

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BCL2 (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007522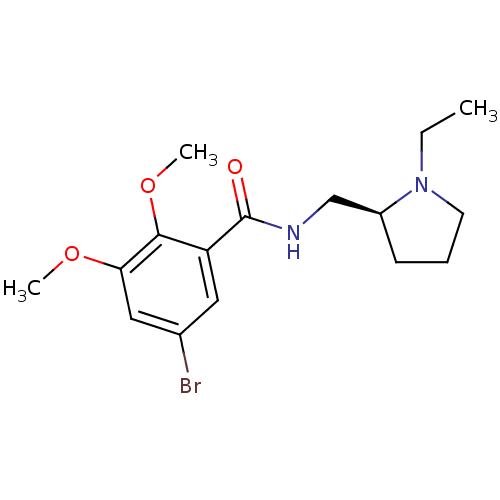 (5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368060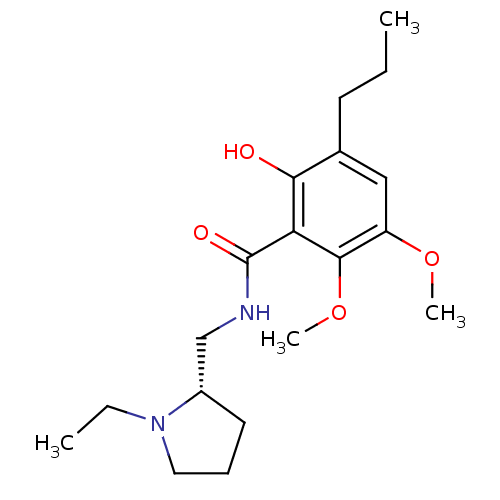 (CHEMBL1907695) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007508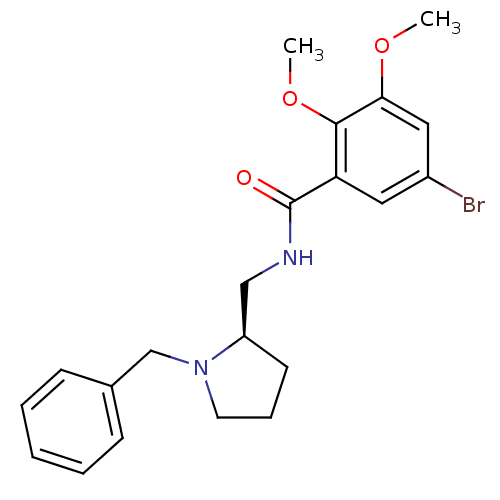 ((R) N-(1-Benzyl-pyrrolidin-2-ylmethyl)-5-bromo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368067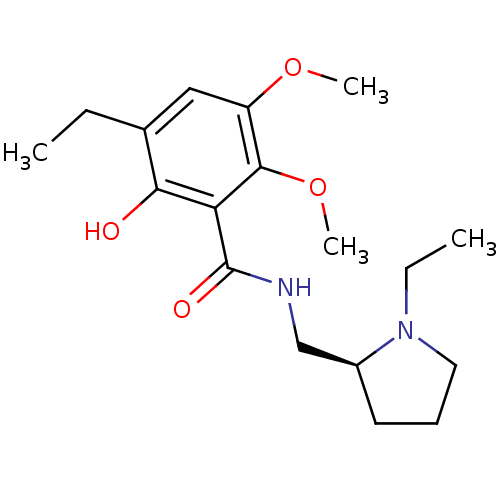 (CHEMBL1907702) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256265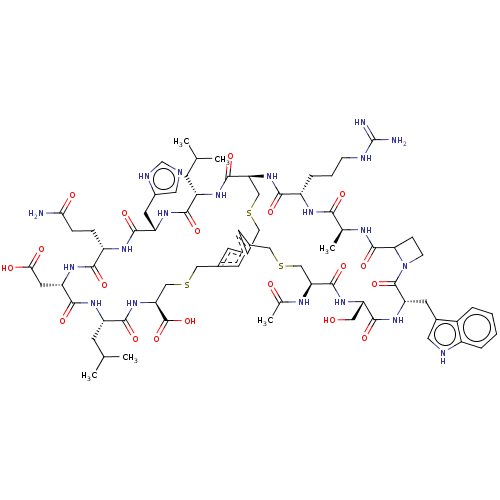 (CHEMBL4089486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007517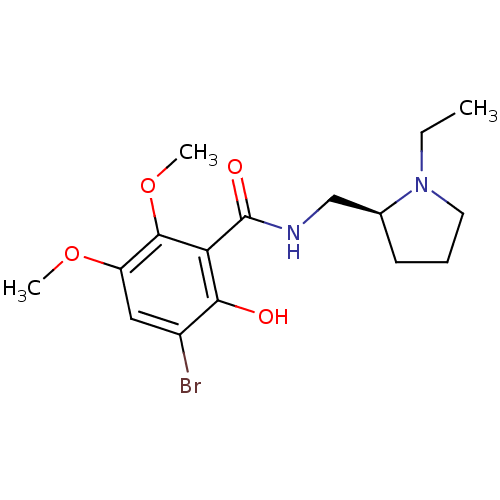 ((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007517 ((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256283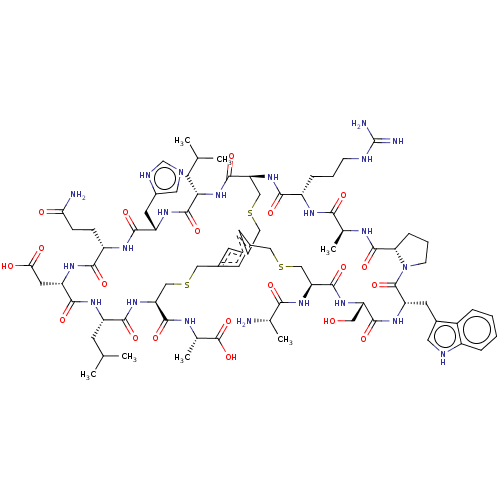 (CHEMBL4079711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50546262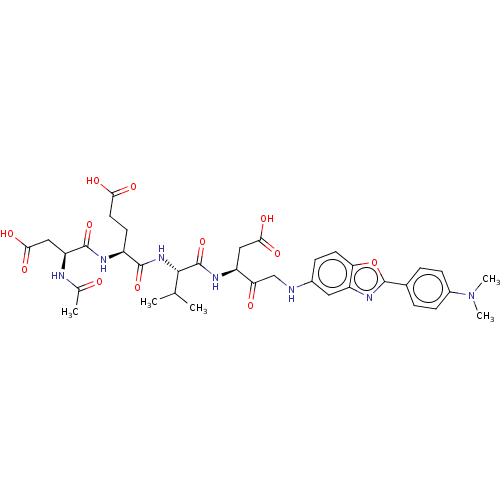 (CHEMBL4751195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368065 (CHEMBL1907692) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256264 (CHEMBL4099333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007509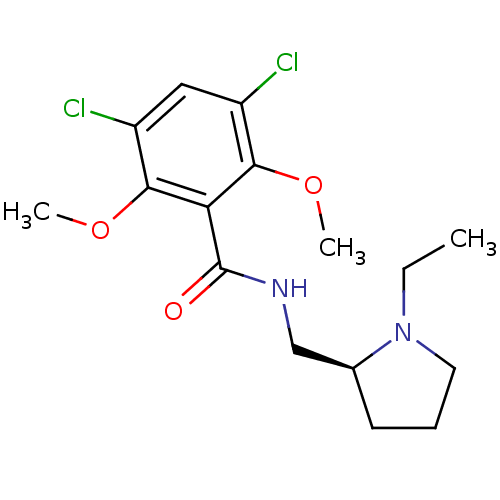 (3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256277 (CHEMBL4093698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007510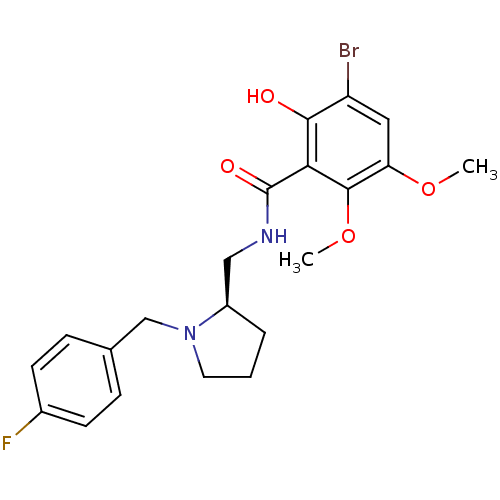 ((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50209809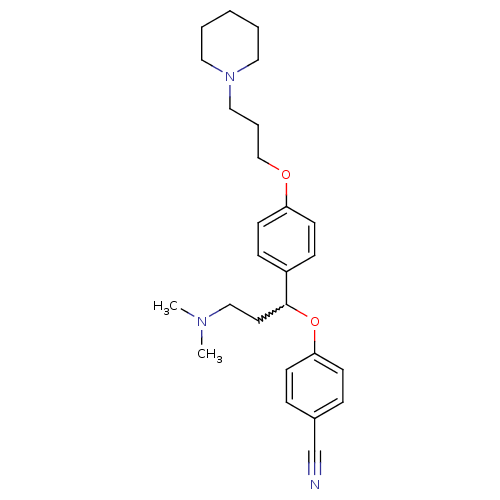 (4-(3-(dimethylamino)-1-(4-(3-(piperidin-1-yl)propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 3130-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.034 BindingDB Entry DOI: 10.7270/Q2H131QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256286 (CHEMBL4076199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256262 (CHEMBL4070056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM82517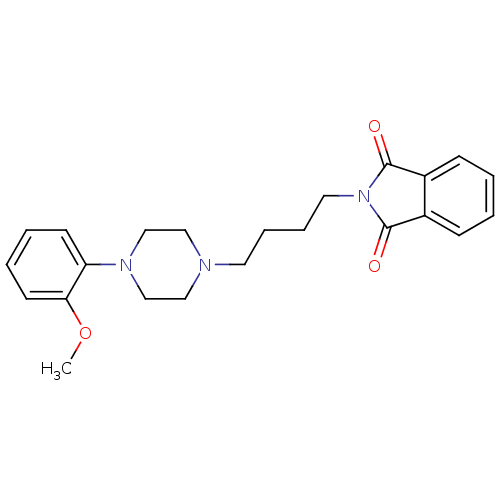 (2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256260 (CHEMBL4094403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256276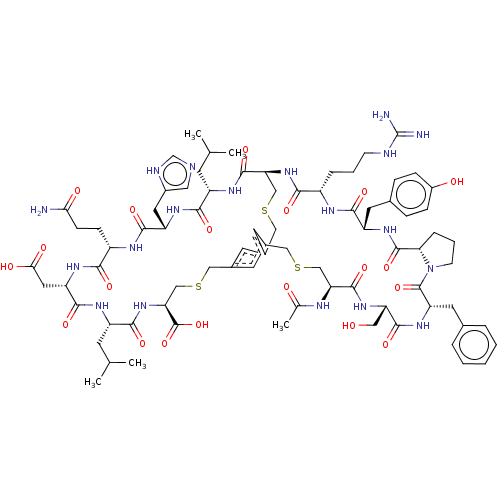 (CHEMBL4079260) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371662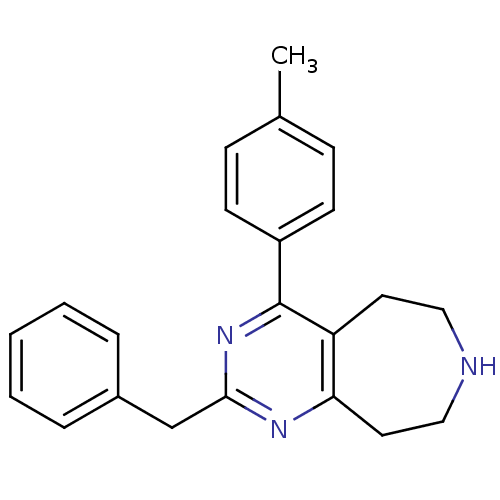 (CHEMBL269974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256284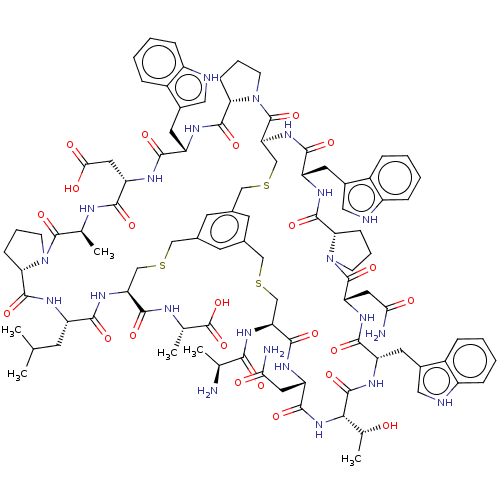 (CHEMBL4075544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 16: 897-900 (2006) Article DOI: 10.1016/j.bmcl.2005.11.003 BindingDB Entry DOI: 10.7270/Q2H131M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007524 ((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256288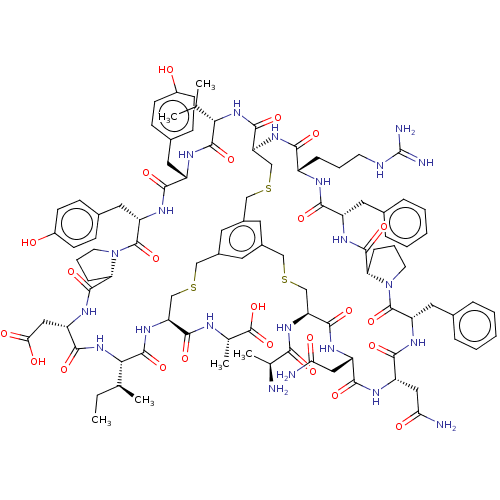 (CHEMBL4085408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50410342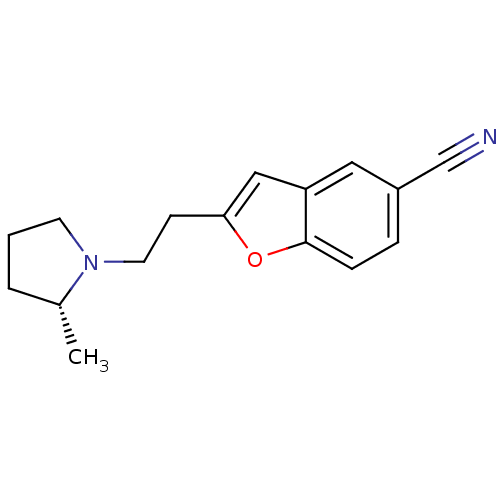 (CHEMBL195408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by PDSP Ki Database | Neuron 11: 449-58 (1993) Article DOI: 10.1016/0896-6273(93)90149-l BindingDB Entry DOI: 10.7270/Q269723D | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22530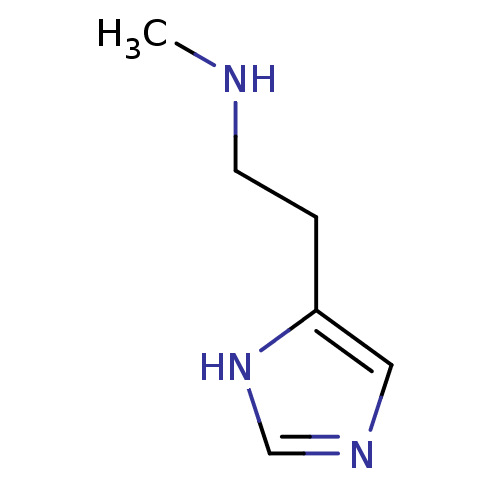 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50177731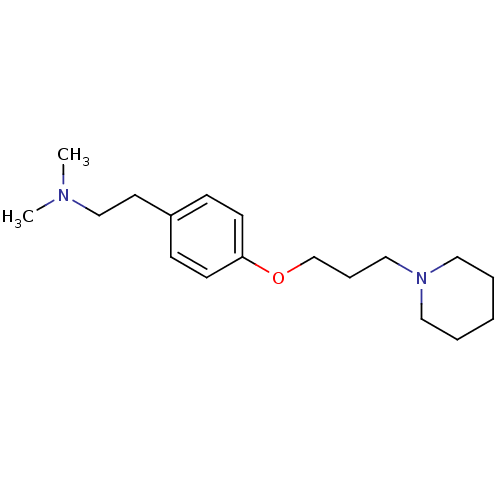 (CHEMBL204872 | dimethyl-{2-[4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 16: 897-900 (2006) Article DOI: 10.1016/j.bmcl.2005.11.003 BindingDB Entry DOI: 10.7270/Q2H131M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50270877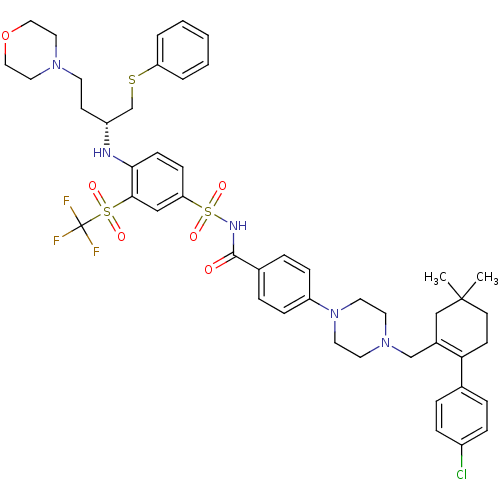 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BCL2 (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028059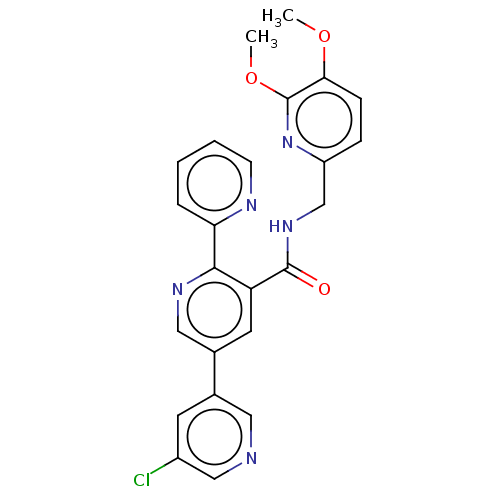 (CHEMBL3338866) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... | J Med Chem 58: 5620-36 (2015) Article DOI: 10.1021/acs.jmedchem.5b00742 BindingDB Entry DOI: 10.7270/Q2QR4ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200636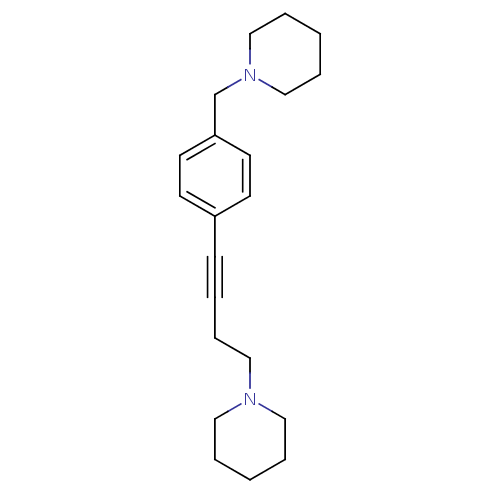 (1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007534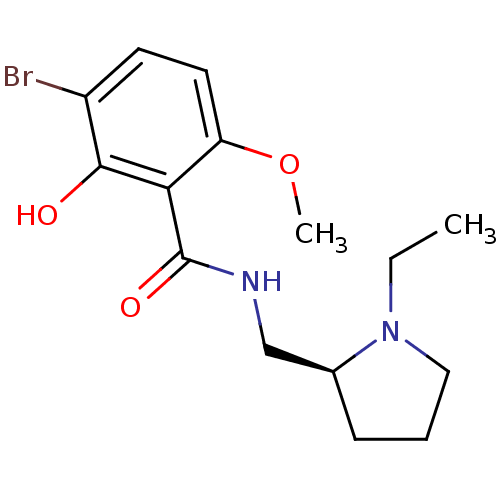 (3-Bromo-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007534 (3-Bromo-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414797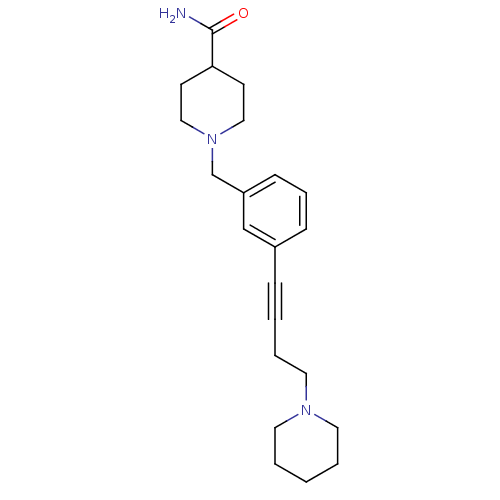 (CHEMBL582977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205280 (2,6-diphenyl-8-isobutyl-1-deazapurine | CHEMBL2214...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11407 total ) | Next | Last >> |