

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells assessed as inhibition of insulin-mediated cell proliferation after 6 days by Hoechst 33258 dye-... | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285009 (3-{3-[(3R,5R)-1-(tert-Butylcarbamoyl-methyl)-5-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285009 (3-{3-[(3R,5R)-1-(tert-Butylcarbamoyl-methyl)-5-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-A (CCK-A) receptor in guinea pig pancreas | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481062 (CHEMBL583904) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481056 (CHEMBL572032) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269484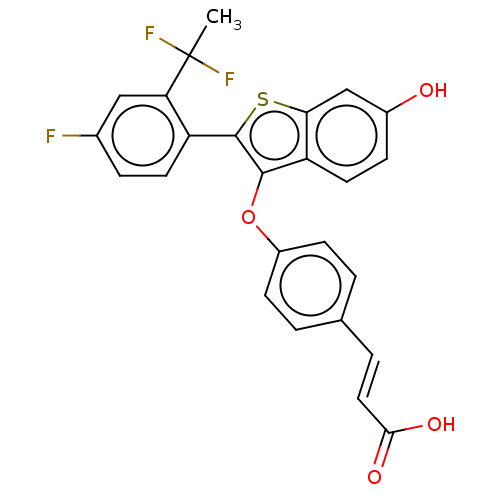 (US10058534, 139) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269467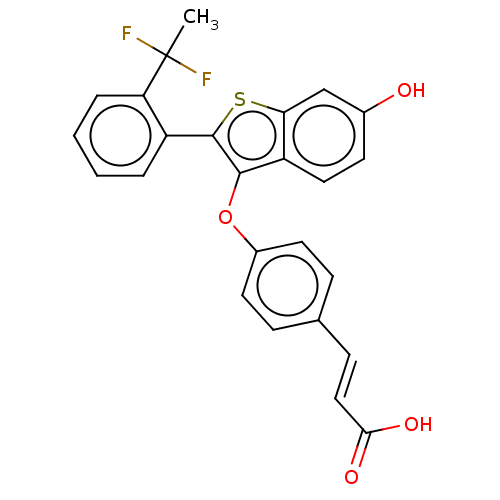 ((E)-3-(4-((2-(2-(1,1- difluoroethyl)phenyl)-6- hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481086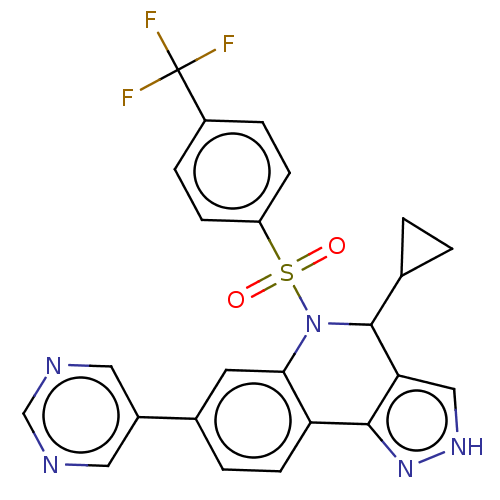 (CHEMBL571602) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481087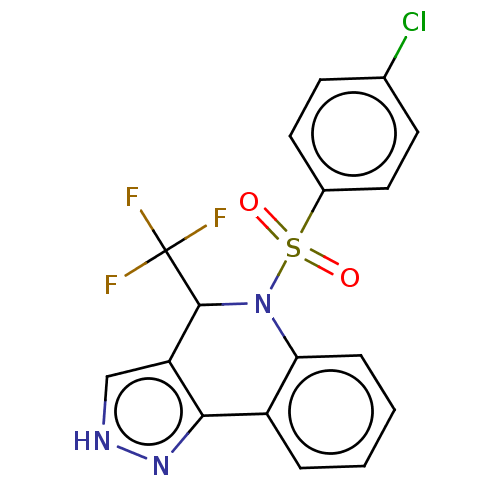 (CHEMBL571145) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285019 (CHEMBL294161 | N-tert-Butyl-2-((3R,5R)-5-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481063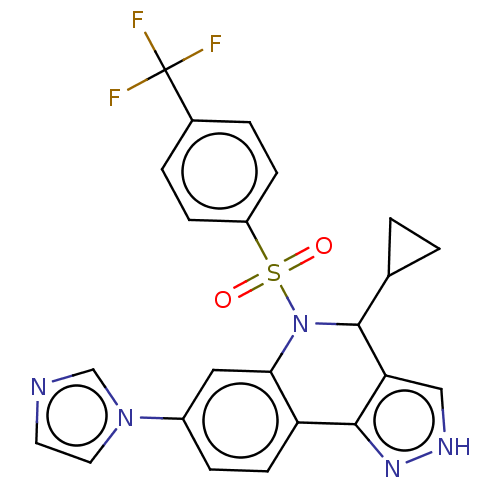 (CHEMBL583662) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285011 (CHEMBL294252 | N-tert-Butyl-2-{(3R,5R)-5-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481055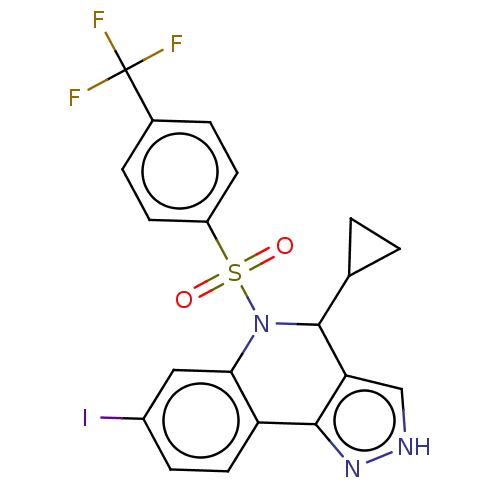 (CHEMBL571821) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481089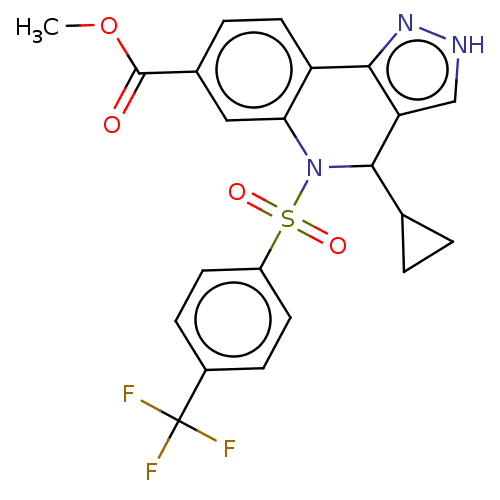 (CHEMBL584330) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481058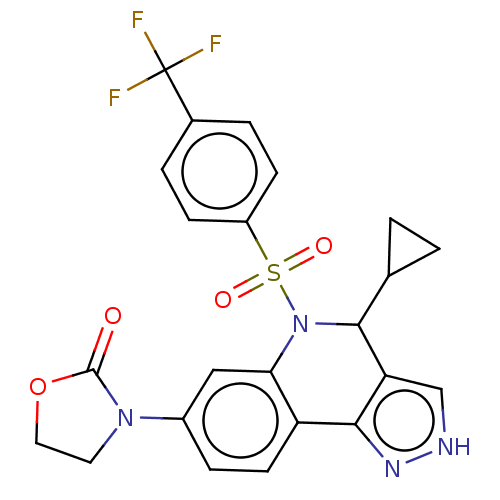 (CHEMBL570694) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481061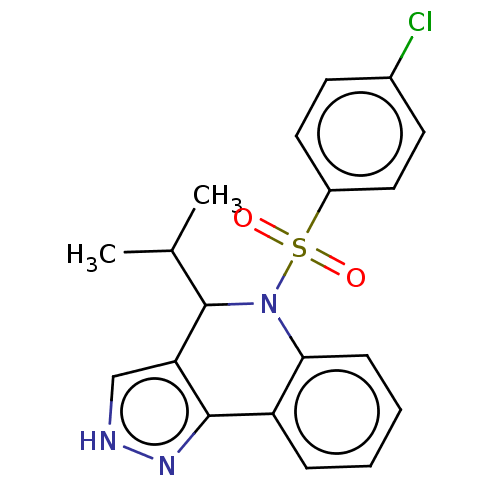 (CHEMBL570379) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269456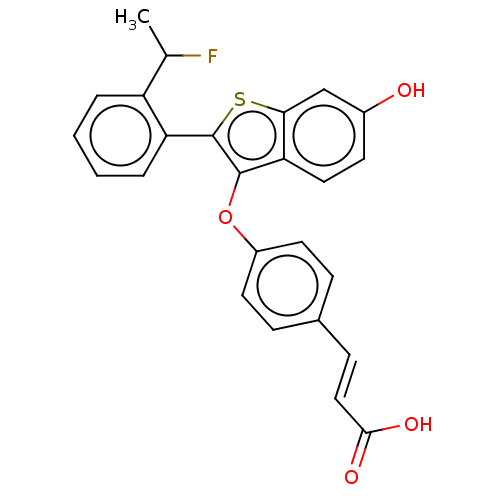 ((E)-3-(4-((2-(2-(1- fluoroethyl)phenyl)-6- hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213578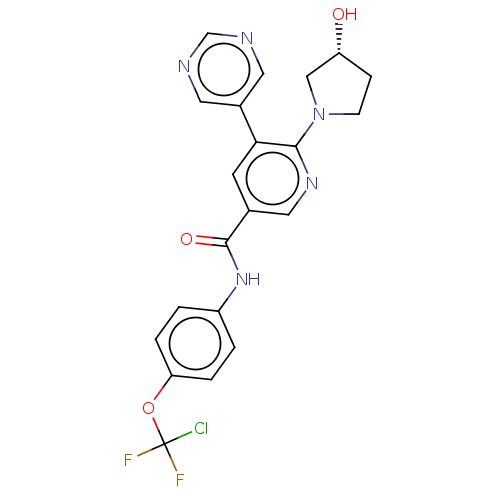 (US9278981, 170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481081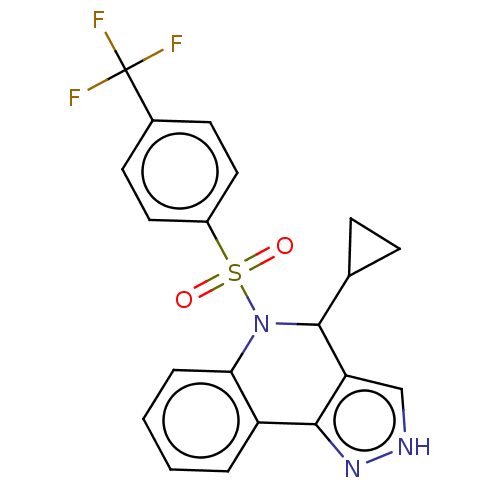 (CHEMBL584956) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481070 (CHEMBL571591) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481068 (CHEMBL584113) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213594 (US9278981, 186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50237304 (CHEMBL4093325) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro binding affinity at human cloned dopamine receptor D2 (short) stably expressed in CHO cells by [3H]spiperone displacement. | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269308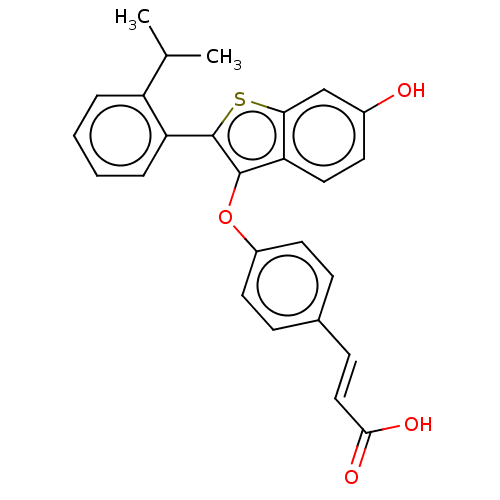 ((E)-3-(4-((6-hydroxy-2- (2-isopropylphenyl)- benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16384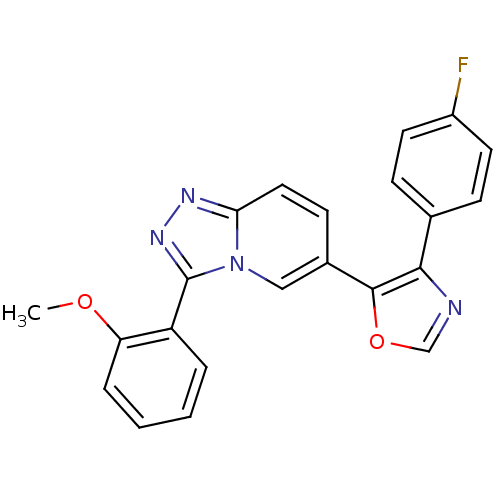 (4-(4-fluorophenyl)-5-[3-(2-methoxyphenyl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50237322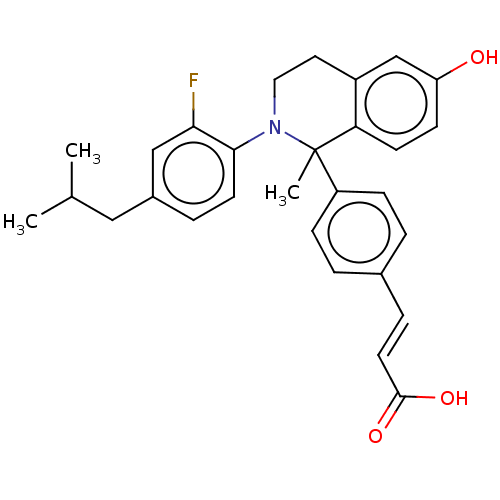 (CHEMBL4088736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16385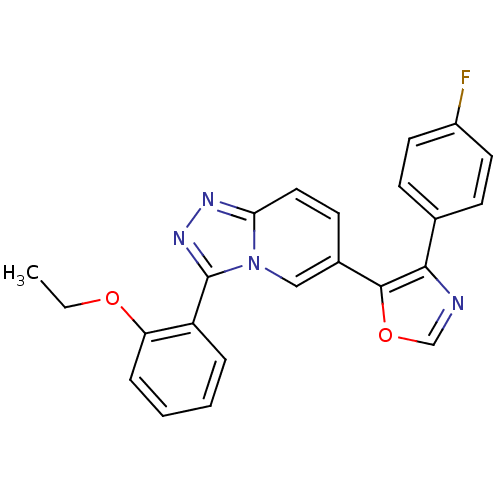 (5-[3-(2-ethoxyphenyl)-[1,2,4]triazolo[3,4-a]pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481064 (CHEMBL585713) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481043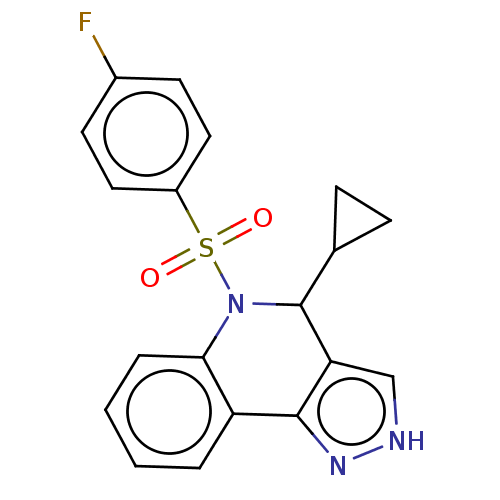 (CHEMBL583660) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481069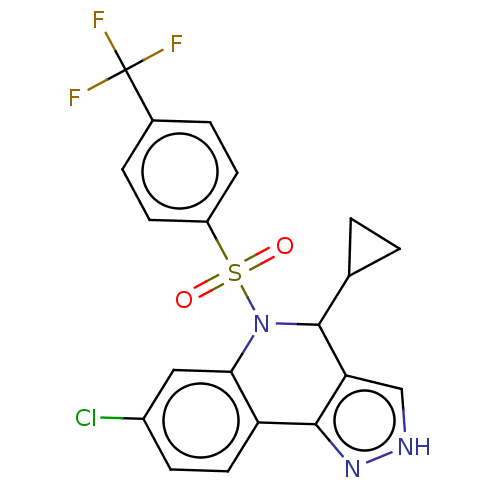 (CHEMBL571175) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481075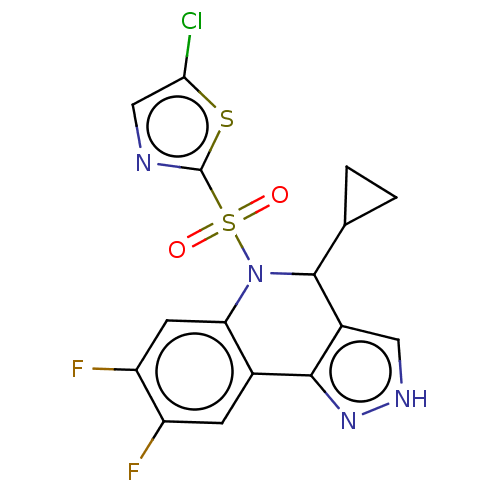 (CHEMBL571817) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50037849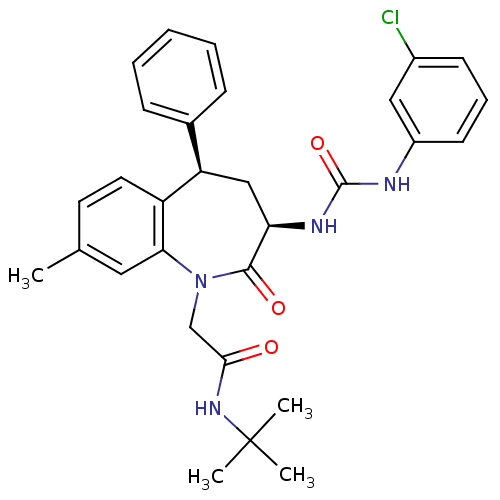 (CHEMBL291458 | N-tert-Butyl-2-{(3R,5R)-3-[3-(3-chl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50237317 (CHEMBL4098286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM213656 (US9278981, 248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50459091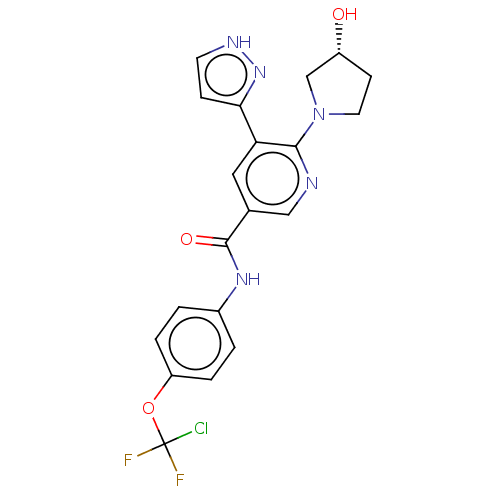 (ABL-001 | ABL001 | ABL001-NX | Asciminib | NVP-ABL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50090462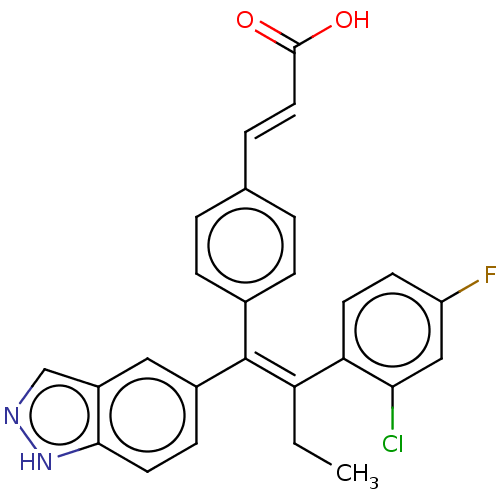 (CHEMBL3581693 | US20240043442, Example GDC-0810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481048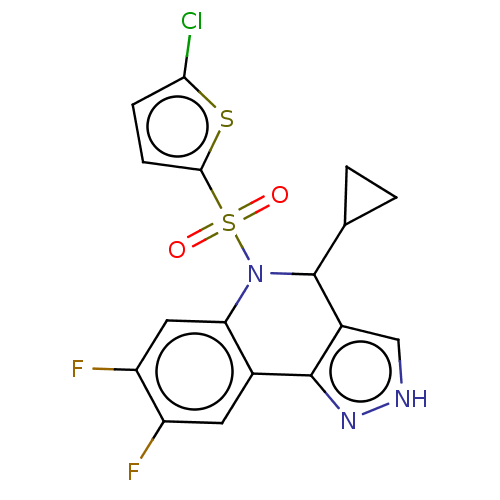 (CHEMBL584115) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481088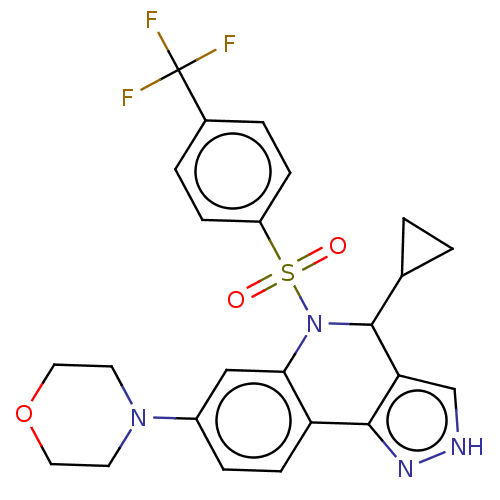 (CHEMBL568891) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM3183 (2-({2,6-dihydroxy-4-[({2-[(4-hydroxybenzene)amido]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | J Med Chem 39: 5215-27 (1996) Article DOI: 10.1021/jm960581w BindingDB Entry DOI: 10.7270/Q2G73BVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM3153 (2-{[2,6-dihydroxy-4-({[(1R,2R)-2-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein Kinase C eta | Bioorg Med Chem Lett 5: 2155-2160 (1995) Article DOI: 10.1016/0960-894X(95)00367-3 BindingDB Entry DOI: 10.7270/Q22N52RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50237332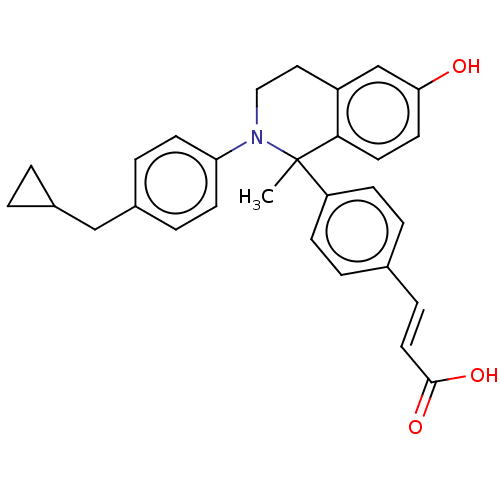 (CHEMBL4078937) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285014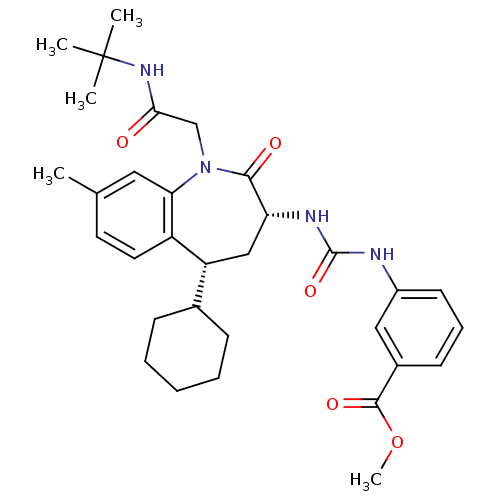 (3-{3-[(3R,5R)-1-(tert-Butylcarbamoyl-methyl)-5-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50237305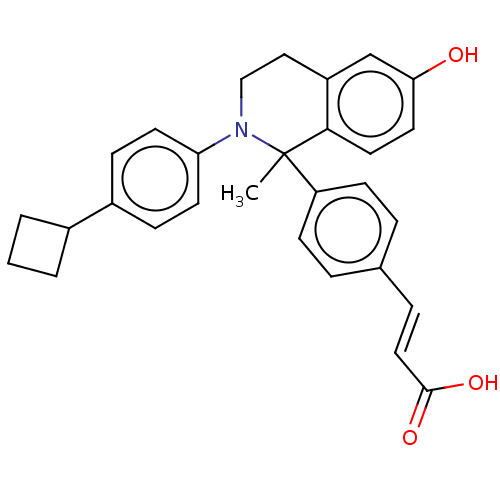 (CHEMBL4072611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... | J Med Chem 60: 2790-2818 (2017) Article DOI: 10.1021/acs.jmedchem.6b01468 BindingDB Entry DOI: 10.7270/Q2DB845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481065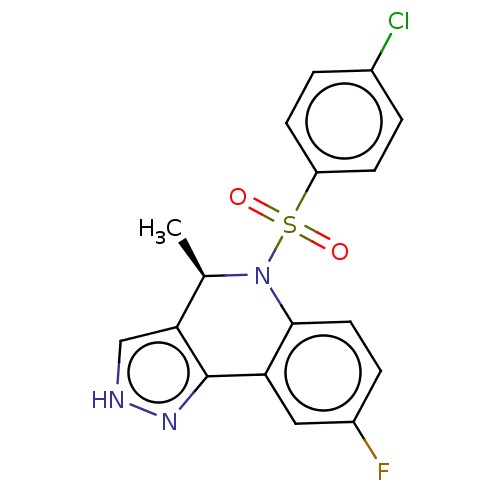 (CHEMBL571818) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481054 (CHEMBL583674) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481079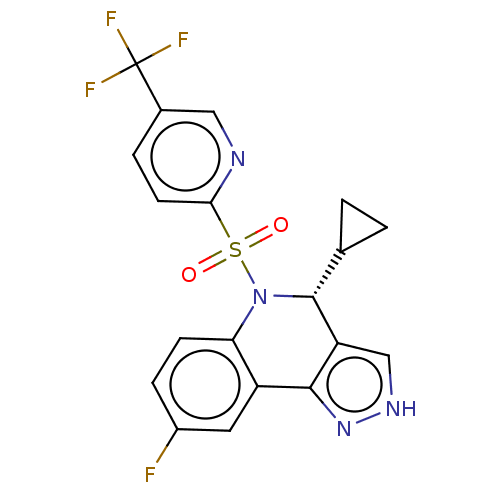 (CHEMBL585538) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50459090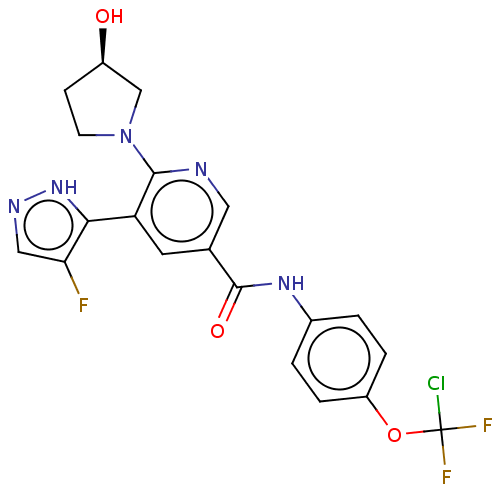 (CHEMBL4213152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16390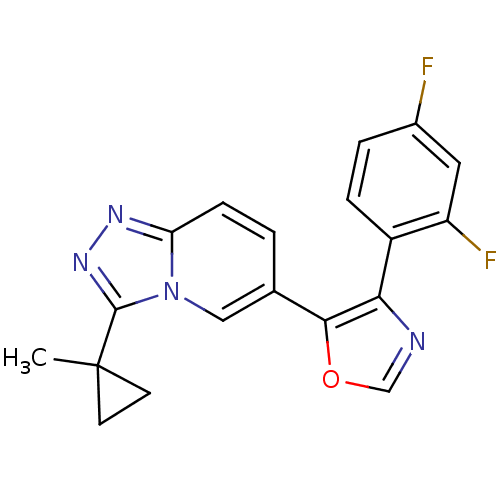 (4-(2,4-difluorophenyl)-5-[3-(1-methylcyclopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50285010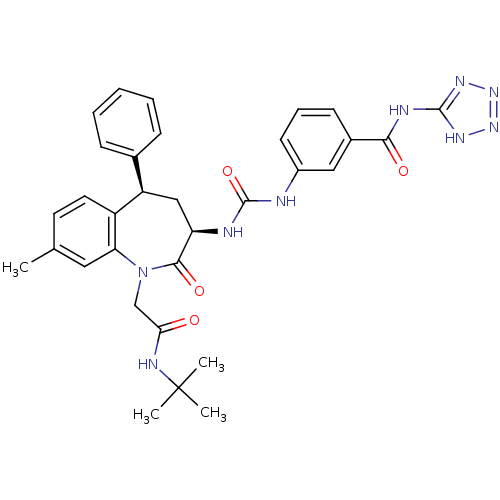 (3-{3-[(3R,5R)-1-(tert-Butylcarbamoyl-methyl)-8-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-B (CCK-B) receptor in guinea pig cortex | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50481067 (CHEMBL571387) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Gamma-secretase in human IMR-32 cells after 2 hrs by ELISA assay | Bioorg Med Chem Lett 19: 4920-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.092 BindingDB Entry DOI: 10.7270/Q2DR2ZBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1706 total ) | Next | Last >> |