

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029903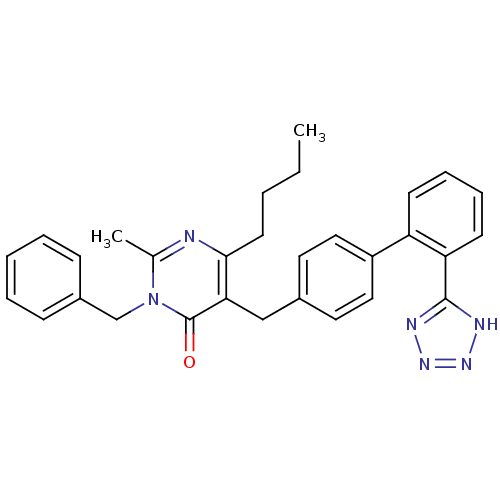 (3-Benzyl-6-butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029911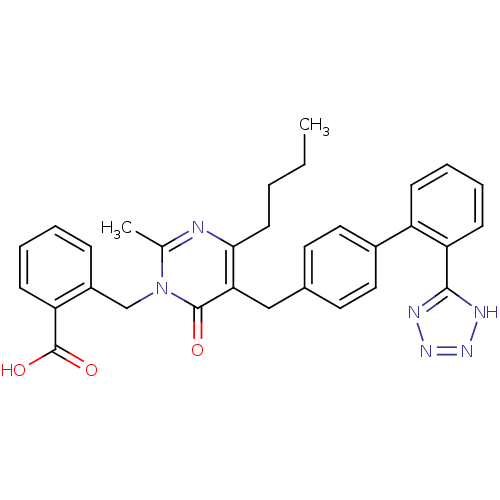 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029896 (4'-(2-Butyl-6-oxo-6H-pyrimidin-1-ylmethyl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241491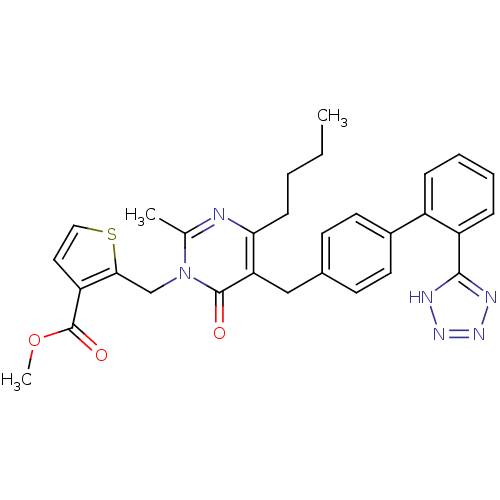 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241501 (6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029892 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029901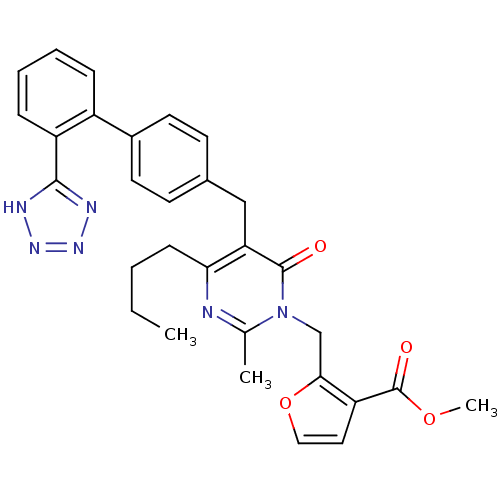 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029897 (4'-[4-Butyl-1-(4-carboxy-benzyl)-6-oxo-1,6-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029902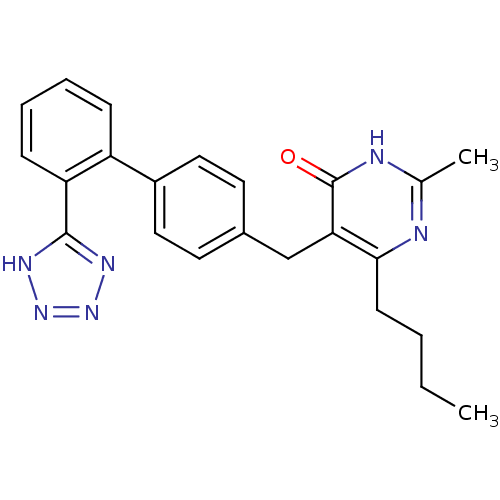 (6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029904 (4'-[4-Butyl-1-(2-carboxy-benzyl)-2-methyl-6-oxo-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029894 (4'-(4-Butyl-2-methyl-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029895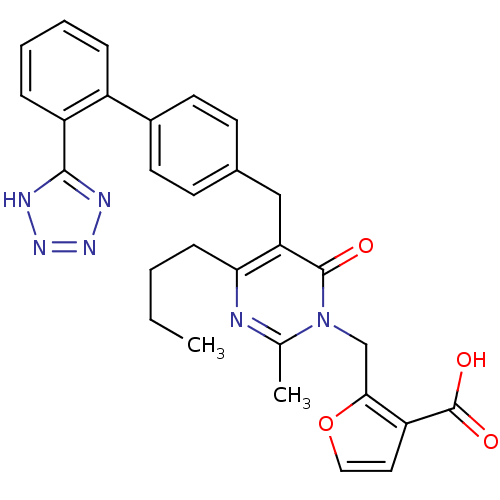 (2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029905 (4'-(2-Butyl-4-methyl-6-oxo-6H-pyrimidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029899 (3-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029908 (4'-(4-Butyl-6-oxo-1-thiophen-2-ylmethyl-1,6-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029893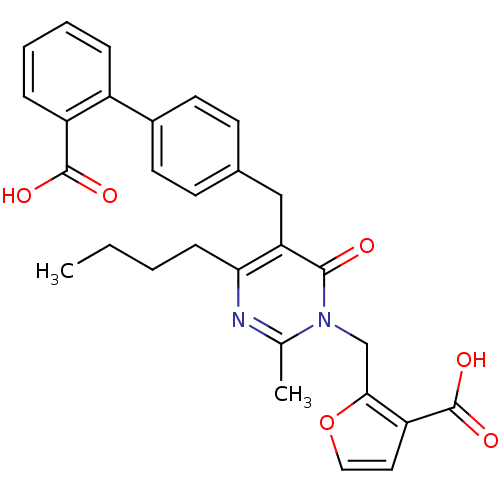 (2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029906 (2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029909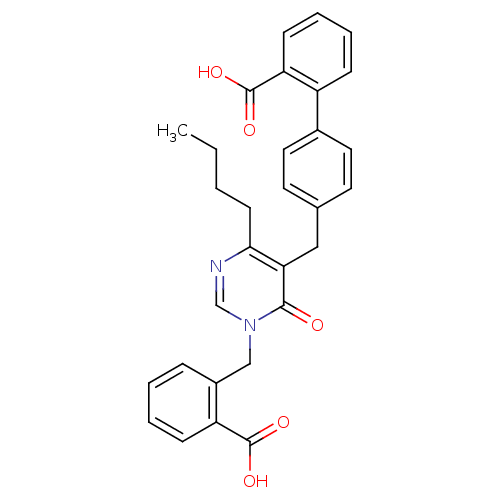 (4'-[4-Butyl-1-(2-carboxy-benzyl)-6-oxo-1,6-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029907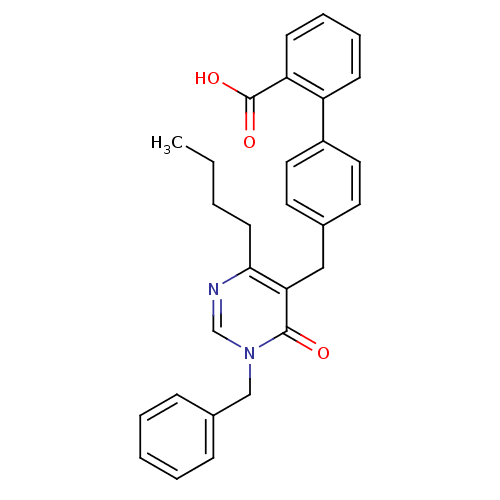 (4'-(1-Benzyl-4-butyl-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50029910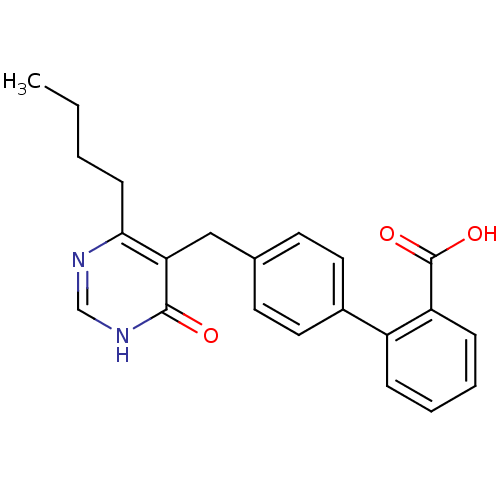 (4'-(4-Butyl-6-oxo-1,6-dihydro-pyrimidin-5-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive | J Med Chem 38: 4806-20 (1996) BindingDB Entry DOI: 10.7270/Q2JQ101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13211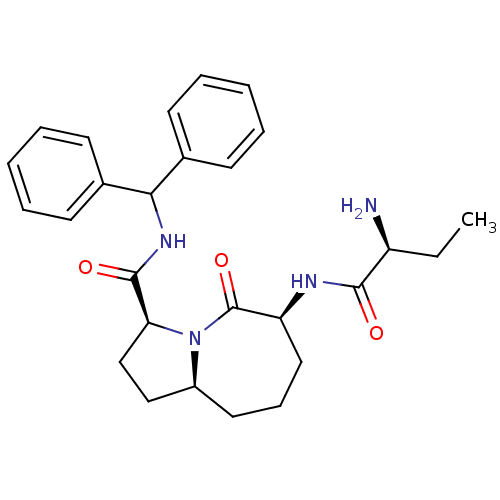 ((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.1 | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13212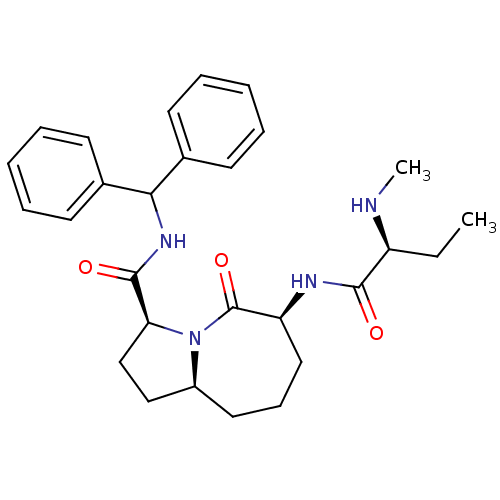 ((3S,6S,9aS)-N-(diphenylmethyl)-6-[(2S)-2-(methylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | -40.9 | 530 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50003399 (4'-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470054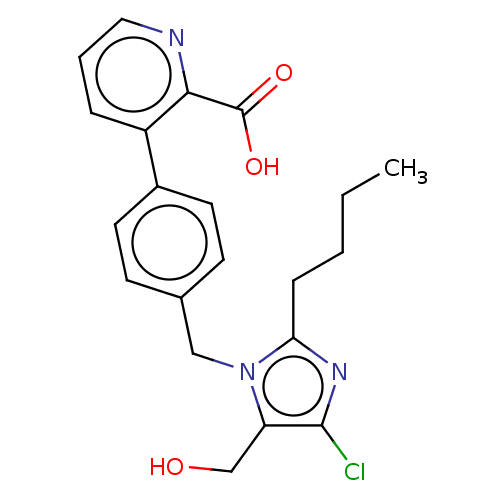 (CHEMBL129225) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470049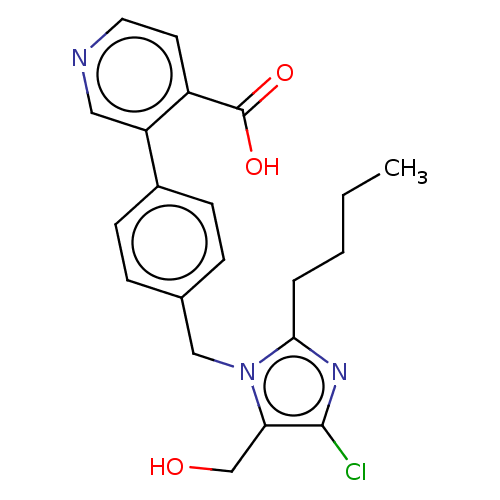 (CHEMBL340682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470051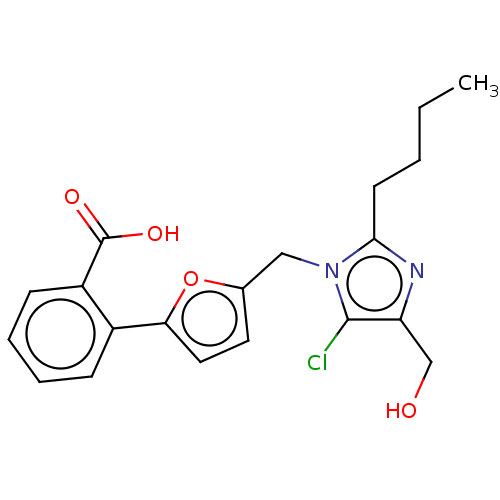 (CHEMBL339044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470050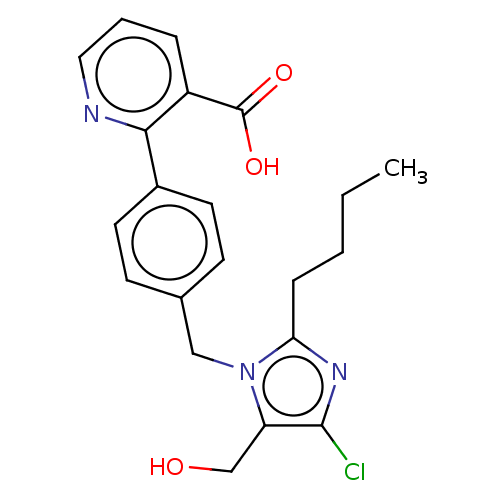 (CHEMBL129170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470052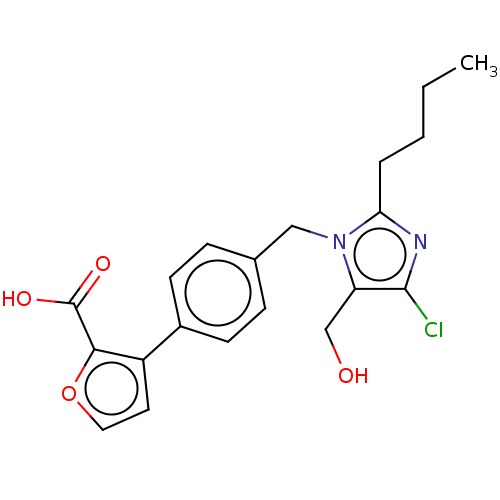 (CHEMBL127108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470055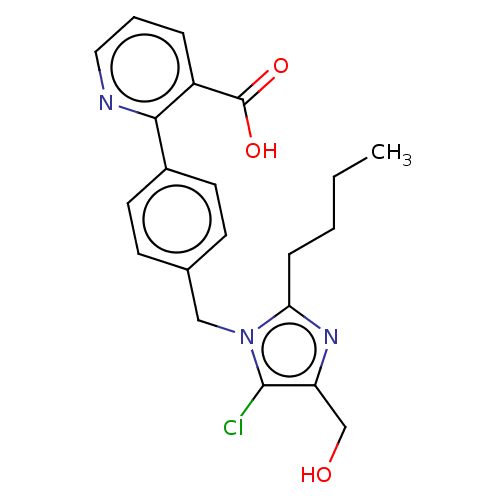 (CHEMBL125790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470047 (CHEMBL50135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470058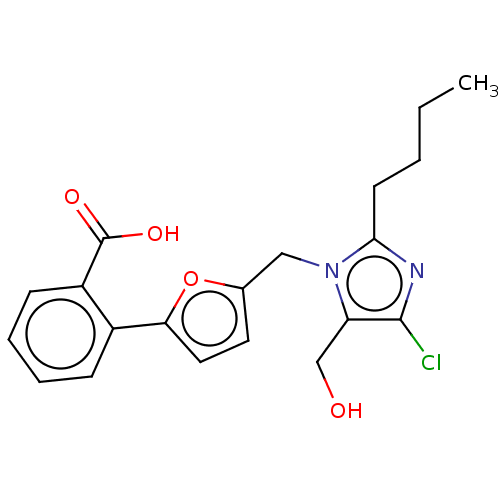 (CHEMBL129005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470048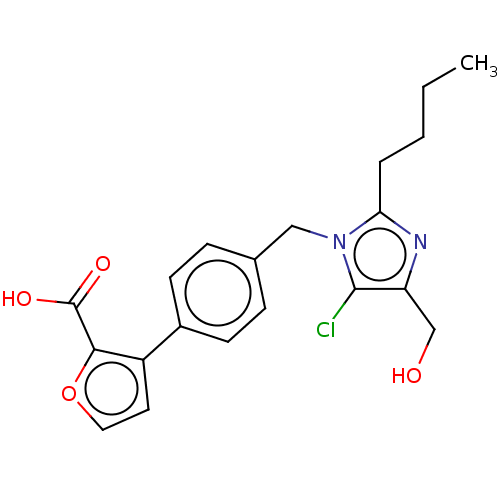 (CHEMBL128291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470057 (CHEMBL129336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470059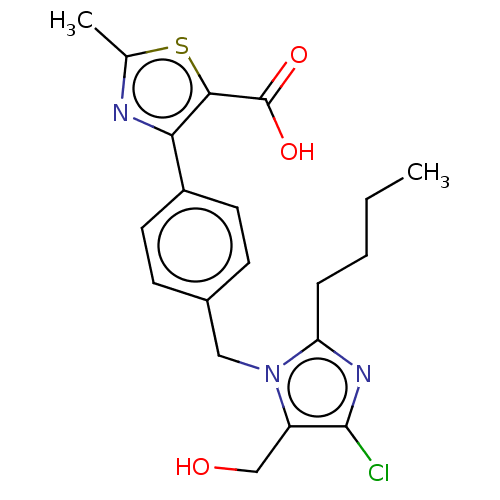 (CHEMBL338840) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470060 (CHEMBL125789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470053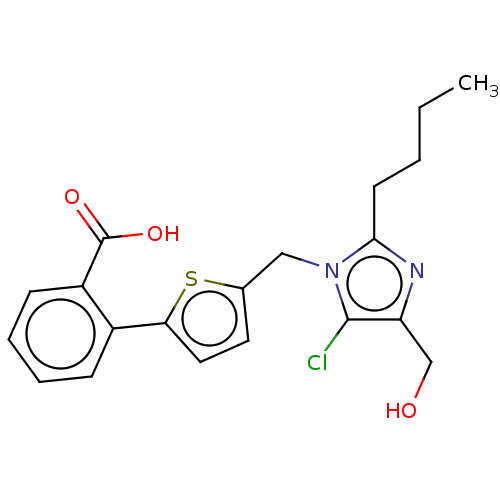 (CHEMBL125621) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470056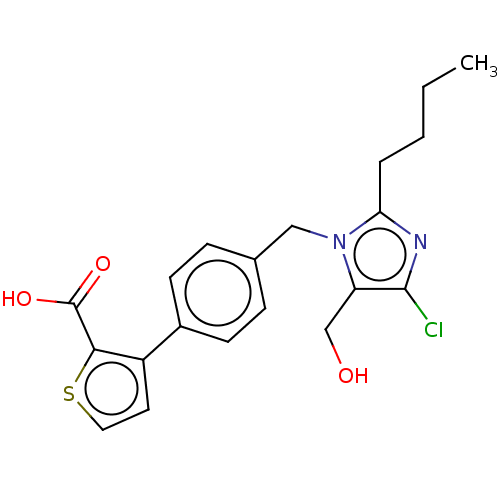 (CHEMBL129006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50237601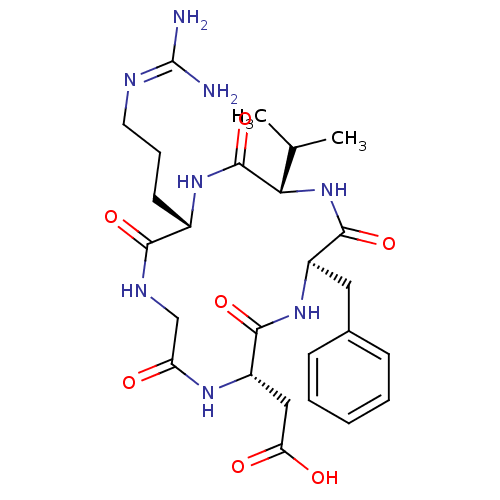 (CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50235980 (2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50399923 (CHEMBL2180979) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis | J Med Chem 55: 10460-74 (2012) Article DOI: 10.1021/jm301058f BindingDB Entry DOI: 10.7270/Q2WD41Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50399922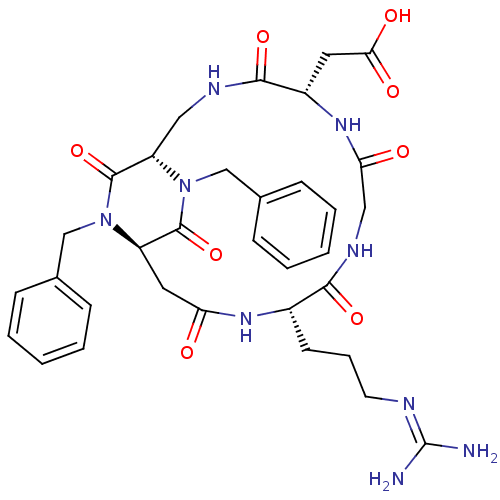 (CHEMBL2180974) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis | J Med Chem 55: 10460-74 (2012) Article DOI: 10.1021/jm301058f BindingDB Entry DOI: 10.7270/Q2WD41Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50399922 (CHEMBL2180974) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis | J Med Chem 55: 10460-74 (2012) Article DOI: 10.1021/jm301058f BindingDB Entry DOI: 10.7270/Q2WD41Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/beta-2 (Homo sapiens (Human)) | BDBM50550223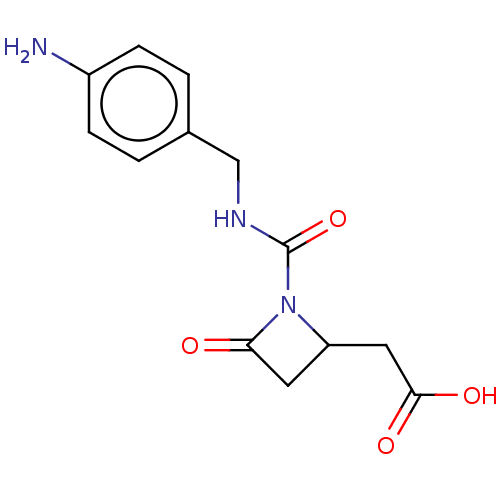 (CHEMBL4794300) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at alphaLbeta2 in human Jurkat E6.1 cells assessed as reduction in cell adhesion to ICAM1 pre-incubated for 30 mins before ICAM1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50177887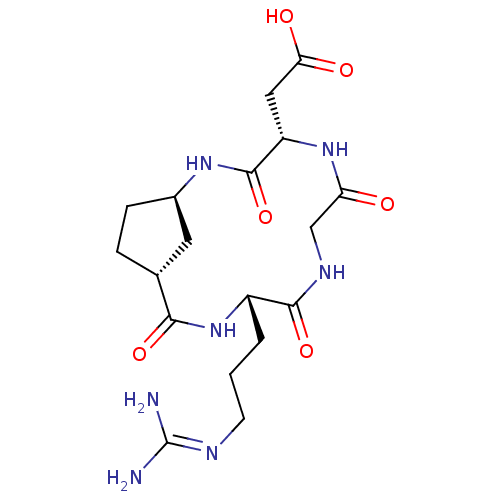 (CHEMBL269913 | CHEMBL556402 | c-[-Arg-Gly-Asp-Acpc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Parma Curated by ChEMBL | Assay Description Displacement of [125I]-echistatin from alpha-v-beta-5 integrin receptor | J Med Chem 48: 7675-87 (2005) Article DOI: 10.1021/jm050698x BindingDB Entry DOI: 10.7270/Q2GX4CBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50439683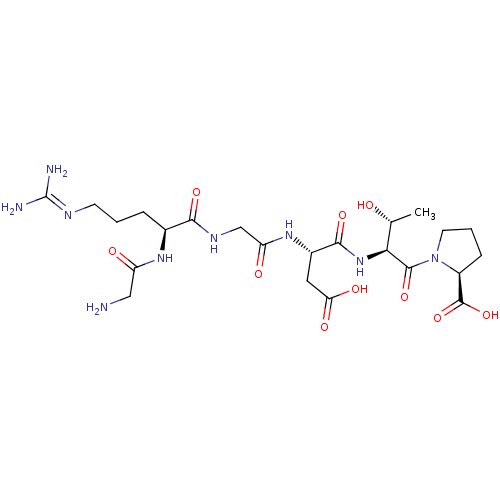 (CHEMBL2418221) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin-mediated human SK-MEL-24 cell adhesion to fibronectin incubated for 30 mins before plating measured after 1 hr | Eur J Med Chem 66: 258-68 (2013) Article DOI: 10.1016/j.ejmech.2013.05.050 BindingDB Entry DOI: 10.7270/Q21Z45VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-5/beta-1 (Homo sapiens (Human)) | BDBM50439683 (CHEMBL2418221) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at alpha5beta1 in human K562 cells assessed as reduction in cell adhesion to fibronectin pre-incubated for 30 mins before fibrone... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/beta-2 (Homo sapiens (Human)) | BDBM50074661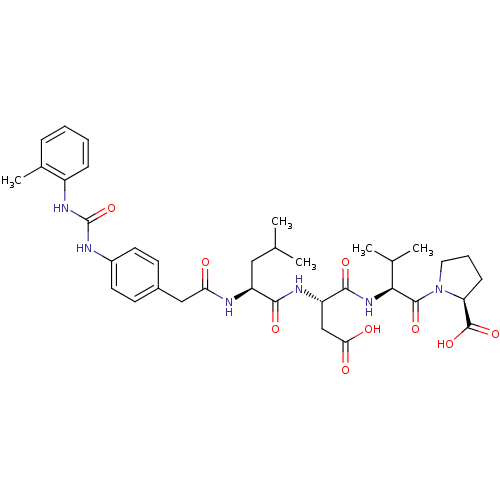 ((S)-1-{(S)-2-[(S)-3-Carboxy-2-((S)-4-methyl-2-{2-[...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at alphaLbeta2 in human Jurkat E6.1 cells assessed as reduction in cell adhesion to ICAM1 pre-incubated for 30 mins before ICAM1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50399919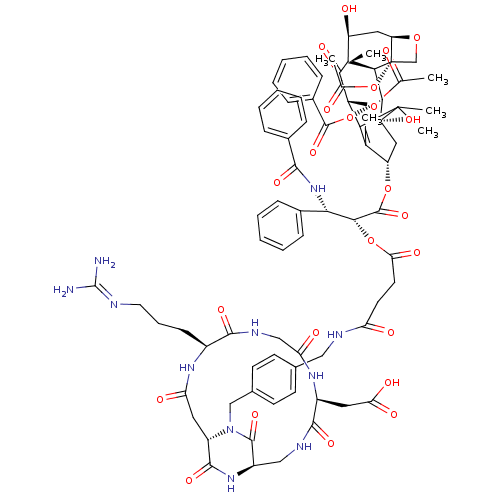 (CHEMBL2180983) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis | J Med Chem 55: 10460-74 (2012) Article DOI: 10.1021/jm301058f BindingDB Entry DOI: 10.7270/Q2WD41Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50177879 (CHEMBL200182 | ST-1646) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysis | J Med Chem 55: 10460-74 (2012) Article DOI: 10.1021/jm301058f BindingDB Entry DOI: 10.7270/Q2WD41Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50505781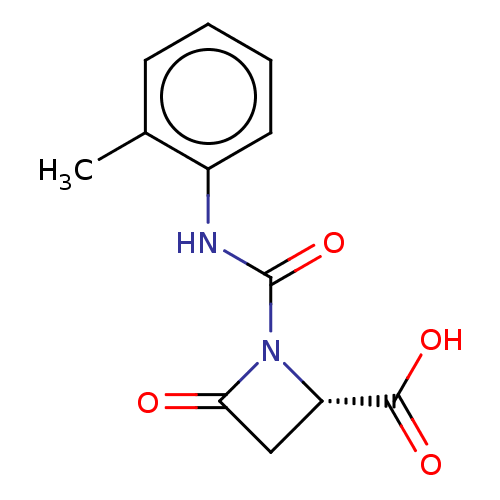 (CHEMBL4461668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fibronectin binding to alpha4bbeta1 isolated from human Jurkat E6.1 cells incubated for 1 hr by scintillation-proximity assay based com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 542 total ) | Next | Last >> |