

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224191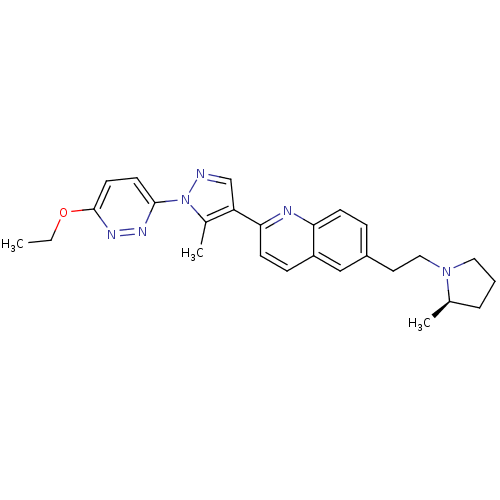 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224192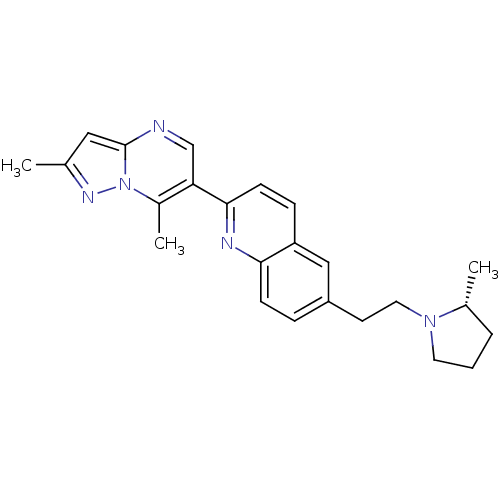 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224187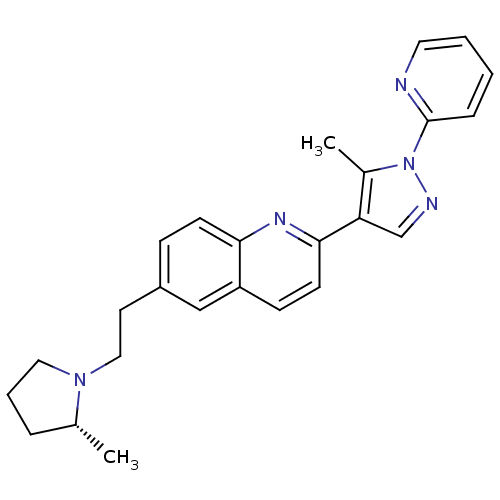 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224189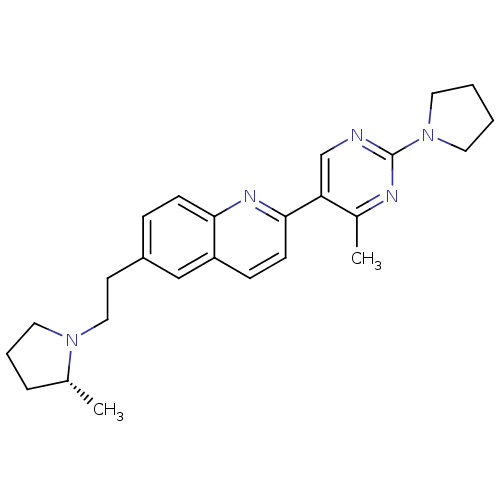 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224188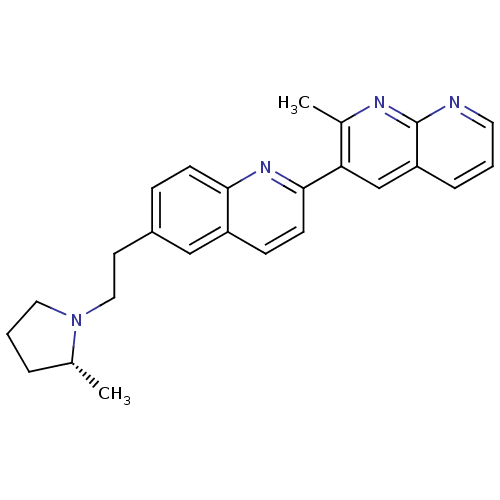 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391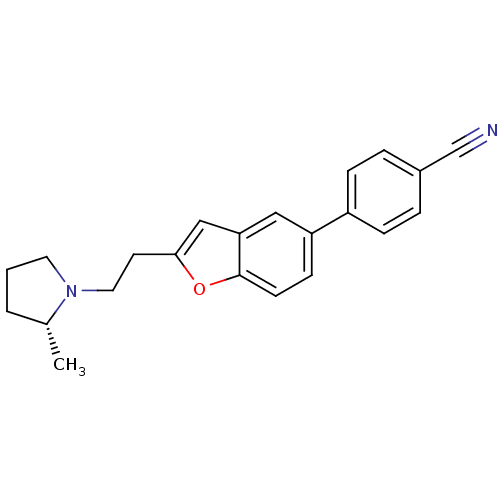 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224190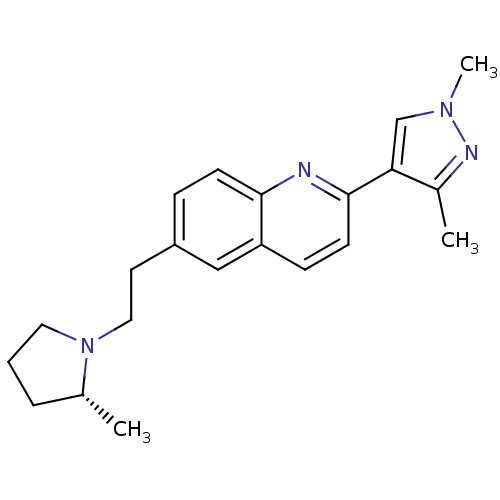 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM50187697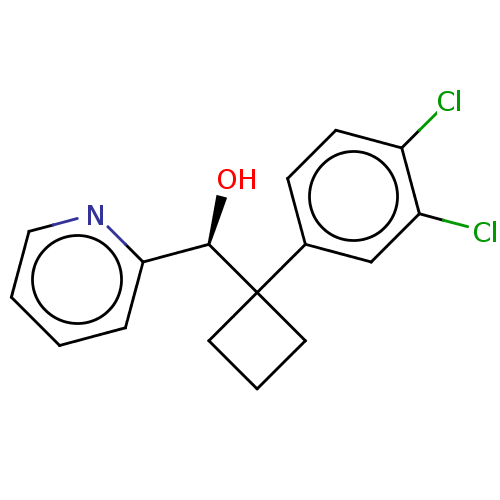 (CHEMBL3828278) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV3 expressed in recombinant HEK293 cells assessed as inhibition of 2-APB induced calcium flux by FLIPR analysis | J Med Chem 59: 4926-47 (2016) Article DOI: 10.1021/acs.jmedchem.6b00287 BindingDB Entry DOI: 10.7270/Q2765H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM50187699 (CHEMBL3828340) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV3 expressed in recombinant HEK293 cells assessed as inhibition of 2-APB induced currents by patch-clamp electrophysi... | J Med Chem 59: 4926-47 (2016) Article DOI: 10.1021/acs.jmedchem.6b00287 BindingDB Entry DOI: 10.7270/Q2765H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM50187699 (CHEMBL3828340) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV3 expressed in recombinant HEK293 cells assessed as inhibition of 2-APB induced calcium flux by FLIPR analysis | J Med Chem 59: 4926-47 (2016) Article DOI: 10.1021/acs.jmedchem.6b00287 BindingDB Entry DOI: 10.7270/Q2765H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.24 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 17.4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50187698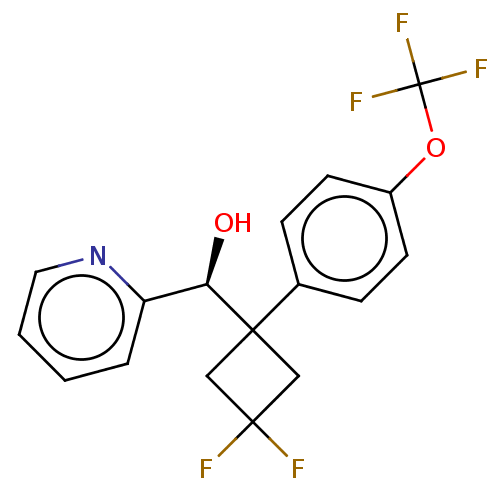 (CHEMBL3827928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Activation of human PXR | J Med Chem 59: 4926-47 (2016) Article DOI: 10.1021/acs.jmedchem.6b00287 BindingDB Entry DOI: 10.7270/Q2765H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.75 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human histamine H3 receptor expressed in HEK239 cells assessed as reduction of basal [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||