 Found 710 hits with Last Name = 'bentley' and Initial = 'j'
Found 710 hits with Last Name = 'bentley' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417035

(CHEMBL1257993)Show SMILES Fc1ccccc1-c1cnc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)cn1 |r,wU:13.13,wD:16.22,(15.52,-7.53,;14.76,-6.18,;13.23,-6.17,;12.47,-4.82,;13.26,-3.49,;14.8,-3.52,;15.55,-4.86,;17.09,-4.88,;17.88,-3.56,;19.42,-3.58,;20.17,-4.93,;21.71,-4.96,;22.45,-6.3,;23.99,-6.33,;24.79,-5.01,;26.33,-5.03,;27.07,-6.38,;28.2,-5.34,;29.54,-6.12,;29.22,-7.62,;30.25,-8.77,;27.69,-7.78,;30.95,-5.5,;32.19,-6.4,;33.6,-5.78,;33.76,-4.25,;32.51,-3.34,;31.11,-3.97,;26.27,-7.7,;24.74,-7.67,;19.38,-6.25,;17.85,-6.23,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-14-29-21(15-27-20)28-13-17-8-10-24(11-9-17)16-30(23(31)32-24)22-7-3-4-12-26-22/h1-7,12,14-15,17H,8-11,13,16H2,(H,28,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417033
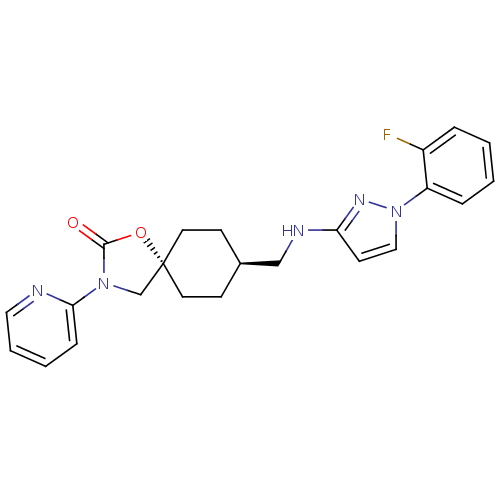
(CHEMBL1258111)Show SMILES Fc1ccccc1-n1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)n1 |r,wU:13.13,wD:16.22,(15.91,-.57,;14.51,.08,;13.26,-.81,;11.85,-.16,;11.72,1.37,;12.98,2.26,;14.38,1.6,;15.63,2.49,;15.65,4.03,;17.12,4.48,;18.01,3.23,;19.55,3.2,;20.3,1.86,;21.84,1.83,;22.63,3.15,;24.17,3.13,;24.91,1.78,;26.05,2.82,;27.39,2.04,;27.06,.54,;28.09,-.61,;25.53,.38,;28.79,2.66,;30.04,1.75,;31.44,2.38,;31.61,3.91,;30.35,4.82,;28.95,4.19,;24.12,.46,;22.59,.49,;17.08,1.99,)| Show InChI InChI=1S/C23H24FN5O2/c24-18-5-1-2-6-19(18)29-14-10-20(27-29)26-15-17-8-11-23(12-9-17)16-28(22(30)31-23)21-7-3-4-13-25-21/h1-7,10,13-14,17H,8-9,11-12,15-16H2,(H,26,27)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417056
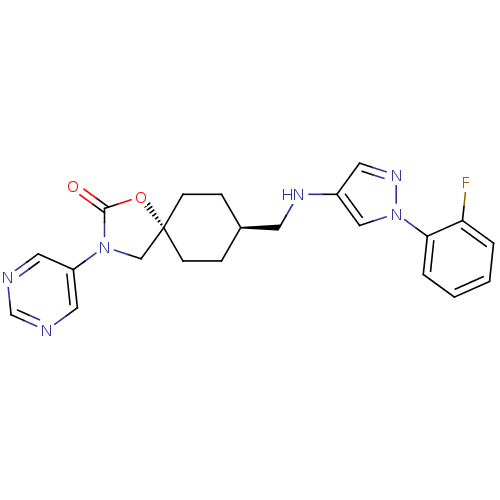
(CHEMBL1258225)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cncnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-5.79,-9.02,;-7.19,-8.37,;-8.44,-9.26,;-9.85,-8.61,;-9.98,-7.07,;-8.73,-6.19,;-7.33,-6.84,;-6.08,-5.96,;-4.62,-6.46,;-3.69,-5.22,;-2.15,-5.25,;-1.4,-6.59,;.14,-6.62,;.94,-5.3,;2.48,-5.31,;3.21,-6.67,;4.35,-5.63,;5.69,-6.41,;5.37,-7.91,;6.4,-9.06,;3.84,-8.08,;7.1,-5.79,;8.34,-6.69,;9.75,-6.07,;9.91,-4.54,;8.66,-3.63,;7.26,-4.26,;2.42,-7.99,;.89,-7.96,;-4.58,-3.96,;-6.05,-4.42,)| Show InChI InChI=1S/C22H23FN6O2/c23-19-3-1-2-4-20(19)29-13-17(10-27-29)26-9-16-5-7-22(8-6-16)14-28(21(30)31-22)18-11-24-15-25-12-18/h1-4,10-13,15-16,26H,5-9,14H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417051

(CHEMBL1258787)Show SMILES Cc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(17.45,-6.84,;16.7,-5.5,;15.16,-5.48,;14.41,-4.13,;15.2,-2.81,;16.74,-2.83,;17.48,-4.18,;19.03,-4.2,;19.78,-5.55,;21.32,-5.57,;22.1,-4.25,;23.64,-4.27,;24.39,-5.62,;25.93,-5.64,;26.72,-4.32,;28.26,-4.34,;29,-5.69,;30.14,-4.66,;31.48,-5.43,;31.16,-6.94,;32.19,-8.08,;29.62,-7.1,;32.88,-4.81,;34.13,-5.72,;35.54,-5.1,;35.7,-3.56,;34.44,-2.66,;33.04,-3.29,;28.21,-7.01,;26.68,-6.98,;21.36,-2.9,;19.82,-2.87,)| Show InChI InChI=1S/C24H26N6O2/c1-17-5-2-3-6-19(17)20-8-9-21(28-27-20)25-15-18-10-12-24(13-11-18)16-30(23(31)32-24)22-7-4-14-26-29-22/h2-9,14,18H,10-13,15-16H2,1H3,(H,25,28)/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417036

(CHEMBL1257992)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nn1 |r,wU:13.13,wD:16.22,(-7.93,-14.34,;-8.69,-13,;-10.22,-12.98,;-10.98,-11.63,;-10.19,-10.31,;-8.65,-10.33,;-7.9,-11.68,;-6.36,-11.69,;-5.6,-13.04,;-4.07,-13.06,;-3.28,-11.75,;-1.74,-11.77,;-1,-13.12,;.54,-13.14,;1.34,-11.82,;2.88,-11.84,;3.62,-13.19,;4.75,-12.16,;6.09,-12.93,;5.77,-14.44,;6.8,-15.58,;4.24,-14.6,;7.5,-12.31,;8.74,-13.22,;10.15,-12.59,;10.31,-11.06,;9.06,-10.15,;7.66,-10.78,;2.82,-14.51,;1.29,-14.48,;-4.03,-10.4,;-5.57,-10.37,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-8-9-21(29-28-20)27-15-17-10-12-24(13-11-17)16-30(23(31)32-24)22-7-3-4-14-26-22/h1-9,14,17H,10-13,15-16H2,(H,27,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417045

(CHEMBL1258341)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cnccn3)CC2)cn1 |r,wU:12.12,wD:15.21,(-6.54,-17.19,;-7.94,-16.54,;-9.19,-17.43,;-10.59,-16.78,;-10.73,-15.24,;-9.48,-14.36,;-8.08,-15.01,;-6.82,-14.13,;-5.37,-14.63,;-4.44,-13.39,;-2.9,-13.41,;-2.15,-14.76,;-.61,-14.79,;.19,-13.47,;1.73,-13.48,;2.46,-14.84,;3.6,-13.8,;4.94,-14.58,;4.62,-16.08,;5.64,-17.23,;3.09,-16.24,;6.35,-13.96,;7.58,-14.86,;8.99,-14.24,;9.15,-12.71,;7.91,-11.8,;6.5,-12.43,;1.67,-16.16,;.14,-16.13,;-5.33,-12.13,;-6.8,-12.59,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-3-1-2-4-19(18)29-14-17(12-27-29)26-11-16-5-7-22(8-6-16)15-28(21(30)31-22)20-13-24-9-10-25-20/h1-4,9-10,12-14,16,26H,5-8,11,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417032

(CHEMBL1258110)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1nc(cs1)-c1ccccn1)CC2 |r,wU:14.16,wD:3.2,(5.76,.15,;4.73,1.3,;3.2,1.14,;2.57,2.54,;3.71,3.58,;5.05,2.8,;6.45,3.43,;7.7,2.52,;9.11,3.14,;9.27,4.67,;8.02,5.58,;6.61,4.95,;1.83,3.89,;.29,3.91,;-.5,2.59,;-2.04,2.62,;-2.79,3.96,;-4.33,3.99,;-5.25,2.75,;-6.71,3.25,;-6.69,4.79,;-5.21,5.24,;-7.96,2.37,;-7.82,.84,;-9.08,-.05,;-10.48,.6,;-10.62,2.14,;-9.36,3.02,;.25,1.25,;1.78,1.22,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-6-2-4-12-24-19)15-22(29-21)9-7-16(8-10-22)13-25-20-26-18(14-30-20)17-5-1-3-11-23-17/h1-6,11-12,14,16H,7-10,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417046

(CHEMBL1258453)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)cn1 |r,wU:12.12,wD:15.21,(16.1,-16.72,;14.7,-16.07,;13.45,-16.97,;12.04,-16.31,;11.91,-14.78,;13.16,-13.9,;14.56,-14.55,;15.81,-13.67,;17.27,-14.16,;18.2,-12.92,;19.74,-12.95,;20.49,-14.3,;22.03,-14.32,;22.82,-13,;24.36,-13.02,;25.1,-14.37,;26.24,-13.33,;27.58,-14.11,;27.26,-15.62,;28.28,-16.76,;25.73,-15.78,;28.99,-13.49,;30.23,-14.4,;31.63,-13.78,;31.8,-12.24,;30.55,-11.33,;29.15,-11.97,;24.31,-15.69,;22.78,-15.66,;17.31,-11.67,;15.84,-12.13,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-4-1-2-5-19(18)29-14-17(13-26-29)24-12-16-7-9-22(10-8-16)15-28(21(30)31-22)20-6-3-11-25-27-20/h1-6,11,13-14,16,24H,7-10,12,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417050

(CHEMBL1258674)Show SMILES Fc1cc(F)cc(c1)-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:14.14,wD:17.23,(-9.07,-1.62,;-8.32,-2.96,;-9.11,-4.29,;-8.36,-5.64,;-9.14,-6.96,;-6.82,-5.65,;-6.04,-4.34,;-6.78,-2.99,;-4.49,-4.35,;-3.74,-5.7,;-2.2,-5.72,;-1.42,-4.4,;.12,-4.43,;.87,-5.77,;2.41,-5.8,;3.2,-4.48,;4.74,-4.5,;5.48,-5.85,;6.62,-4.81,;7.96,-5.59,;7.64,-7.09,;8.67,-8.24,;6.1,-7.25,;9.36,-4.97,;10.61,-5.88,;12.02,-5.25,;12.18,-3.72,;10.92,-2.81,;9.52,-3.44,;4.69,-7.17,;3.16,-7.14,;-2.16,-3.06,;-3.7,-3.03,)| Show InChI InChI=1S/C23H22F2N6O2/c24-17-10-16(11-18(25)12-17)19-3-4-20(29-28-19)26-13-15-5-7-23(8-6-15)14-31(22(32)33-23)21-2-1-9-27-30-21/h1-4,9-12,15H,5-8,13-14H2,(H,26,29)/t15-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417042

(CHEMBL1257636)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-7.01,-.66,;-8.41,-.01,;-9.65,-.9,;-11.06,-.25,;-11.21,1.29,;-9.94,2.17,;-8.54,1.52,;-7.29,2.4,;-5.83,1.9,;-4.9,3.14,;-3.36,3.12,;-2.62,1.77,;-1.08,1.74,;-.28,3.07,;1.26,3.05,;2,1.7,;3.14,2.73,;4.48,1.96,;4.16,.45,;5.18,-.7,;2.62,.29,;5.88,2.57,;7.12,1.67,;8.53,2.29,;8.69,3.82,;7.44,4.73,;6.04,4.1,;1.21,.37,;-.33,.41,;-5.79,4.4,;-7.27,3.94,)| Show InChI InChI=1S/C23H24FN5O2/c24-20-5-1-2-6-21(20)29-15-18(13-27-29)26-12-17-7-9-23(10-8-17)16-28(22(30)31-23)19-4-3-11-25-14-19/h1-6,11,13-15,17,26H,7-10,12,16H2/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417044
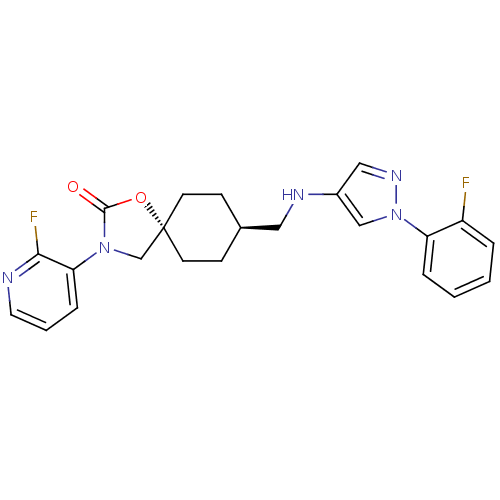
(CHEMBL1258340)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3F)CC2)cn1 |r,wU:12.12,wD:15.21,(17.14,-9.3,;15.74,-8.65,;14.5,-9.54,;13.09,-8.89,;12.95,-7.35,;14.21,-6.47,;15.61,-7.12,;16.86,-6.24,;18.32,-6.74,;19.25,-5.5,;20.79,-5.52,;21.54,-6.87,;23.08,-6.9,;23.87,-5.57,;25.41,-5.59,;26.15,-6.95,;27.29,-5.92,;28.63,-6.69,;28.31,-8.19,;29.34,-9.34,;26.78,-8.35,;30.04,-6.07,;31.28,-6.97,;32.69,-6.35,;32.85,-4.82,;31.6,-3.91,;30.2,-4.54,;28.95,-3.63,;25.36,-8.27,;23.83,-8.24,;18.36,-4.24,;16.89,-4.7,)| Show InChI InChI=1S/C23H23F2N5O2/c24-18-4-1-2-5-19(18)30-14-17(13-28-30)27-12-16-7-9-23(10-8-16)15-29(22(31)32-23)20-6-3-11-26-21(20)25/h1-6,11,13-14,16,27H,7-10,12,15H2/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417041

(CHEMBL1257637)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1nccs1)CC2 |r,wU:14.16,wD:3.2,(30.13,-31.33,;29.1,-30.19,;27.57,-30.35,;26.95,-28.94,;28.09,-27.91,;29.43,-28.68,;30.83,-28.06,;32.08,-28.97,;33.48,-28.34,;33.65,-26.81,;32.39,-25.9,;30.99,-26.53,;26.21,-27.59,;24.67,-27.57,;23.88,-28.89,;22.34,-28.87,;21.59,-27.52,;20.05,-27.5,;19.26,-28.81,;17.73,-28.79,;16.97,-27.44,;17.77,-26.12,;19.3,-26.15,;15.43,-27.43,;14.51,-28.66,;13.05,-28.17,;13.06,-26.63,;14.53,-26.17,;24.62,-30.23,;26.16,-30.26,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-3-1-2-10-23-19)15-22(29-21)8-6-16(7-9-22)13-25-18-5-4-17(14-26-18)20-24-11-12-30-20/h1-5,10-12,14,16H,6-9,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417054

(CHEMBL1258673)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(14.72,.44,;13.96,1.78,;12.43,1.8,;11.67,3.15,;12.46,4.47,;14,4.45,;14.75,3.1,;16.29,3.08,;17.04,1.73,;18.58,1.71,;19.36,3.03,;20.9,3.01,;21.65,1.66,;23.19,1.64,;23.99,2.96,;25.53,2.94,;26.26,1.59,;27.4,2.62,;28.74,1.85,;28.42,.34,;29.45,-.8,;26.89,.18,;30.15,2.47,;31.39,1.56,;32.8,2.18,;32.96,3.72,;31.71,4.62,;30.31,3.99,;25.47,.27,;23.94,.3,;18.62,4.38,;17.08,4.41,)| Show InChI InChI=1S/C23H23FN6O2/c24-18-5-2-1-4-17(18)19-7-8-20(28-27-19)25-14-16-9-11-23(12-10-16)15-30(22(31)32-23)21-6-3-13-26-29-21/h1-8,13,16H,9-12,14-15H2,(H,25,28)/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417039
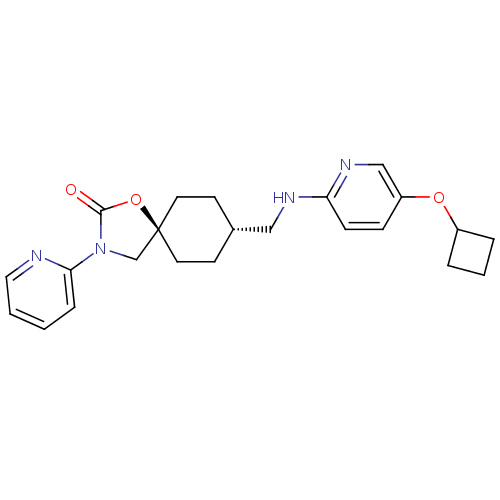
(CHEMBL1257761)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(OC3CCC3)cn1)CC2 |r,wU:14.16,wD:3.2,(29.67,-25.14,;28.64,-23.99,;27.1,-24.16,;26.48,-22.75,;27.62,-21.72,;28.96,-22.49,;30.36,-21.87,;31.61,-22.78,;33.02,-22.15,;33.18,-20.62,;31.92,-19.71,;30.52,-20.34,;25.74,-21.4,;24.2,-21.38,;23.41,-22.7,;21.87,-22.68,;21.12,-21.33,;19.58,-21.3,;18.8,-22.62,;17.26,-22.6,;16.51,-21.25,;14.96,-21.24,;14.22,-19.89,;14.64,-18.41,;13.16,-17.99,;12.74,-19.47,;17.3,-19.93,;18.84,-19.96,;24.16,-24.04,;25.69,-24.07,)| Show InChI InChI=1S/C23H28N4O3/c28-22-27(21-6-1-2-13-24-21)16-23(30-22)11-9-17(10-12-23)14-25-20-8-7-19(15-26-20)29-18-4-3-5-18/h1-2,6-8,13,15,17-18H,3-5,9-12,14,16H2,(H,25,26)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417390
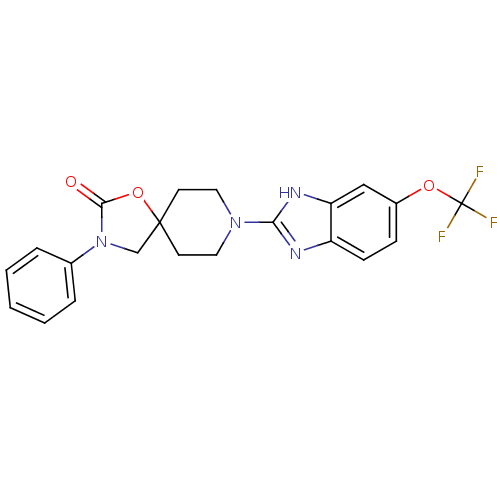
(CHEMBL1289154)Show SMILES FC(F)(F)Oc1ccc2nc([nH]c2c1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C21H19F3N4O3/c22-21(23,24)30-15-6-7-16-17(12-15)26-18(25-16)27-10-8-20(9-11-27)13-28(19(29)31-20)14-4-2-1-3-5-14/h1-7,12H,8-11,13H2,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417040
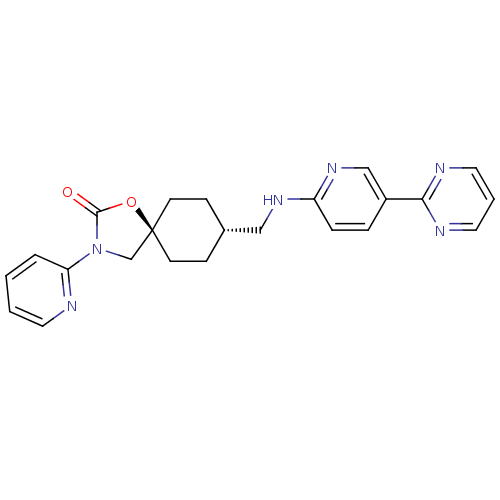
(CHEMBL1257760)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1ncccn1)CC2 |r,wU:14.16,wD:3.2,(7.1,-28.95,;6.07,-27.81,;4.54,-27.97,;3.92,-26.56,;5.06,-25.53,;6.4,-26.3,;7.8,-25.68,;9.05,-26.59,;10.45,-25.96,;10.62,-24.43,;9.36,-23.52,;7.96,-24.15,;3.18,-25.21,;1.64,-25.19,;.85,-26.51,;-.69,-26.49,;-1.44,-25.14,;-2.98,-25.12,;-3.77,-26.43,;-5.3,-26.41,;-6.06,-25.06,;-5.26,-23.74,;-3.73,-23.77,;-7.6,-25.05,;-8.35,-23.7,;-9.89,-23.68,;-10.68,-25,;-9.92,-26.35,;-8.38,-26.37,;1.59,-27.85,;3.13,-27.88,)| Show InChI InChI=1S/C23H24N6O2/c30-22-29(20-4-1-2-11-24-20)16-23(31-22)9-7-17(8-10-23)14-27-19-6-5-18(15-28-19)21-25-12-3-13-26-21/h1-6,11-13,15,17H,7-10,14,16H2,(H,27,28)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417047

(CHEMBL1258454)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccnnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-7.1,-25.51,;-8.5,-24.86,;-9.75,-25.75,;-11.16,-25.1,;-11.3,-23.56,;-10.04,-22.68,;-8.64,-23.33,;-7.38,-22.45,;-5.93,-22.95,;-5,-21.71,;-3.45,-21.73,;-2.71,-23.08,;-1.17,-23.1,;-.37,-21.78,;1.17,-21.8,;1.9,-23.15,;3.04,-22.12,;4.38,-22.89,;4.06,-24.4,;5.09,-25.55,;2.53,-24.56,;5.79,-22.28,;7.03,-23.18,;8.44,-22.56,;8.6,-21.03,;7.35,-20.12,;5.95,-20.75,;1.11,-24.48,;-.42,-24.44,;-5.89,-20.45,;-7.36,-20.91,)| Show InChI InChI=1S/C22H23FN6O2/c23-19-3-1-2-4-20(19)29-14-17(12-27-29)24-11-16-5-8-22(9-6-16)15-28(21(30)31-22)18-7-10-25-26-13-18/h1-4,7,10,12-14,16,24H,5-6,8-9,11,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417407

(CHEMBL1289153)Show SMILES Fc1ccc(cc1)-c1ccc2[nH]c(nc2c1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C26H23FN4O2/c27-20-9-6-18(7-10-20)19-8-11-22-23(16-19)29-24(28-22)30-14-12-26(13-15-30)17-31(25(32)33-26)21-4-2-1-3-5-21/h1-11,16H,12-15,17H2,(H,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417048
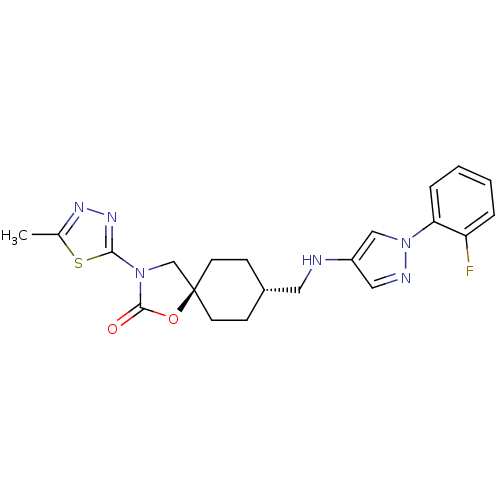
(CHEMBL1258562)Show SMILES Cc1nnc(s1)N1C[C@@]2(CC[C@H](CNc3cnn(c3)-c3ccccc3F)CC2)OC1=O |r,wU:11.12,wD:8.31,(32.21,-20.6,;30.68,-20.6,;29.77,-19.35,;28.3,-19.82,;28.3,-21.36,;29.77,-21.84,;26.9,-21.98,;25.57,-21.2,;24.42,-22.24,;23.69,-20.88,;22.15,-20.86,;21.35,-22.19,;19.81,-22.16,;19.07,-20.81,;17.53,-20.79,;16.64,-19.53,;15.17,-19.99,;15.14,-21.53,;16.6,-22.03,;13.89,-22.41,;12.49,-21.76,;11.24,-22.64,;11.37,-24.18,;12.78,-24.83,;14.02,-23.94,;15.42,-24.59,;22.1,-23.53,;23.64,-23.56,;25.05,-23.64,;26.58,-23.48,;27.61,-24.63,)| Show InChI InChI=1S/C21H23FN6O2S/c1-14-25-26-19(31-14)27-13-21(30-20(27)29)8-6-15(7-9-21)10-23-16-11-24-28(12-16)18-5-3-2-4-17(18)22/h2-5,11-12,15,23H,6-10,13H2,1H3/t15-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417055

(CHEMBL1258788)Show SMILES Cc1cccc(n1)-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nc1 |r,wU:13.13,wD:16.22,(-8.85,-9.35,;-8.11,-10.7,;-8.9,-12.02,;-8.14,-13.37,;-6.6,-13.38,;-5.82,-12.07,;-6.57,-10.72,;-4.28,-12.08,;-3.52,-13.43,;-1.98,-13.45,;-1.2,-12.13,;.34,-12.16,;1.09,-13.51,;2.63,-13.53,;3.42,-12.21,;4.96,-12.23,;5.7,-13.58,;6.84,-12.55,;8.18,-13.32,;7.85,-14.82,;8.88,-15.97,;6.32,-14.99,;9.58,-12.7,;10.83,-13.61,;12.23,-12.98,;12.4,-11.45,;11.14,-10.54,;9.74,-11.17,;4.91,-14.9,;3.37,-14.87,;-1.95,-10.79,;-3.48,-10.76,)| Show InChI InChI=1S/C24H26N6O2/c1-17-4-2-5-20(28-17)19-7-8-21(26-15-19)25-14-18-9-11-24(12-10-18)16-30(23(31)32-24)22-6-3-13-27-29-22/h2-8,13,15,18H,9-12,14,16H2,1H3,(H,25,26)/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50169264
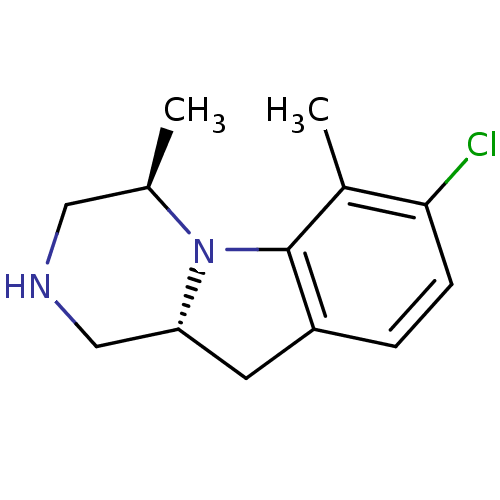
((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...)Show InChI InChI=1S/C13H17ClN2/c1-8-6-15-7-11-5-10-3-4-12(14)9(2)13(10)16(8)11/h3-4,8,11,15H,5-7H2,1-2H3/t8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5HT from human recombinant 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 1207-11 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.083
BindingDB Entry DOI: 10.7270/Q21R6Q3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50169264

((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...)Show InChI InChI=1S/C13H17ClN2/c1-8-6-15-7-11-5-10-3-4-12(14)9(2)13(10)16(8)11/h3-4,8,11,15H,5-7H2,1-2H3/t8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand |
Bioorg Med Chem Lett 15: 3604-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.074
BindingDB Entry DOI: 10.7270/Q2GQ6X87 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417387

(CHEMBL1289386)Show SMILES Fc1ccc(cc1)N1C[C@@]2(CC[C@@H](CC2)c2nc3ccc(OC(F)(F)F)cc3[nH]2)OC1=O |r,wU:9.32,wD:12.16,(48.29,-13.3,;46.95,-14.06,;46.93,-15.6,;45.59,-16.35,;44.26,-15.57,;44.28,-14.03,;45.61,-13.28,;42.93,-16.32,;41.53,-15.68,;40.48,-16.81,;39.74,-18.16,;38.21,-18.19,;37.41,-16.88,;38.14,-15.53,;39.68,-15.5,;35.87,-16.91,;34.99,-18.18,;33.51,-17.74,;32.19,-18.54,;30.84,-17.8,;30.81,-16.26,;29.46,-15.52,;29.42,-13.98,;28.07,-13.24,;30.74,-13.18,;29.46,-12.44,;32.12,-15.46,;33.47,-16.19,;34.93,-15.67,;41.24,-18.15,;42.75,-17.85,;43.88,-18.89,)| Show InChI InChI=1S/C22H19F4N3O3/c23-14-1-3-15(4-2-14)29-12-21(32-20(29)30)9-7-13(8-10-21)19-27-17-6-5-16(11-18(17)28-19)31-22(24,25)26/h1-6,11,13H,7-10,12H2,(H,27,28)/t13-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417034
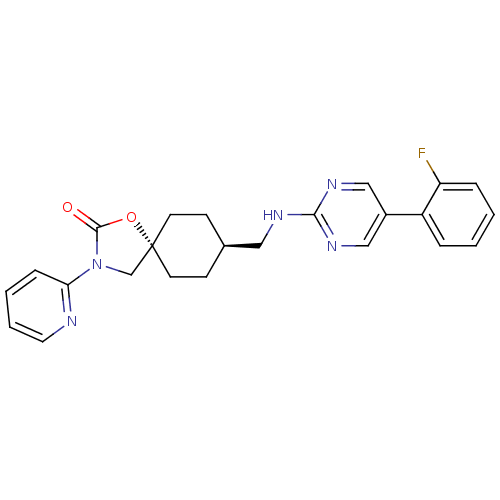
(CHEMBL1258224)Show SMILES Fc1ccccc1-c1cnc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nc1 |r,wU:13.13,wD:16.22,(-7.82,-7.93,;-8.57,-6.59,;-10.11,-6.57,;-10.86,-5.22,;-10.07,-3.9,;-8.53,-3.92,;-7.79,-5.27,;-6.24,-5.29,;-5.45,-3.96,;-3.91,-3.99,;-3.17,-5.34,;-1.63,-5.36,;-.88,-6.71,;.66,-6.73,;1.45,-5.41,;2.99,-5.43,;3.73,-6.78,;4.87,-5.75,;6.21,-6.52,;5.89,-8.03,;6.92,-9.17,;4.35,-8.19,;7.61,-5.9,;8.86,-6.81,;10.27,-6.19,;10.43,-4.65,;9.17,-3.75,;7.77,-4.37,;2.94,-8.1,;1.41,-8.07,;-3.95,-6.65,;-5.49,-6.64,)| Show InChI InChI=1S/C24H24FN5O2/c25-20-6-2-1-5-19(20)18-14-28-22(29-15-18)27-13-17-8-10-24(11-9-17)16-30(23(31)32-24)21-7-3-4-12-26-21/h1-7,12,14-15,17H,8-11,13,16H2,(H,27,28,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417405

(CHEMBL1289267)Show SMILES FC(F)(F)Oc1ccc2nc([nH]c2c1)[C@H]1CC[C@@]2(CN(C(=O)O2)c2ccccc2)CC1 |r,wU:17.24,wD:14.15,(-14.1,-12.22,;-12.74,-12.96,;-11.43,-12.16,;-12.7,-11.42,;-12.72,-14.5,;-11.36,-15.24,;-11.32,-16.78,;-9.97,-17.52,;-8.66,-16.71,;-7.17,-17.16,;-6.29,-15.88,;-7.23,-14.65,;-8.69,-15.17,;-10.04,-14.44,;-4.76,-15.85,;-3.96,-17.17,;-2.43,-17.14,;-1.69,-15.79,;-.64,-14.66,;.76,-15.3,;.58,-16.83,;1.71,-17.87,;-.93,-17.13,;2.09,-14.55,;3.41,-15.33,;4.75,-14.58,;4.77,-13.04,;3.44,-12.26,;2.1,-13.01,;-2.48,-14.47,;-4.03,-14.5,)| Show InChI InChI=1S/C22H20F3N3O3/c23-22(24,25)30-16-6-7-17-18(12-16)27-19(26-17)14-8-10-21(11-9-14)13-28(20(29)31-21)15-4-2-1-3-5-15/h1-7,12,14H,8-11,13H2,(H,26,27)/t14-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417406

(CHEMBL1290037)Show SMILES O=C1O[C@@]2(CN1c1ccccc1)CC[C@@H](CC2)c1nc2ccc(cc2[nH]1)C#N |r,wU:3.2,wD:14.19,(44.12,-48.11,;42.99,-47.06,;41.48,-47.37,;40.72,-46.03,;41.77,-44.89,;43.17,-45.54,;44.5,-44.78,;45.83,-45.57,;47.17,-44.82,;47.18,-43.28,;45.85,-42.49,;44.51,-43.25,;39.98,-47.38,;38.45,-47.41,;37.65,-46.09,;38.38,-44.74,;39.92,-44.71,;36.11,-46.12,;35.23,-47.4,;33.75,-46.95,;32.43,-47.76,;31.08,-47.02,;31.05,-45.47,;32.36,-44.67,;33.71,-45.4,;35.17,-44.89,;29.7,-44.74,;28.35,-43.96,)| Show InChI InChI=1S/C22H20N4O2/c23-13-15-6-7-18-19(12-15)25-20(24-18)16-8-10-22(11-9-16)14-26(21(27)28-22)17-4-2-1-3-5-17/h1-7,12,16H,8-11,14H2,(H,24,25)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417052

(CHEMBL1258907)Show SMILES Fc1cccnc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nc1 |r,wU:13.13,wD:16.22,(16.94,-15.12,;16.19,-13.77,;14.65,-13.76,;13.89,-12.41,;14.68,-11.08,;16.22,-11.11,;16.97,-12.46,;18.51,-12.47,;19.27,-13.82,;20.8,-13.84,;21.59,-12.52,;23.13,-12.55,;23.88,-13.89,;25.42,-13.92,;26.21,-12.6,;27.75,-12.62,;28.49,-13.97,;29.63,-12.93,;30.97,-13.71,;30.64,-15.21,;31.67,-16.36,;29.11,-15.37,;32.37,-13.09,;33.62,-14,;35.02,-13.37,;35.19,-11.84,;33.93,-10.93,;32.53,-11.56,;27.7,-15.29,;26.16,-15.26,;20.84,-11.18,;19.31,-11.15,)| Show InChI InChI=1S/C23H23FN6O2/c24-18-3-1-11-25-21(18)17-5-6-19(27-14-17)26-13-16-7-9-23(10-8-16)15-30(22(31)32-23)20-4-2-12-28-29-20/h1-6,11-12,14,16H,7-10,13,15H2,(H,26,27)/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416448

(CHEMBL1209159)Show SMILES CC(C(=O)N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1)c1ccccc1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15(16-7-3-2-4-8-16)22(28)27-12-18-17(19(18)13-27)11-25-23-26-21(14-29-23)20-9-5-6-10-24-20/h2-10,14-15,17-19H,11-13H2,1H3,(H,25,26)/t15?,17-,18+,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417404
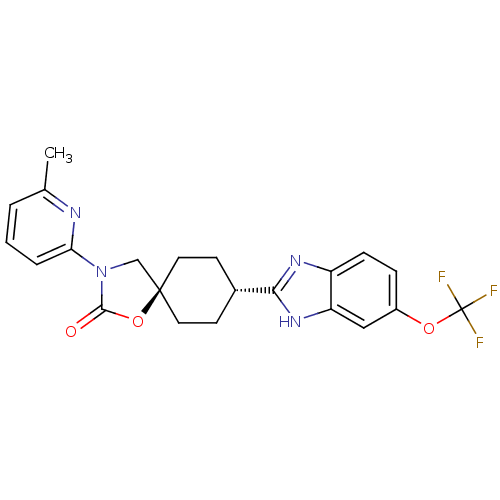
(CHEMBL1289609)Show SMILES Cc1cccc(n1)N1C[C@@]2(CC[C@@H](CC2)c2nc3ccc(OC(F)(F)F)cc3[nH]2)OC1=O |r,wU:9.32,wD:12.16,(4.72,-25.12,;4.71,-26.66,;6.04,-27.44,;6.02,-28.99,;4.68,-29.74,;3.36,-28.95,;3.37,-27.42,;2.02,-29.7,;.63,-29.06,;-.42,-30.2,;-1.16,-31.54,;-2.7,-31.58,;-3.49,-30.26,;-2.76,-28.91,;-1.22,-28.88,;-5.03,-30.29,;-5.91,-31.56,;-7.39,-31.12,;-8.71,-31.92,;-10.06,-31.19,;-10.09,-29.64,;-11.46,-28.9,;-11.48,-27.37,;-12.84,-26.63,;-10.17,-26.56,;-11.44,-25.82,;-8.78,-28.84,;-7.43,-29.57,;-5.97,-29.06,;.34,-31.53,;1.85,-31.23,;2.98,-32.28,)| Show InChI InChI=1S/C22H21F3N4O3/c1-13-3-2-4-18(26-13)29-12-21(32-20(29)30)9-7-14(8-10-21)19-27-16-6-5-15(11-17(16)28-19)31-22(23,24)25/h2-6,11,14H,7-10,12H2,1H3,(H,27,28)/t14-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417403
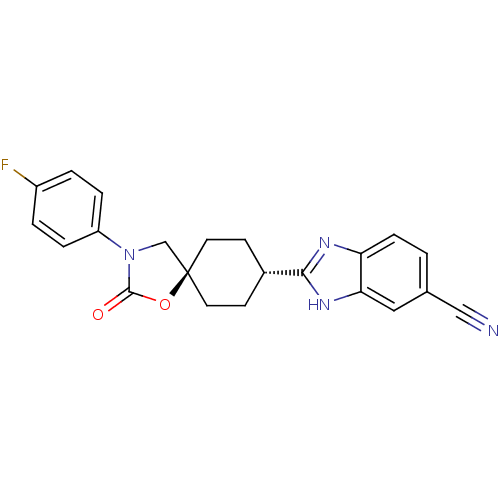
(CHEMBL1290038)Show SMILES Fc1ccc(cc1)N1C[C@@]2(CC[C@@H](CC2)c2nc3ccc(cc3[nH]2)C#N)OC1=O |r,wU:9.29,wD:12.16,(8.45,-40.09,;7.11,-40.85,;7.09,-42.39,;5.75,-43.14,;4.42,-42.35,;4.44,-40.82,;5.77,-40.06,;3.09,-43.11,;1.69,-42.46,;.65,-43.6,;-.1,-44.95,;-1.63,-44.98,;-2.43,-43.66,;-1.7,-42.31,;-.15,-42.28,;-3.96,-43.69,;-4.85,-44.97,;-6.33,-44.52,;-7.64,-45.33,;-8.99,-44.59,;-9.03,-43.05,;-7.72,-42.24,;-6.37,-42.98,;-4.9,-42.46,;-10.38,-42.31,;-11.72,-41.54,;1.41,-44.94,;2.91,-44.64,;4.05,-45.68,)| Show InChI InChI=1S/C22H19FN4O2/c23-16-2-4-17(5-3-16)27-13-22(29-21(27)28)9-7-15(8-10-22)20-25-18-6-1-14(12-24)11-19(18)26-20/h1-6,11,15H,7-10,13H2,(H,25,26)/t15-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417386
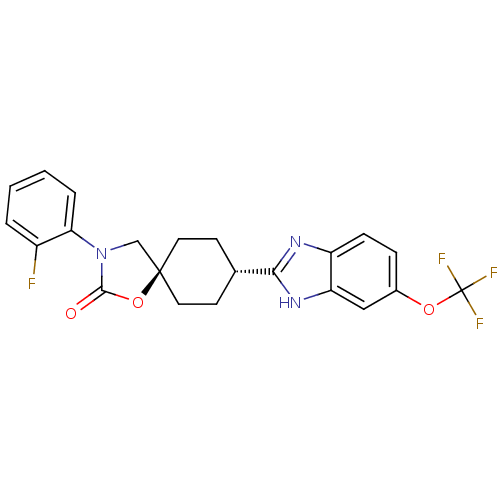
(CHEMBL1289268)Show SMILES Fc1ccccc1N1C[C@@]2(CC[C@@H](CC2)c2nc3ccc(OC(F)(F)F)cc3[nH]2)OC1=O |r,wU:9.32,wD:12.16,(21.81,-13.52,;23.13,-14.3,;24.47,-13.55,;25.8,-14.33,;25.78,-15.87,;24.44,-16.62,;23.12,-15.84,;21.78,-16.59,;20.39,-15.95,;19.34,-17.08,;18.6,-18.43,;17.06,-18.46,;16.27,-17.15,;17,-15.79,;18.54,-15.76,;14.73,-17.18,;13.85,-18.45,;12.37,-18.01,;11.05,-18.81,;9.7,-18.07,;9.67,-16.53,;8.32,-15.79,;8.28,-14.25,;6.93,-13.51,;9.59,-13.45,;8.32,-12.71,;10.98,-15.73,;12.33,-16.46,;13.79,-15.94,;20.1,-18.42,;21.61,-18.12,;22.74,-19.16,)| Show InChI InChI=1S/C22H19F4N3O3/c23-15-3-1-2-4-18(15)29-12-21(32-20(29)30)9-7-13(8-10-21)19-27-16-6-5-14(11-17(16)28-19)31-22(24,25)26/h1-6,11,13H,7-10,12H2,(H,27,28)/t13-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416460

(CHEMBL1209345)Show SMILES Fc1cc(F)cc(CN2C[C@H]3[C@@H](CNc4nc(cs4)-c4ccccn4)[C@H]3C2)c1 |r| Show InChI InChI=1S/C21H20F2N4S/c22-14-5-13(6-15(23)7-14)9-27-10-17-16(18(17)11-27)8-25-21-26-20(12-28-21)19-3-1-2-4-24-19/h1-7,12,16-18H,8-11H2,(H,25,26)/t16-,17+,18- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417043
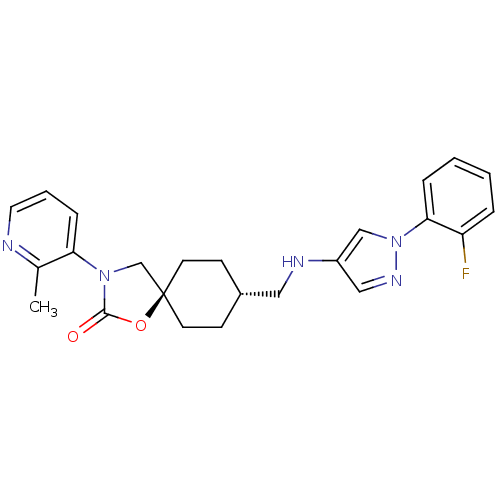
(CHEMBL1257520)Show SMILES Cc1ncccc1N1C[C@@]2(CC[C@H](CNc3cnn(c3)-c3ccccc3F)CC2)OC1=O |r,wU:12.13,wD:9.32,(27.95,3.78,;29.2,2.88,;30.61,3.51,;31.86,2.6,;31.69,1.07,;30.29,.45,;29.05,1.35,;27.64,.73,;26.3,1.5,;25.16,.47,;24.42,1.83,;22.88,1.84,;22.08,.52,;20.54,.55,;19.8,1.89,;18.26,1.92,;17.37,3.18,;15.89,2.72,;15.87,1.18,;17.33,.68,;14.61,.3,;13.22,.95,;11.96,.07,;12.1,-1.47,;13.5,-2.12,;14.75,-1.23,;16.15,-1.88,;22.84,-.82,;24.37,-.85,;25.79,-.94,;27.32,-.77,;28.34,-1.92,)| Show InChI InChI=1S/C24H26FN5O2/c1-17-21(7-4-12-26-17)29-16-24(32-23(29)31)10-8-18(9-11-24)13-27-19-14-28-30(15-19)22-6-3-2-5-20(22)25/h2-7,12,14-15,18,27H,8-11,13,16H2,1H3/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416445

(CHEMBL1209162)Show SMILES O=C(CCc1ccccc1)N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1 |r| Show InChI InChI=1S/C23H24N4OS/c28-22(10-9-16-6-2-1-3-7-16)27-13-18-17(19(18)14-27)12-25-23-26-21(15-29-23)20-8-4-5-11-24-20/h1-8,11,15,17-19H,9-10,12-14H2,(H,25,26)/t17-,18+,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417402

(CHEMBL1289820)Show SMILES FC(F)(F)c1ccc2nc([nH]c2c1)[C@H]1CC[C@@]2(CN(C(=O)O2)c2ccccc2)CC1 |r,wU:16.23,wD:13.14,(28.53,-36.29,;28.56,-37.83,;27.25,-38.63,;27.22,-37.06,;29.91,-38.57,;29.95,-40.11,;31.3,-40.85,;32.61,-40.05,;34.1,-40.49,;34.98,-39.22,;34.04,-37.99,;32.58,-38.5,;31.22,-37.77,;36.51,-39.19,;37.31,-40.51,;38.84,-40.47,;39.59,-39.12,;40.63,-37.99,;42.03,-38.63,;41.86,-40.16,;42.99,-41.2,;40.35,-40.46,;43.37,-37.88,;44.69,-38.67,;46.03,-37.91,;46.05,-36.37,;44.71,-35.59,;43.38,-36.35,;38.79,-37.81,;37.24,-37.84,)| Show InChI InChI=1S/C22H20F3N3O2/c23-22(24,25)15-6-7-17-18(12-15)27-19(26-17)14-8-10-21(11-9-14)13-28(20(29)30-21)16-4-2-1-3-5-16/h1-7,12,14H,8-11,13H2,(H,26,27)/t14-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416447

(CHEMBL1209160)Show SMILES O=C(N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1)C1(CC1)c1ccccc1 |r| Show InChI InChI=1S/C24H24N4OS/c29-22(24(9-10-24)16-6-2-1-3-7-16)28-13-18-17(19(18)14-28)12-26-23-27-21(15-30-23)20-8-4-5-11-25-20/h1-8,11,15,17-19H,9-10,12-14H2,(H,26,27)/t17-,18+,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416443

(CHEMBL1209216)Show SMILES O=C([C@H]1C[C@@H]1c1ccccc1)N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1 |r| Show InChI InChI=1S/C24H24N4OS/c29-23(17-10-16(17)15-6-2-1-3-7-15)28-12-19-18(20(19)13-28)11-26-24-27-22(14-30-24)21-8-4-5-9-25-21/h1-9,14,16-20H,10-13H2,(H,26,27)/t16-,17+,18-,19+,20-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50179077
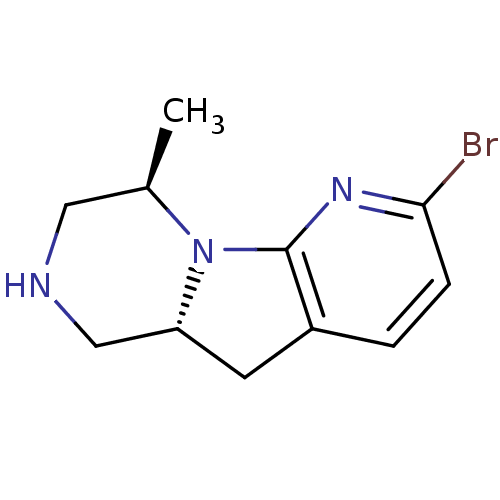
((4R,9aR)-6-bromo-4-methyl-1,2,3,4,9,9a-hexahydro-2...)Show InChI InChI=1S/C11H14BrN3/c1-7-5-13-6-9-4-8-2-3-10(12)14-11(8)15(7)9/h2-3,7,9,13H,4-6H2,1H3/t7-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5HT from human recombinant 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 1207-11 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.083
BindingDB Entry DOI: 10.7270/Q21R6Q3T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417038

(CHEMBL1257881)Show SMILES FC(F)(F)c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nc1 |r,wU:10.9,wD:13.18,(-9.58,-17.43,;-8.84,-18.78,;-9.62,-20.1,;-10.38,-18.78,;-7.29,-18.8,;-6.54,-20.15,;-5,-20.16,;-4.22,-18.85,;-2.68,-18.87,;-1.93,-20.22,;-.39,-20.24,;.4,-18.92,;1.94,-18.94,;2.68,-20.29,;3.82,-19.26,;5.16,-20.03,;4.84,-21.54,;5.87,-22.68,;3.3,-21.7,;6.56,-19.41,;7.81,-20.32,;9.22,-19.7,;9.38,-18.16,;8.12,-17.26,;6.72,-17.88,;1.89,-21.61,;.36,-21.58,;-4.96,-17.5,;-6.5,-17.47,)| Show InChI InChI=1S/C20H21F3N4O2/c21-20(22,23)15-4-5-16(26-12-15)25-11-14-6-8-19(9-7-14)13-27(18(28)29-19)17-3-1-2-10-24-17/h1-5,10,12,14H,6-9,11,13H2,(H,25,26)/t14-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416454

(CHEMBL1209052)Show SMILES Fc1cccc(c1)C(=O)N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1 |r| Show InChI InChI=1S/C21H19FN4OS/c22-14-5-3-4-13(8-14)20(27)26-10-16-15(17(16)11-26)9-24-21-25-19(12-28-21)18-6-1-2-7-23-18/h1-8,12,15-17H,9-11H2,(H,24,25)/t15-,16+,17- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416450
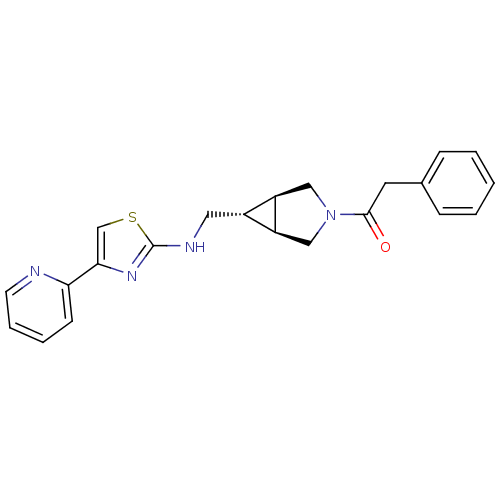
(CHEMBL1209101)Show SMILES O=C(Cc1ccccc1)N1C[C@H]2[C@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1 |r| Show InChI InChI=1S/C22H22N4OS/c27-21(10-15-6-2-1-3-7-15)26-12-17-16(18(17)13-26)11-24-22-25-20(14-28-22)19-8-4-5-9-23-19/h1-9,14,16-18H,10-13H2,(H,24,25)/t16-,17-,18+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416449

(CHEMBL1209102)Show SMILES O=C(Cc1ccccc1)N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1 |r| Show InChI InChI=1S/C22H22N4OS/c27-21(10-15-6-2-1-3-7-15)26-12-17-16(18(17)13-26)11-24-22-25-20(14-28-22)19-8-4-5-9-23-19/h1-9,14,16-18H,10-13H2,(H,24,25)/t16-,17+,18- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50416436
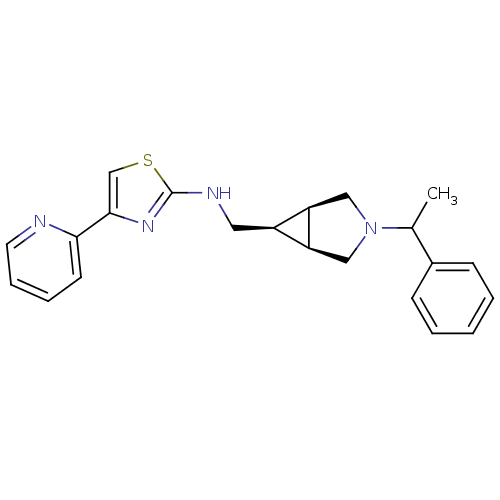
(CHEMBL1209346)Show SMILES CC(N1C[C@H]2[C@@H](CNc3nc(cs3)-c3ccccn3)[C@H]2C1)c1ccccc1 |r| Show InChI InChI=1S/C22H24N4S/c1-15(16-7-3-2-4-8-16)26-12-18-17(19(18)13-26)11-24-22-25-21(14-27-22)20-9-5-6-10-23-20/h2-10,14-15,17-19H,11-13H2,1H3,(H,24,25)/t15?,17-,18+,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 4741-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.140
BindingDB Entry DOI: 10.7270/Q2JS9QMP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417394

(CHEMBL1289038)Show SMILES Brc1ccc2nc([nH]c2c1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C20H19BrN4O2/c21-14-6-7-16-17(12-14)23-18(22-16)24-10-8-20(9-11-24)13-25(19(26)27-20)15-4-2-1-3-5-15/h1-7,12H,8-11,13H2,(H,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417388

(CHEMBL1289714)Show SMILES Fc1cccc(n1)N1C[C@@]2(CC[C@@H](CC2)c2nc3ccc(OC(F)(F)F)cc3[nH]2)OC1=O |r,wU:9.32,wD:12.16,(45.18,-25.24,;45.16,-26.78,;46.5,-27.56,;46.48,-29.1,;45.14,-29.86,;43.82,-29.07,;43.83,-27.54,;42.48,-29.82,;41.08,-29.18,;40.04,-30.32,;39.29,-31.66,;37.76,-31.7,;36.96,-30.38,;37.69,-29.03,;39.24,-29,;35.43,-30.41,;34.55,-31.68,;33.06,-31.24,;31.75,-32.04,;30.4,-31.3,;30.36,-29.76,;29.01,-29.02,;28.98,-27.48,;27.62,-26.75,;30.29,-26.68,;29.02,-25.94,;31.67,-28.96,;33.03,-29.69,;34.49,-29.18,;40.8,-31.65,;42.31,-31.35,;43.44,-32.39,)| Show InChI InChI=1S/C21H18F4N4O3/c22-16-2-1-3-17(28-16)29-11-20(32-19(29)30)8-6-12(7-9-20)18-26-14-5-4-13(10-15(14)27-18)31-21(23,24)25/h1-5,10,12H,6-9,11H2,(H,26,27)/t12-,20- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417408

(CHEMBL1289503)Show SMILES FC(F)(F)Oc1ccc2nc([nH]c2c1)[C@H]1CC[C@@]2(CN(C(=O)O2)c2ccccn2)CC1 |r,wU:17.24,wD:14.15,(27.51,-20,;28.86,-20.74,;30.17,-19.93,;28.9,-19.19,;28.9,-22.27,;30.25,-23.01,;30.28,-24.56,;31.63,-25.29,;32.95,-24.49,;34.43,-24.93,;35.31,-23.66,;34.37,-22.43,;32.91,-22.94,;31.56,-22.21,;36.84,-23.63,;37.64,-24.95,;39.18,-24.92,;39.92,-23.57,;40.97,-22.43,;42.36,-23.07,;42.19,-24.6,;43.32,-25.65,;40.68,-24.9,;43.7,-22.32,;45.02,-23.11,;46.36,-22.36,;46.38,-20.81,;45.05,-20.03,;43.71,-20.79,;39.12,-22.25,;37.58,-22.28,)| Show InChI InChI=1S/C21H19F3N4O3/c22-21(23,24)30-14-4-5-15-16(11-14)27-18(26-15)13-6-8-20(9-7-13)12-28(19(29)31-20)17-3-1-2-10-25-17/h1-5,10-11,13H,6-9,12H2,(H,26,27)/t13-,20- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50169263
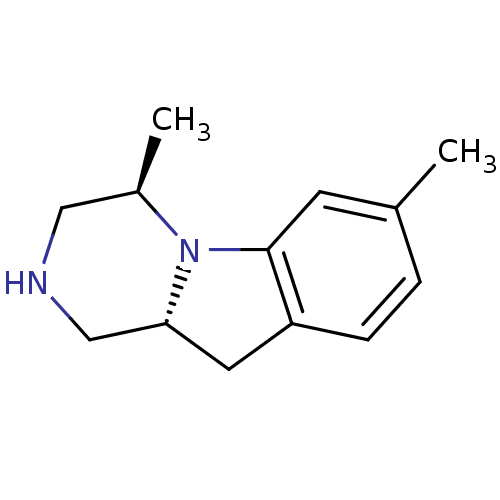
((4R,10aR)-4,7-Dimethyl-1,2,3,4,10,10a-hexahydro-py...)Show InChI InChI=1S/C13H18N2/c1-9-3-4-11-6-12-8-14-7-10(2)15(12)13(11)5-9/h3-5,10,12,14H,6-8H2,1-2H3/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand |
Bioorg Med Chem Lett 15: 3604-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.074
BindingDB Entry DOI: 10.7270/Q2GQ6X87 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50169266
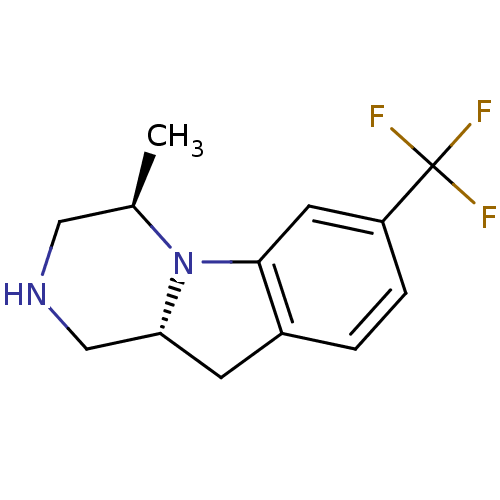
((4R,10aR)-4-Methyl-7-trifluoromethyl-1,2,3,4,10,10...)Show InChI InChI=1S/C13H15F3N2/c1-8-6-17-7-11-4-9-2-3-10(13(14,15)16)5-12(9)18(8)11/h2-3,5,8,11,17H,4,6-7H2,1H3/t8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand |
Bioorg Med Chem Lett 15: 3604-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.074
BindingDB Entry DOI: 10.7270/Q2GQ6X87 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50169267
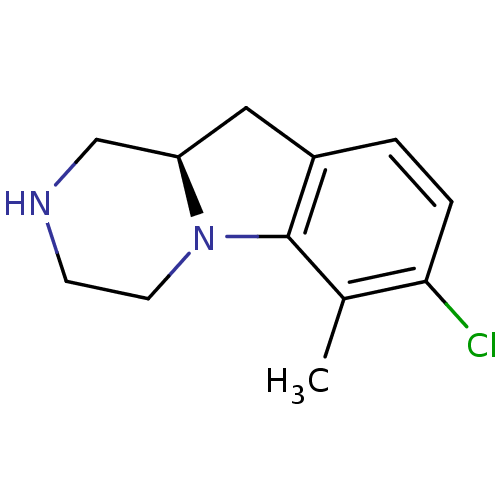
((R)-7-Chloro-6-methyl-1,2,3,4,10,10a-hexahydro-pyr...)Show InChI InChI=1S/C12H15ClN2/c1-8-11(13)3-2-9-6-10-7-14-4-5-15(10)12(8)9/h2-3,10,14H,4-7H2,1H3/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand |
Bioorg Med Chem Lett 15: 3604-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.074
BindingDB Entry DOI: 10.7270/Q2GQ6X87 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417391

(CHEMBL1290706)Show SMILES FC(F)(F)c1ccc2nc([nH]c2c1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C21H19F3N4O2/c22-21(23,24)14-6-7-16-17(12-14)26-18(25-16)27-10-8-20(9-11-27)13-28(19(29)30-20)15-4-2-1-3-5-15/h1-7,12H,8-11,13H2,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data