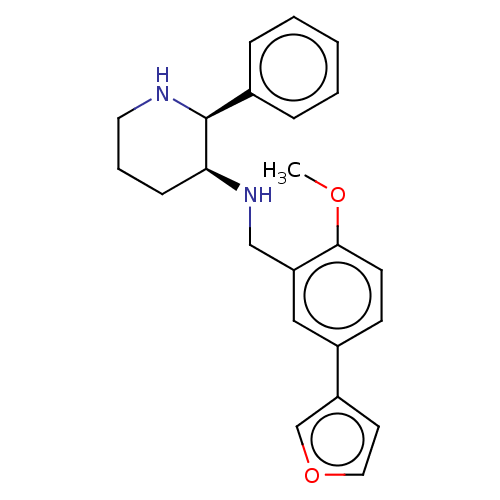
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470683
(CHEMBL356786)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccoc1 Show InChI InChI=1S/C23H26N2O2/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50254239
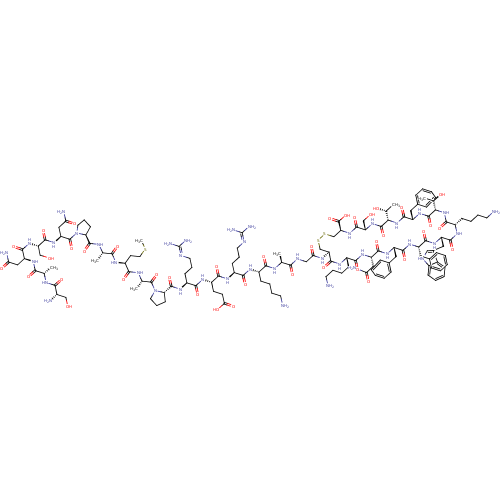
(somatostatin-28)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)[C@@H](C)O)C(O)=O |r,wU:42.43,28.31,13.12,4.4,66.67,86.87,106.108,115.116,194.201,172.177,149.152,134.136,124.126,120.224,wD:47.48,34.39,20.25,8.8,54.55,62.64,77.78,97.98,202.209,183.189,158.161,145.218,211.220,130.221,214.223,(-22.23,-7.83,;-23.64,-7.23,;-24.87,-8.16,;-26.29,-7.56,;-27.51,-8.49,;-28.93,-7.9,;-29.12,-6.37,;-27.9,-5.44,;-30.54,-5.78,;-31.77,-6.71,;-30.73,-4.25,;-32.15,-3.66,;-33.4,-4.59,;-32.34,-2.13,;-31.06,-1.26,;-31.8,.25,;-33.45,.03,;-33.78,-1.63,;-35.09,-2.39,;-35.19,-3.93,;-36.39,-1.55,;-37.77,-2.23,;-37.83,-3.76,;-39.2,-4.44,;-36.56,-4.61,;-36.28,-.01,;-37.58,.85,;-38.94,.16,;-37.46,2.37,;-36.09,3.05,;-34.83,2.21,;-38.75,3.22,;-38.65,4.76,;-37.27,5.45,;-39.93,5.6,;-41.31,4.92,;-41.42,3.4,;-40.12,2.54,;-42.8,2.71,;-39.84,7.14,;-41.12,8,;-42.5,7.31,;-41.02,9.53,;-39.65,10.21,;-42.31,10.39,;-42.21,11.93,;-40.83,12.61,;-43.49,12.76,;-43.38,14.3,;-44.87,12.08,;-44.96,10.56,;-27.32,-10.02,;-28.56,-10.95,;-25.9,-10.61,;-25.71,-12.14,;-26.93,-13.07,;-24.29,-12.75,;-23.09,-11.81,;-24.1,-14.26,;-25.25,-15.51,;-24.43,-16.97,;-22.76,-16.63,;-22.84,-15.08,;-21.53,-14.32,;-21.53,-12.78,;-20.18,-15.08,;-18.84,-14.32,;-18.84,-12.78,;-17.53,-12.02,;-17.53,-10.47,;-16.2,-9.71,;-16.2,-8.17,;-17.52,-7.41,;-14.86,-7.41,;-17.53,-15.08,;-17.53,-16.63,;-16.18,-14.32,;-14.85,-15.09,;-14.85,-16.63,;-13.54,-17.39,;-13.54,-18.94,;-14.87,-19.72,;-12.18,-19.71,;-13.54,-14.32,;-13.54,-12.78,;-12.19,-15.09,;-10.86,-14.33,;-10.86,-12.79,;-9.51,-12.02,;-9.51,-10.48,;-8.18,-9.72,;-8.18,-8.18,;-9.52,-7.42,;-6.84,-7.41,;-9.51,-15.11,;-9.51,-16.63,;-8.2,-14.33,;-6.85,-15.11,;-6.85,-16.64,;-5.52,-17.42,;-5.52,-18.94,;-4.2,-19.72,;-4.2,-21.27,;-5.52,-14.33,;-5.52,-12.79,;-4.19,-15.11,;-2.85,-14.33,;-2.85,-12.81,;-1.53,-15.11,;-1.53,-16.64,;-.2,-14.34,;1.14,-15.12,;2.46,-14.34,;2.46,-12.82,;3.81,-15.12,;5.13,-14.34,;5.14,-3.92,;6.49,-3.13,;50.46,-3.19,;50.41,-14.4,;49.07,-15.18,;47.74,-14.4,;46.4,-15.18,;46.4,-16.7,;45.07,-14.39,;45.07,-12.87,;46.4,-12.09,;43.75,-15.17,;42.41,-14.39,;42.41,-12.87,;41.08,-15.17,;39.75,-14.39,;38.42,-15.15,;38.42,-16.7,;37.09,-14.39,;37.09,-12.85,;38.42,-12.08,;39.76,-12.87,;41.08,-12.09,;41.09,-10.55,;39.76,-9.78,;38.42,-10.54,;35.75,-15.15,;34.42,-14.38,;34.42,-12.84,;33.1,-15.15,;31.76,-14.38,;30.44,-15.14,;30.44,-16.69,;29.09,-14.38,;29.09,-12.84,;30.44,-12.08,;30.44,-10.54,;31.76,-9.77,;31.76,-8.23,;27.77,-15.14,;26.44,-14.38,;26.44,-12.84,;25.1,-15.14,;25.1,-16.68,;26.44,-17.45,;27.84,-16.82,;28.87,-17.98,;28.09,-19.3,;28.57,-20.76,;27.53,-21.91,;26.03,-21.59,;25.57,-20.12,;26.59,-18.98,;23.78,-14.38,;22.44,-15.14,;22.44,-16.68,;21.11,-14.37,;21.11,-12.83,;22.44,-12.07,;23.78,-12.83,;25.1,-12.07,;25.1,-10.53,;23.78,-9.77,;22.44,-10.53,;19.79,-15.13,;18.44,-14.37,;18.44,-12.83,;17.12,-15.13,;17.12,-16.68,;18.44,-17.44,;19.76,-16.68,;21.1,-17.45,;21.09,-18.99,;19.75,-19.75,;18.44,-18.98,;15.78,-14.37,;14.45,-15.13,;14.45,-16.67,;13.11,-14.37,;13.11,-12.83,;14.45,-12.04,;15.78,-12.83,;14.45,-10.52,;11.79,-15.13,;10.46,-14.36,;10.46,-12.82,;9.12,-15.12,;9.12,-16.67,;10.46,-17.43,;10.46,-18.98,;11.78,-19.74,;11.78,-21.28,;7.79,-14.36,;6.47,-15.12,;6.47,-16.67,;33.1,-16.69,;31.76,-17.46,;34.42,-17.45,;41.08,-16.7,;39.75,-17.48,;42.41,-17.48,;49.07,-16.71,;47.74,-17.49,;50.41,-17.49,)| Show InChI InChI=1S/C137H207N41O39S3/c1-69(154-113(194)82(37-19-22-47-138)159-115(196)85(40-25-50-149-136(145)146)160-118(199)87(44-45-106(188)189)163-116(197)86(41-26-51-150-137(147)148)164-130(211)101-43-27-52-177(101)133(214)72(4)156-114(195)88(46-54-218-7)158-110(191)71(3)155-129(210)100-42-28-53-178(100)134(215)95(61-104(144)186)171-126(207)96(65-180)172-124(205)93(59-102(142)184)165-111(192)70(2)153-112(193)80(141)64-179)109(190)152-63-105(187)157-98-67-219-220-68-99(135(216)217)174-127(208)97(66-181)173-132(213)108(74(6)183)176-125(206)91(57-77-33-15-10-16-34-77)170-131(212)107(73(5)182)175-119(200)84(39-21-24-49-140)161-122(203)92(58-78-62-151-81-36-18-17-35-79(78)81)168-121(202)90(56-76-31-13-9-14-32-76)166-120(201)89(55-75-29-11-8-12-30-75)167-123(204)94(60-103(143)185)169-117(198)83(162-128(98)209)38-20-23-48-139/h8-18,29-36,62,69-74,80,82-101,107-108,151,179-183H,19-28,37-61,63-68,138-141H2,1-7H3,(H2,142,184)(H2,143,185)(H2,144,186)(H,152,190)(H,153,193)(H,154,194)(H,155,210)(H,156,195)(H,157,187)(H,158,191)(H,159,196)(H,160,199)(H,161,203)(H,162,209)(H,163,197)(H,164,211)(H,165,192)(H,166,201)(H,167,204)(H,168,202)(H,169,198)(H,170,212)(H,171,207)(H,172,205)(H,173,213)(H,174,208)(H,175,200)(H,176,206)(H,188,189)(H,216,217)(H4,145,146,149)(H4,147,148,150)/t69-,70-,71-,72-,73+,74+,80-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,107-,108-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50254239

(somatostatin-28)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)[C@@H](C)O)C(O)=O |r,wU:42.43,28.31,13.12,4.4,66.67,86.87,106.108,115.116,194.201,172.177,149.152,134.136,124.126,120.224,wD:47.48,34.39,20.25,8.8,54.55,62.64,77.78,97.98,202.209,183.189,158.161,145.218,211.220,130.221,214.223,(-22.23,-7.83,;-23.64,-7.23,;-24.87,-8.16,;-26.29,-7.56,;-27.51,-8.49,;-28.93,-7.9,;-29.12,-6.37,;-27.9,-5.44,;-30.54,-5.78,;-31.77,-6.71,;-30.73,-4.25,;-32.15,-3.66,;-33.4,-4.59,;-32.34,-2.13,;-31.06,-1.26,;-31.8,.25,;-33.45,.03,;-33.78,-1.63,;-35.09,-2.39,;-35.19,-3.93,;-36.39,-1.55,;-37.77,-2.23,;-37.83,-3.76,;-39.2,-4.44,;-36.56,-4.61,;-36.28,-.01,;-37.58,.85,;-38.94,.16,;-37.46,2.37,;-36.09,3.05,;-34.83,2.21,;-38.75,3.22,;-38.65,4.76,;-37.27,5.45,;-39.93,5.6,;-41.31,4.92,;-41.42,3.4,;-40.12,2.54,;-42.8,2.71,;-39.84,7.14,;-41.12,8,;-42.5,7.31,;-41.02,9.53,;-39.65,10.21,;-42.31,10.39,;-42.21,11.93,;-40.83,12.61,;-43.49,12.76,;-43.38,14.3,;-44.87,12.08,;-44.96,10.56,;-27.32,-10.02,;-28.56,-10.95,;-25.9,-10.61,;-25.71,-12.14,;-26.93,-13.07,;-24.29,-12.75,;-23.09,-11.81,;-24.1,-14.26,;-25.25,-15.51,;-24.43,-16.97,;-22.76,-16.63,;-22.84,-15.08,;-21.53,-14.32,;-21.53,-12.78,;-20.18,-15.08,;-18.84,-14.32,;-18.84,-12.78,;-17.53,-12.02,;-17.53,-10.47,;-16.2,-9.71,;-16.2,-8.17,;-17.52,-7.41,;-14.86,-7.41,;-17.53,-15.08,;-17.53,-16.63,;-16.18,-14.32,;-14.85,-15.09,;-14.85,-16.63,;-13.54,-17.39,;-13.54,-18.94,;-14.87,-19.72,;-12.18,-19.71,;-13.54,-14.32,;-13.54,-12.78,;-12.19,-15.09,;-10.86,-14.33,;-10.86,-12.79,;-9.51,-12.02,;-9.51,-10.48,;-8.18,-9.72,;-8.18,-8.18,;-9.52,-7.42,;-6.84,-7.41,;-9.51,-15.11,;-9.51,-16.63,;-8.2,-14.33,;-6.85,-15.11,;-6.85,-16.64,;-5.52,-17.42,;-5.52,-18.94,;-4.2,-19.72,;-4.2,-21.27,;-5.52,-14.33,;-5.52,-12.79,;-4.19,-15.11,;-2.85,-14.33,;-2.85,-12.81,;-1.53,-15.11,;-1.53,-16.64,;-.2,-14.34,;1.14,-15.12,;2.46,-14.34,;2.46,-12.82,;3.81,-15.12,;5.13,-14.34,;5.14,-3.92,;6.49,-3.13,;50.46,-3.19,;50.41,-14.4,;49.07,-15.18,;47.74,-14.4,;46.4,-15.18,;46.4,-16.7,;45.07,-14.39,;45.07,-12.87,;46.4,-12.09,;43.75,-15.17,;42.41,-14.39,;42.41,-12.87,;41.08,-15.17,;39.75,-14.39,;38.42,-15.15,;38.42,-16.7,;37.09,-14.39,;37.09,-12.85,;38.42,-12.08,;39.76,-12.87,;41.08,-12.09,;41.09,-10.55,;39.76,-9.78,;38.42,-10.54,;35.75,-15.15,;34.42,-14.38,;34.42,-12.84,;33.1,-15.15,;31.76,-14.38,;30.44,-15.14,;30.44,-16.69,;29.09,-14.38,;29.09,-12.84,;30.44,-12.08,;30.44,-10.54,;31.76,-9.77,;31.76,-8.23,;27.77,-15.14,;26.44,-14.38,;26.44,-12.84,;25.1,-15.14,;25.1,-16.68,;26.44,-17.45,;27.84,-16.82,;28.87,-17.98,;28.09,-19.3,;28.57,-20.76,;27.53,-21.91,;26.03,-21.59,;25.57,-20.12,;26.59,-18.98,;23.78,-14.38,;22.44,-15.14,;22.44,-16.68,;21.11,-14.37,;21.11,-12.83,;22.44,-12.07,;23.78,-12.83,;25.1,-12.07,;25.1,-10.53,;23.78,-9.77,;22.44,-10.53,;19.79,-15.13,;18.44,-14.37,;18.44,-12.83,;17.12,-15.13,;17.12,-16.68,;18.44,-17.44,;19.76,-16.68,;21.1,-17.45,;21.09,-18.99,;19.75,-19.75,;18.44,-18.98,;15.78,-14.37,;14.45,-15.13,;14.45,-16.67,;13.11,-14.37,;13.11,-12.83,;14.45,-12.04,;15.78,-12.83,;14.45,-10.52,;11.79,-15.13,;10.46,-14.36,;10.46,-12.82,;9.12,-15.12,;9.12,-16.67,;10.46,-17.43,;10.46,-18.98,;11.78,-19.74,;11.78,-21.28,;7.79,-14.36,;6.47,-15.12,;6.47,-16.67,;33.1,-16.69,;31.76,-17.46,;34.42,-17.45,;41.08,-16.7,;39.75,-17.48,;42.41,-17.48,;49.07,-16.71,;47.74,-17.49,;50.41,-17.49,)| Show InChI InChI=1S/C137H207N41O39S3/c1-69(154-113(194)82(37-19-22-47-138)159-115(196)85(40-25-50-149-136(145)146)160-118(199)87(44-45-106(188)189)163-116(197)86(41-26-51-150-137(147)148)164-130(211)101-43-27-52-177(101)133(214)72(4)156-114(195)88(46-54-218-7)158-110(191)71(3)155-129(210)100-42-28-53-178(100)134(215)95(61-104(144)186)171-126(207)96(65-180)172-124(205)93(59-102(142)184)165-111(192)70(2)153-112(193)80(141)64-179)109(190)152-63-105(187)157-98-67-219-220-68-99(135(216)217)174-127(208)97(66-181)173-132(213)108(74(6)183)176-125(206)91(57-77-33-15-10-16-34-77)170-131(212)107(73(5)182)175-119(200)84(39-21-24-49-140)161-122(203)92(58-78-62-151-81-36-18-17-35-79(78)81)168-121(202)90(56-76-31-13-9-14-32-76)166-120(201)89(55-75-29-11-8-12-30-75)167-123(204)94(60-103(143)185)169-117(198)83(162-128(98)209)38-20-23-48-139/h8-18,29-36,62,69-74,80,82-101,107-108,151,179-183H,19-28,37-61,63-68,138-141H2,1-7H3,(H2,142,184)(H2,143,185)(H2,144,186)(H,152,190)(H,153,193)(H,154,194)(H,155,210)(H,156,195)(H,157,187)(H,158,191)(H,159,196)(H,160,199)(H,161,203)(H,162,209)(H,163,197)(H,164,211)(H,165,192)(H,166,201)(H,167,204)(H,168,202)(H,169,198)(H,170,212)(H,171,207)(H,172,205)(H,173,213)(H,174,208)(H,175,200)(H,176,206)(H,188,189)(H,216,217)(H4,145,146,149)(H4,147,148,150)/t69-,70-,71-,72-,73+,74+,80-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,107-,108-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
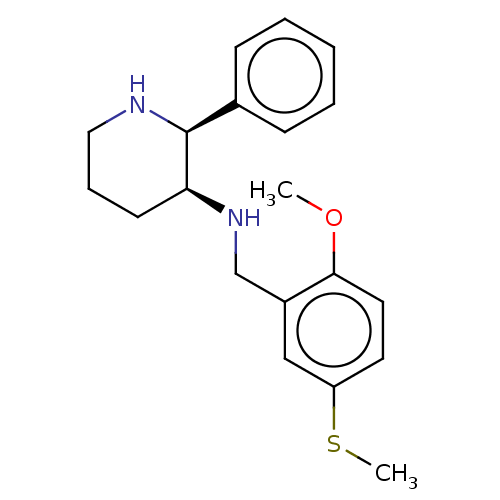
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470672
(CHEMBL434884)Show InChI InChI=1S/C20H26N2OS/c1-23-19-11-10-17(24-2)13-16(19)14-22-18-9-6-12-21-20(18)15-7-4-3-5-8-15/h3-5,7-8,10-11,13,18,20-22H,6,9,12,14H2,1-2H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM82457
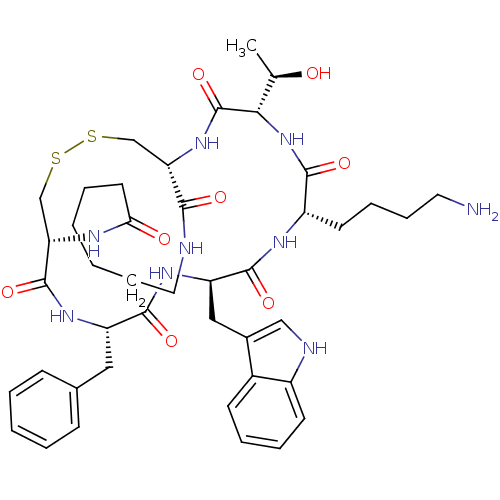
((1R,4S,7S,10R,13S,16R)-4-[(R)-1-Hydroxyethyl]-7-(4...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)NCCCCCCC(=O)N2 Show InChI InChI=1S/C43H59N9O8S2/c1-26(53)37-43(60)51-34-24-61-62-25-35(47-36(54)18-7-2-3-12-20-45-38(34)55)42(59)49-32(21-27-13-5-4-6-14-27)40(57)50-33(22-28-23-46-30-16-9-8-15-29(28)30)41(58)48-31(39(56)52-37)17-10-11-19-44/h4-6,8-9,13-16,23,26,31-35,37,46,53H,2-3,7,10-12,17-22,24-25,44H2,1H3,(H,45,55)(H,47,54)(H,48,58)(H,49,59)(H,50,57)(H,51,60)(H,52,56)/t26-,31+,32+,33-,34+,35+,37+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085671
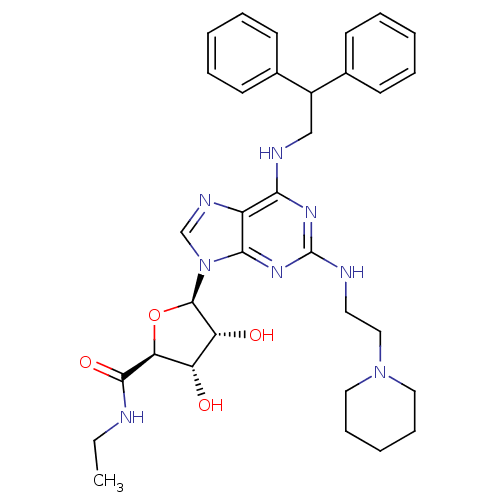
((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCN3CCCCC3)nc12 Show InChI InChI=1S/C33H42N8O4/c1-2-34-31(44)28-26(42)27(43)32(45-28)41-21-37-25-29(38-33(39-30(25)41)35-16-19-40-17-10-5-11-18-40)36-20-24(22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-4,6-9,12-15,21,24,26-28,32,42-43H,2,5,10-11,16-20H2,1H3,(H,34,44)(H2,35,36,38,39)/t26-,27+,28-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163737

(US9062059, 9-2)Show SMILES Cc1nnc(CNc2cc(OC[C@H]3C[C@@H]3c3ccc(cn3)C3CC3)nc(C)n2)s1 |r| Show InChI InChI=1S/C21H24N6OS/c1-12-24-19(23-10-21-27-26-13(2)29-21)8-20(25-12)28-11-16-7-17(16)18-6-5-15(9-22-18)14-3-4-14/h5-6,8-9,14,16-17H,3-4,7,10-11H2,1-2H3,(H,23,24,25)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50094929
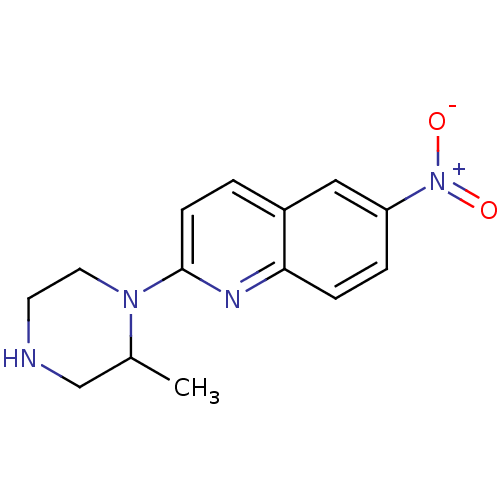
(2-(2-Methyl-piperazin-1-yl)-6-nitro-quinoline | CH...)Show InChI InChI=1S/C14H16N4O2/c1-10-9-15-6-7-17(10)14-5-2-11-8-12(18(19)20)3-4-13(11)16-14/h2-5,8,10,15H,6-7,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Washington University
Curated by ChEMBL
| Assay Description
In vitro radioligand [3H]-paroxetine from rat cortical Serotonin transporter |
Bioorg Med Chem Lett 10: 2643-6 (2000)
BindingDB Entry DOI: 10.7270/Q2W37VJW |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50144221

(CHEMBL3759802)Show InChI InChI=1S/C17H17NO3/c1-11(19)18-8-7-13-10-21-16-6-4-12-3-5-14(20-2)9-15(12)17(13)16/h3-6,9-10H,7-8H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cell membranes after 120 mins |
Eur J Med Chem 109: 360-70 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.047
BindingDB Entry DOI: 10.7270/Q2VM4F3T |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM163735
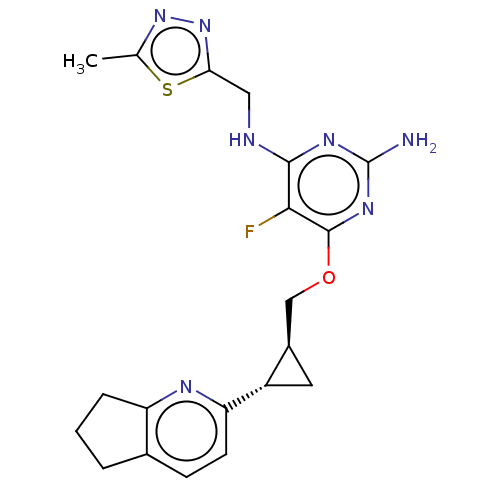
(US9062059, 3-25)Show SMILES Cc1nnc(CNc2nc(N)nc(OC[C@H]3C[C@@H]3c3ccc4CCCc4n3)c2F)s1 |r| Show InChI InChI=1S/C20H22FN7OS/c1-10-27-28-16(30-10)8-23-18-17(21)19(26-20(22)25-18)29-9-12-7-13(12)15-6-5-11-3-2-4-14(11)24-15/h5-6,12-13H,2-4,7-9H2,1H3,(H3,22,23,25,26)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... |
US Patent US9062059 (2015)
BindingDB Entry DOI: 10.7270/Q29022J5 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50230961
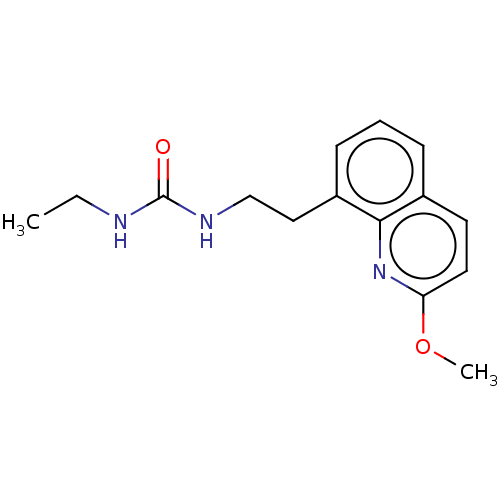
(CHEMBL4062947)Show InChI InChI=1S/C15H19N3O2/c1-3-16-15(19)17-10-9-12-6-4-5-11-7-8-13(20-2)18-14(11)12/h4-8H,3,9-10H2,1-2H3,(H2,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in HEK or CHO cell membranes after 120 mins |
Eur J Med Chem 127: 621-631 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.013
BindingDB Entry DOI: 10.7270/Q24F1SZS |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50230959
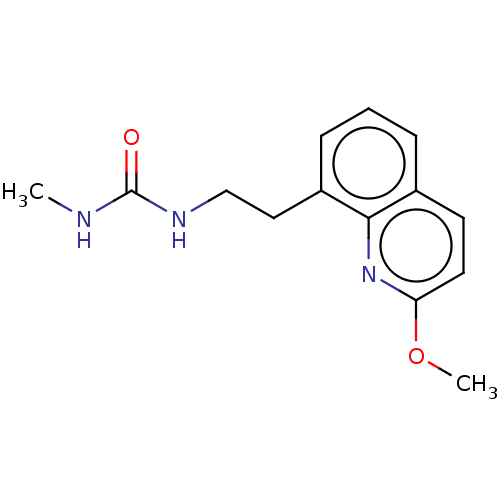
(CHEMBL4084612)Show InChI InChI=1S/C14H17N3O2/c1-15-14(18)16-9-8-11-5-3-4-10-6-7-12(19-2)17-13(10)11/h3-7H,8-9H2,1-2H3,(H2,15,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in HEK or CHO cell membranes after 120 mins |
Eur J Med Chem 127: 621-631 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.013
BindingDB Entry DOI: 10.7270/Q24F1SZS |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50464415
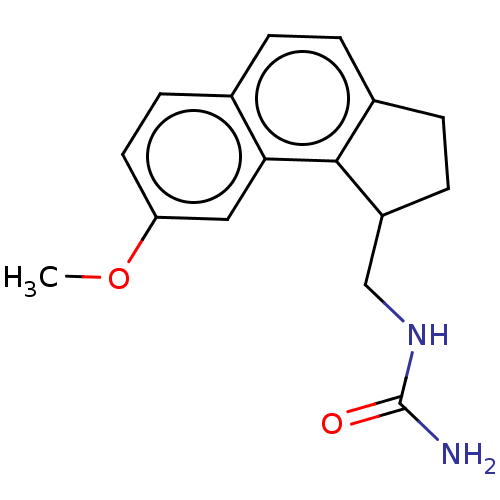
(CHEMBL4278575)Show InChI InChI=1S/C16H18N2O2/c1-20-13-7-6-10-2-3-11-4-5-12(9-18-16(17)19)15(11)14(10)8-13/h2-3,6-8,12H,4-5,9H2,1H3,(H3,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cell membranes after 120 mins by filter binding method |
Eur J Med Chem 141: 552-566 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.025
BindingDB Entry DOI: 10.7270/Q2CC13BX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50464420
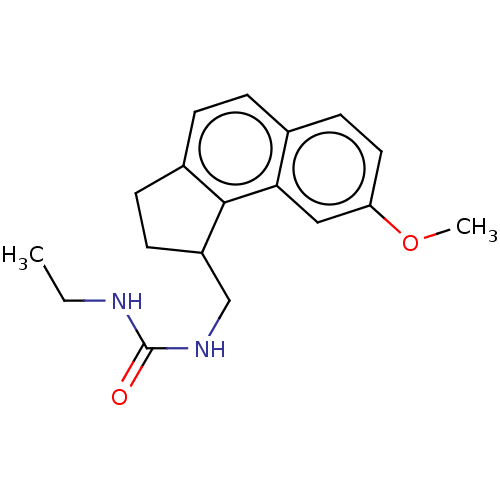
(CHEMBL4290302)Show InChI InChI=1S/C18H22N2O2/c1-3-19-18(21)20-11-14-7-6-13-5-4-12-8-9-15(22-2)10-16(12)17(13)14/h4-5,8-10,14H,3,6-7,11H2,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cell membranes after 120 mins by filter binding method |
Eur J Med Chem 141: 552-566 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.025
BindingDB Entry DOI: 10.7270/Q2CC13BX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50464407
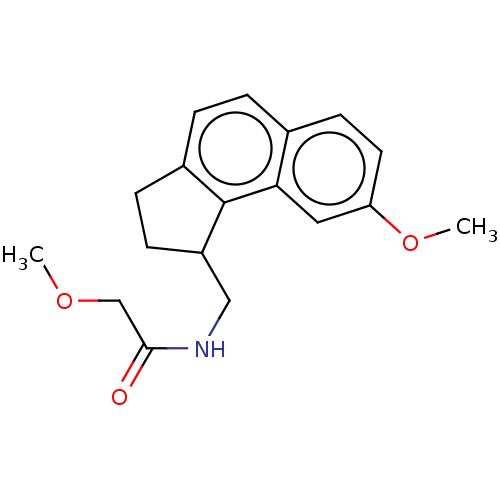
(CHEMBL4292336)Show InChI InChI=1S/C18H21NO3/c1-21-11-17(20)19-10-14-6-5-13-4-3-12-7-8-15(22-2)9-16(12)18(13)14/h3-4,7-9,14H,5-6,10-11H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cell membranes after 120 mins by filter binding method |
Eur J Med Chem 141: 552-566 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.025
BindingDB Entry DOI: 10.7270/Q2CC13BX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50464412
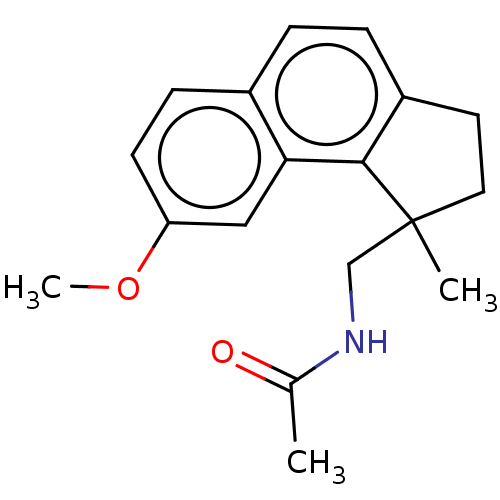
(CHEMBL4278248)Show InChI InChI=1S/C18H21NO2/c1-12(20)19-11-18(2)9-8-14-5-4-13-6-7-15(21-3)10-16(13)17(14)18/h4-7,10H,8-9,11H2,1-3H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cell membranes after 120 mins by filter binding method |
Eur J Med Chem 141: 552-566 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.025
BindingDB Entry DOI: 10.7270/Q2CC13BX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085668

((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@@H]3CCC[C@H]3O)nc12 Show InChI InChI=1S/C17H25N7O5/c1-2-19-15(28)12-10(26)11(27)16(29-12)24-6-20-9-13(18)22-17(23-14(9)24)21-7-4-3-5-8(7)25/h6-8,10-12,16,25-27H,2-5H2,1H3,(H,19,28)(H3,18,21,22,23)/t7-,8-,10+,11-,12+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035179
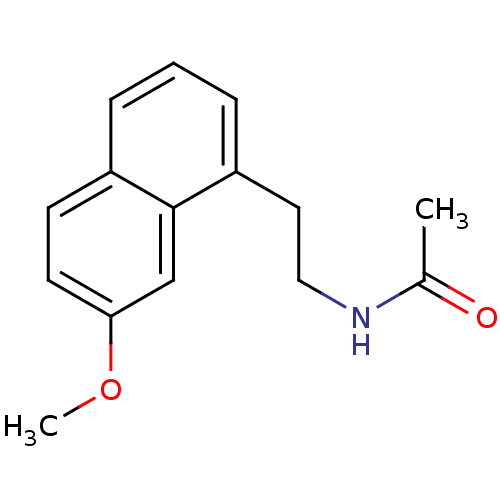
(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in HEK or CHO cell membranes after 120 mins |
Eur J Med Chem 127: 621-631 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.013
BindingDB Entry DOI: 10.7270/Q24F1SZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data