

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371581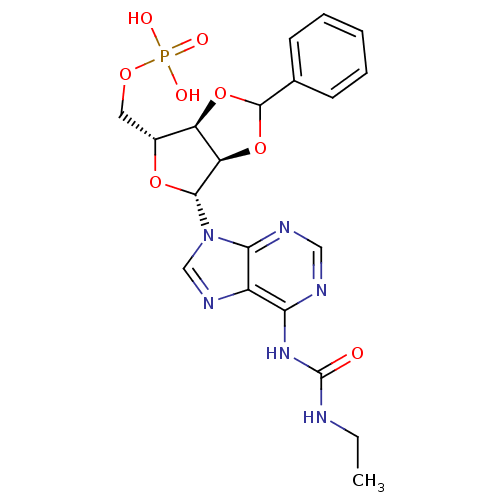 (CHEMBL1162179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371582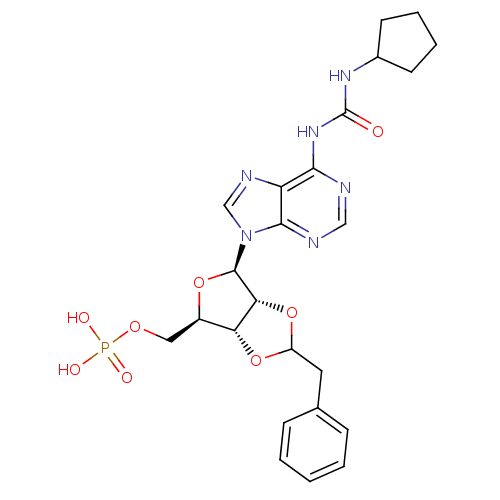 (CHEMBL1162182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371583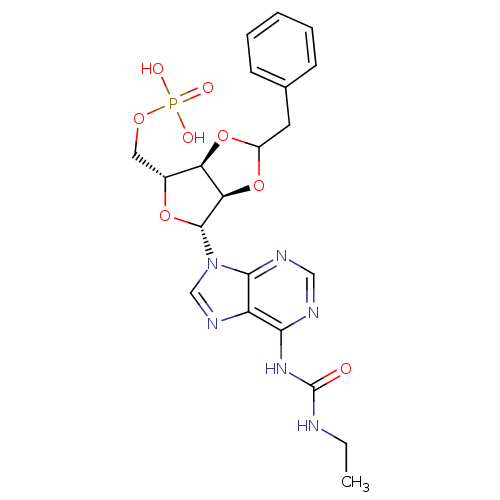 (CHEMBL1162184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371589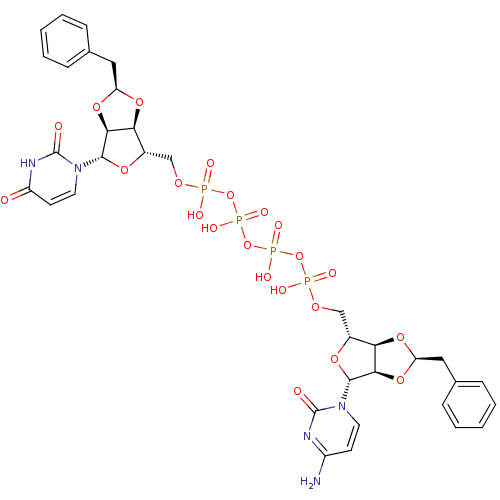 (CHEMBL1162196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371594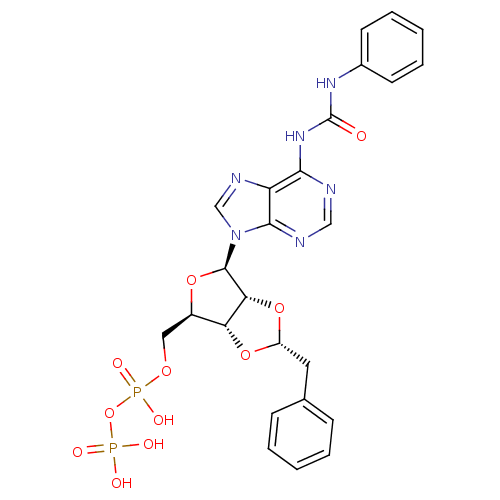 (CHEMBL1162161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371584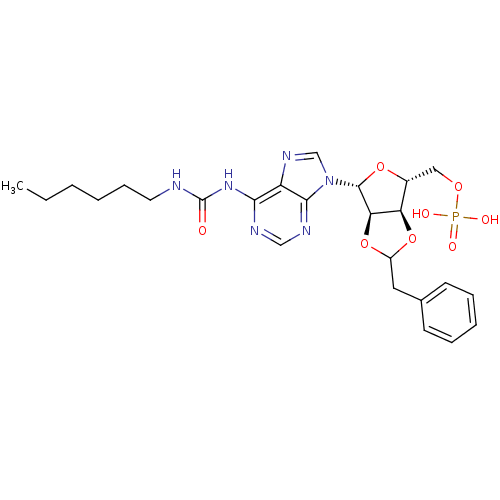 (CHEMBL1162185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371585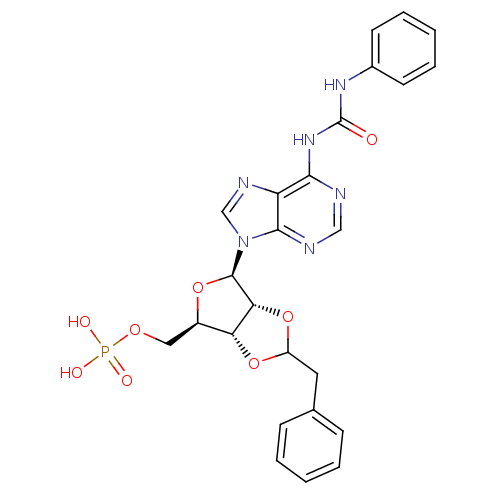 (CHEMBL1162188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371578 (CHEMBL1162172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371577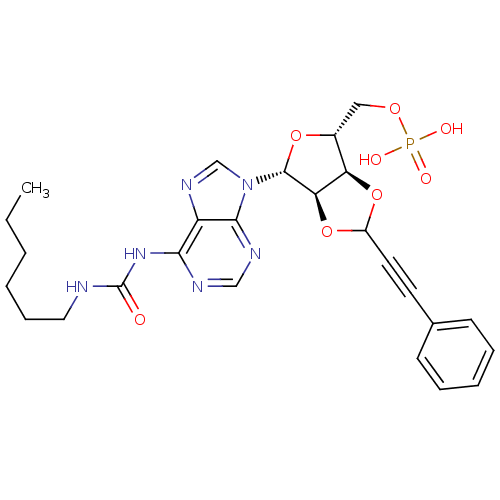 (CHEMBL1162173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371579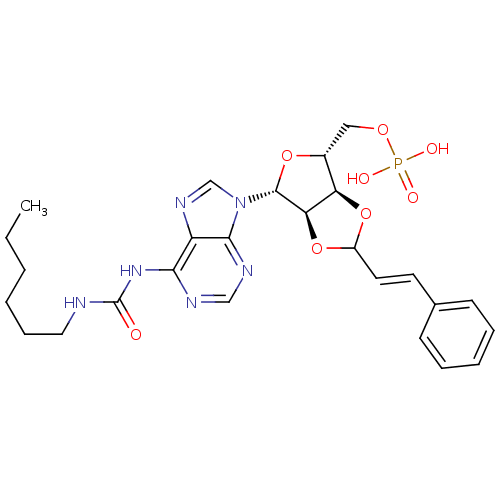 (CHEMBL1162171) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371576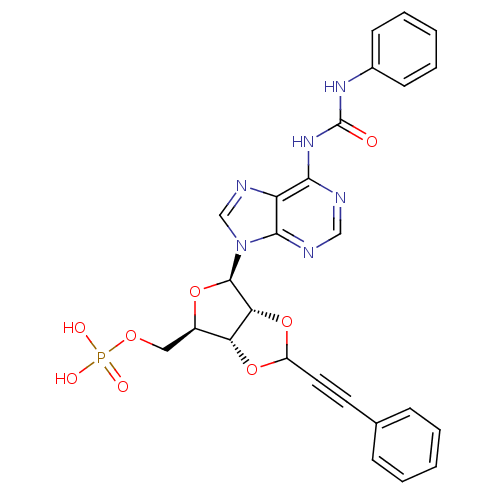 (CHEMBL1162164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371593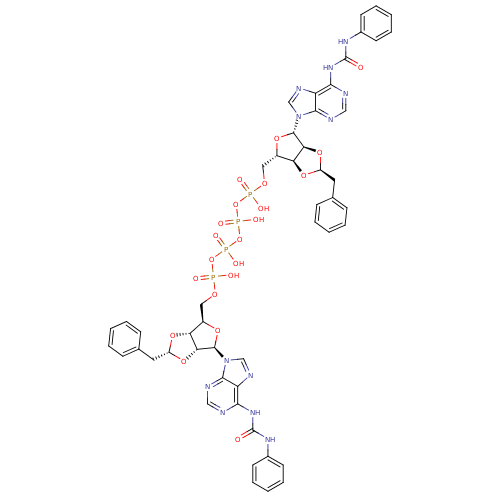 (CHEMBL1162159) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 522 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371603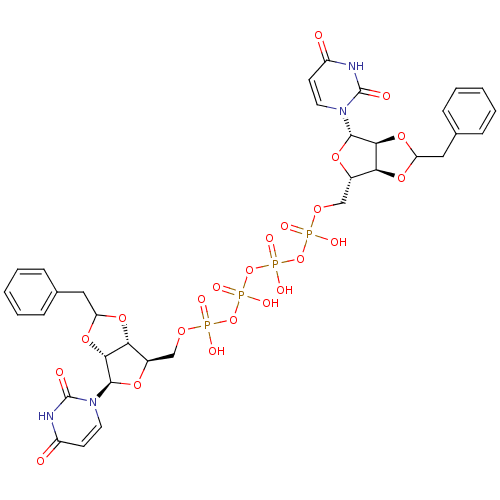 (CHEMBL1162165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371589 (CHEMBL1162196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371588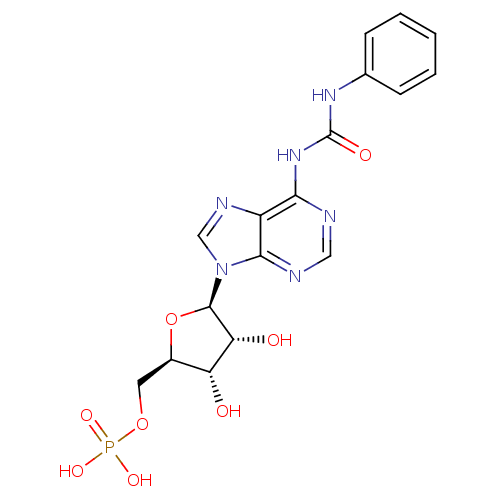 (CHEMBL1162195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371573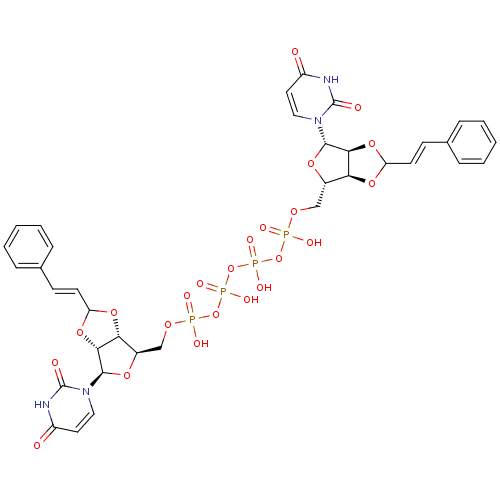 (CHEMBL1162158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371573 (CHEMBL1162158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371599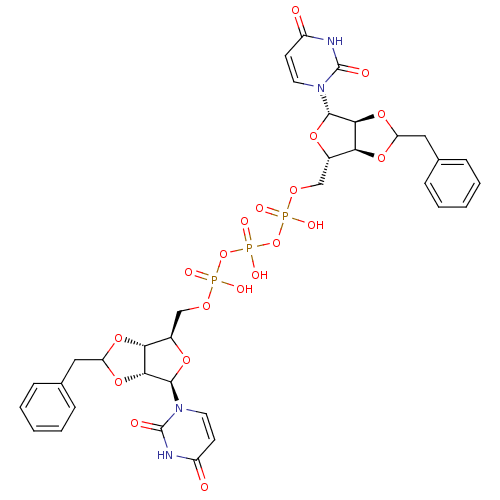 (CHEMBL1162169) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371598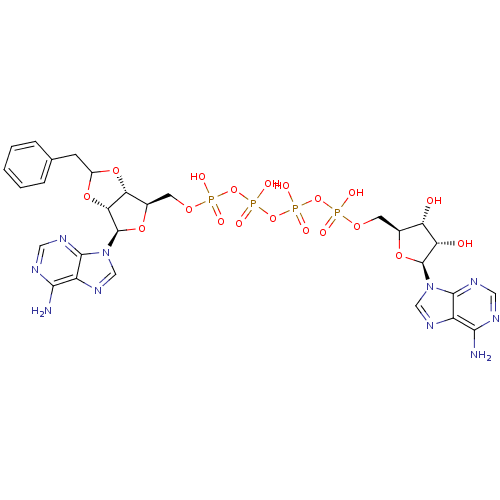 (CHEMBL1162170) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371571 (CHEMBL1162181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371570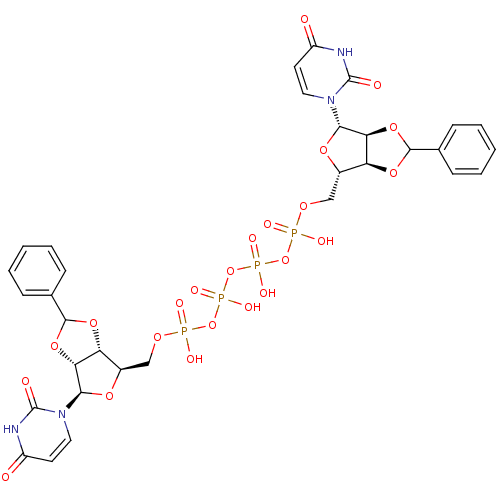 (CHEMBL1162162) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371574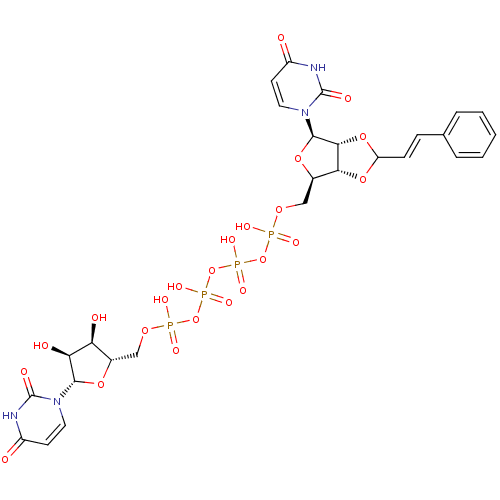 (CHEMBL1162157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371574 (CHEMBL1162157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371601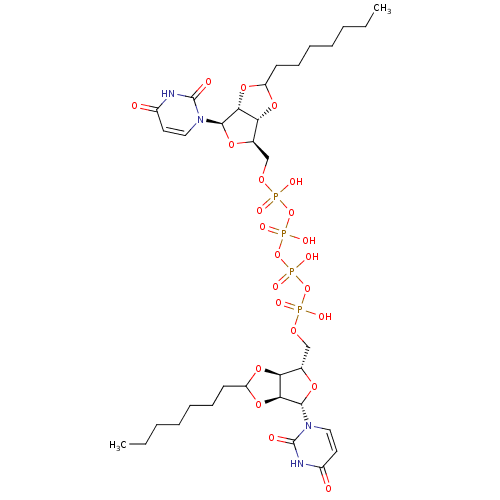 (CHEMBL1162189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371596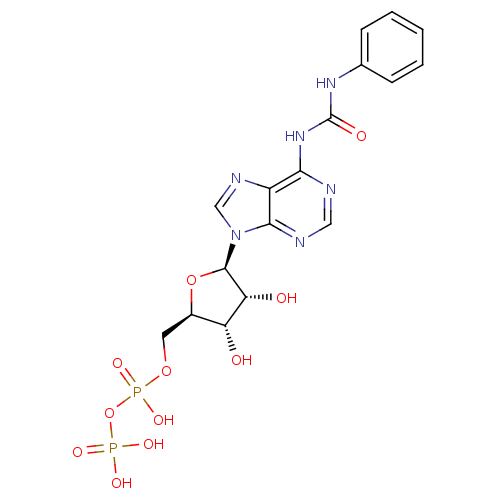 (CHEMBL1162163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371592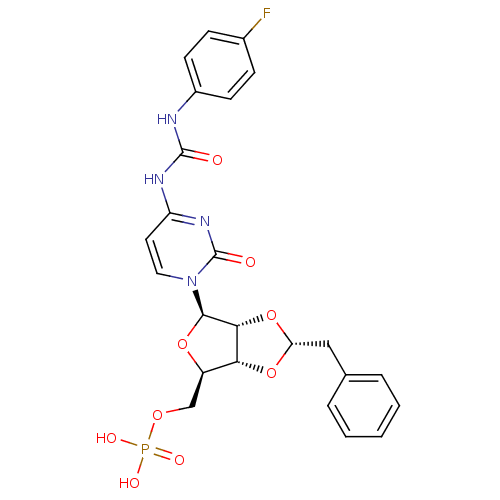 (CHEMBL1162205) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371597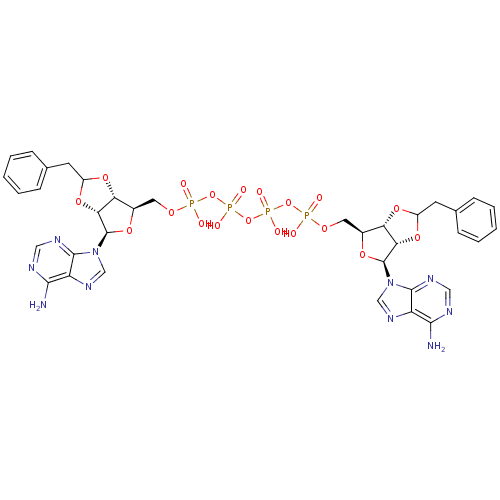 (CHEMBL1162167) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371590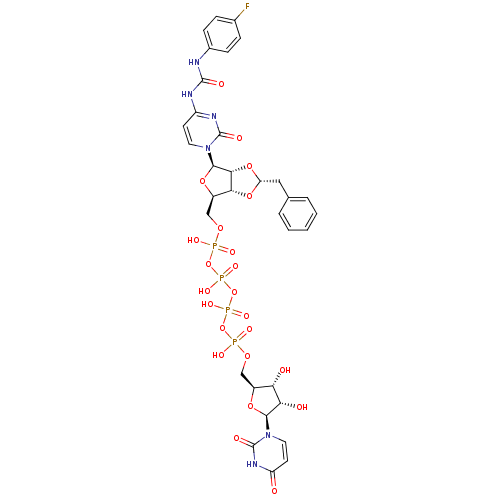 (CHEMBL1162197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371575 (CHEMBL1162166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371602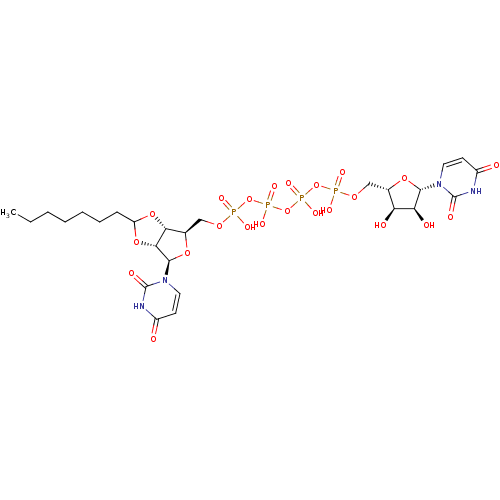 (CHEMBL1162194) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371595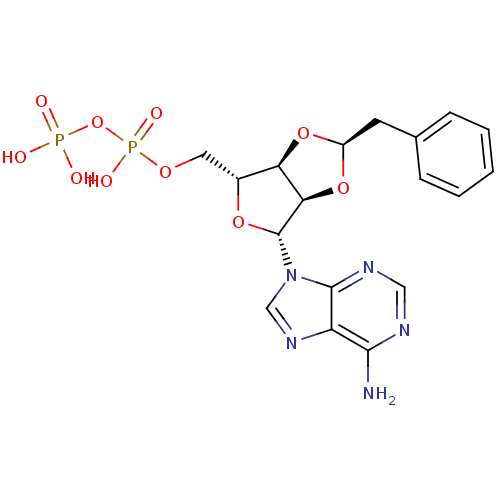 (CHEMBL1162160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371591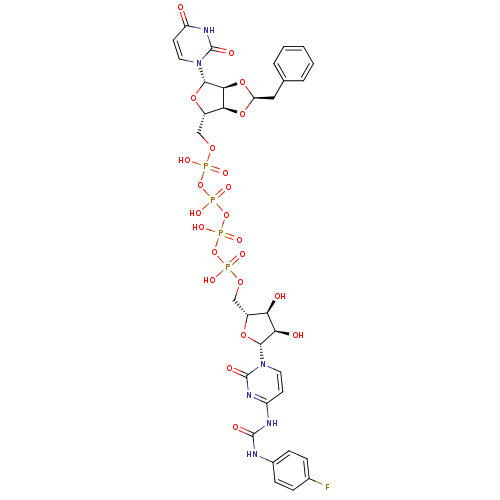 (CHEMBL1162200) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371604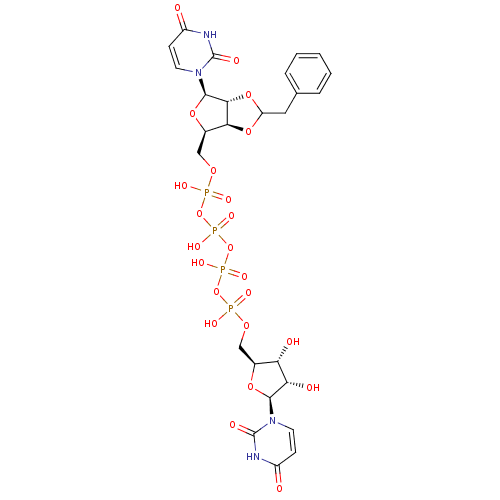 (CHEMBL1162168) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371600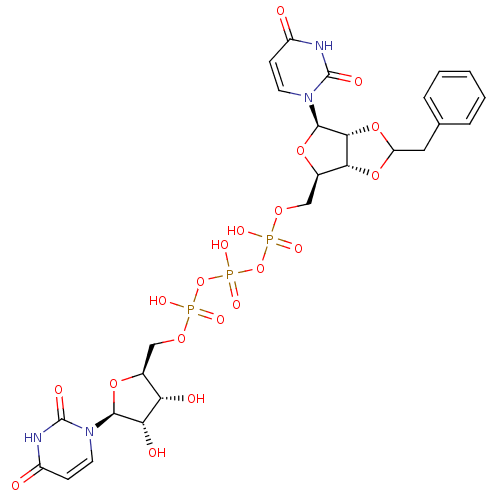 (CHEMBL1162174) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371587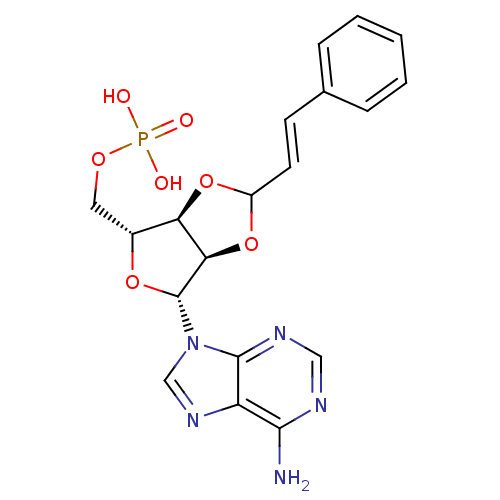 (CHEMBL1162190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371586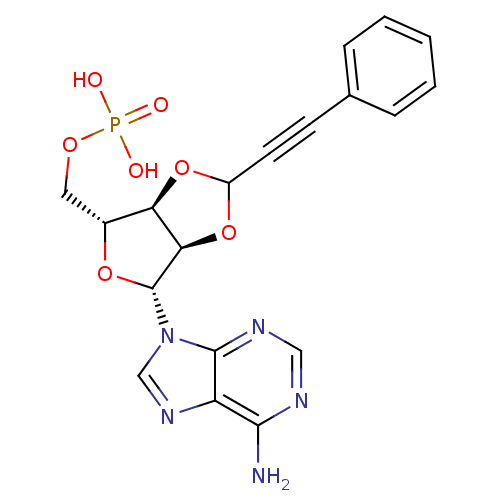 (CHEMBL1162187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50371569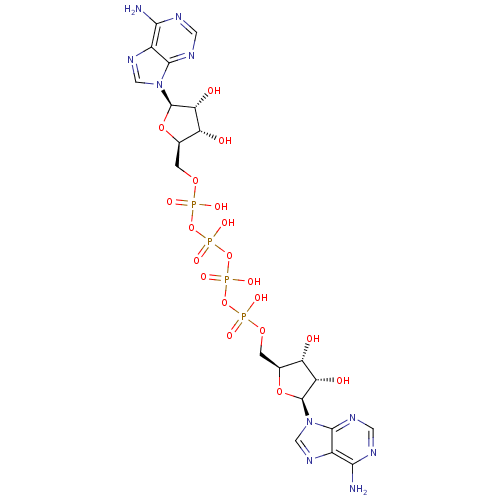 (CHEMBL1162201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 426 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist at human recombinant P2Y1 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371570 (CHEMBL1162162) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371571 (CHEMBL1162181) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371572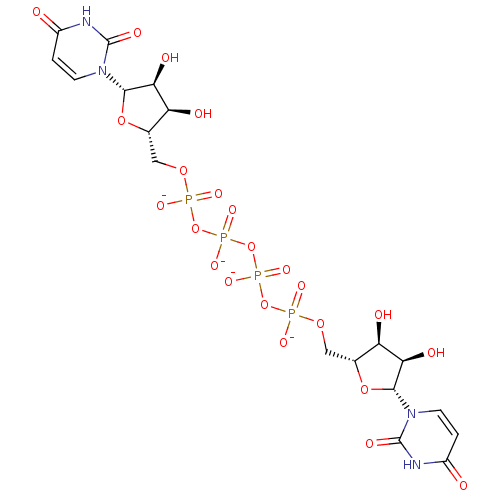 (CHEMBL406266) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371573 (CHEMBL1162158) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371574 (CHEMBL1162157) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371574 (CHEMBL1162157) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371575 (CHEMBL1162166) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 192 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371605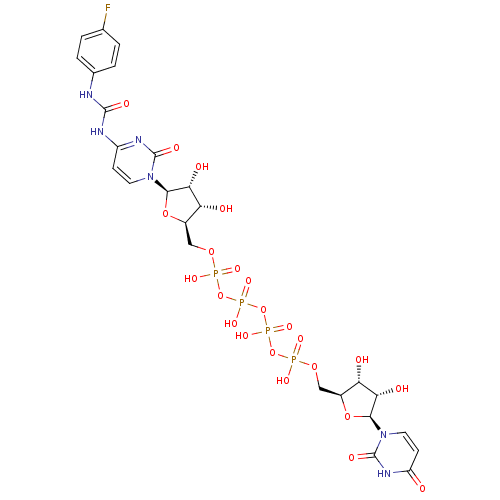 (CHEMBL1162203) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371594 (CHEMBL1162161) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371595 (CHEMBL1162160) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50371596 (CHEMBL1162163) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at P2Y2 receptor expressed in human 1321 cells by calcium mobilization assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |