

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23921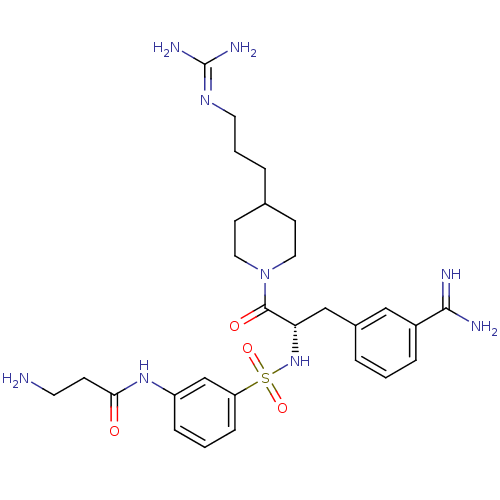 (3-amidinophenylalanine deriv., 63 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23916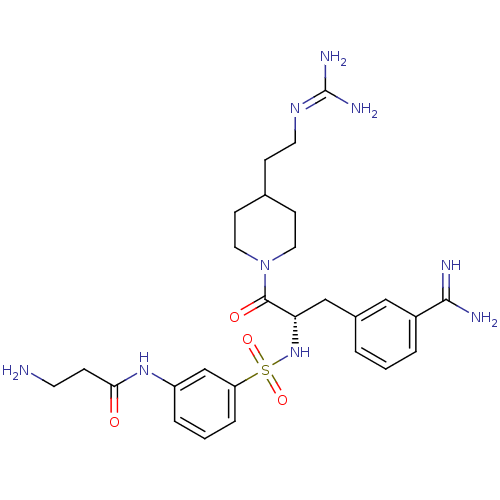 (3-amidinophenylalanine deriv., 12 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50245500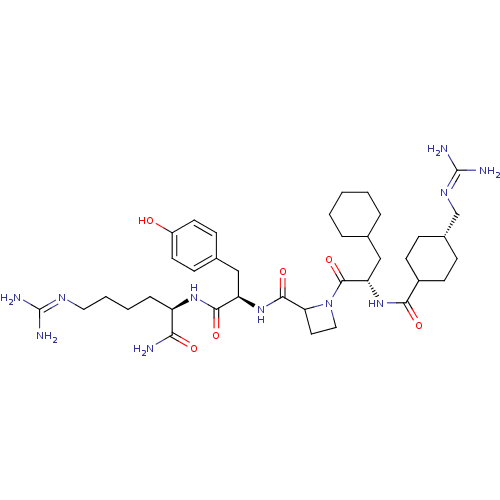 (CHEMBL447547 | N-[(2S)-1-(2-{[(1R)-1-{[(1R)-5-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibition of human thrombin | Eur J Med Chem 43: 1330-5 (2008) Article DOI: 10.1016/j.ejmech.2007.07.019 BindingDB Entry DOI: 10.7270/Q2SB45JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23918 (3-amidinophenylalanine deriv., 60 | 4-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23915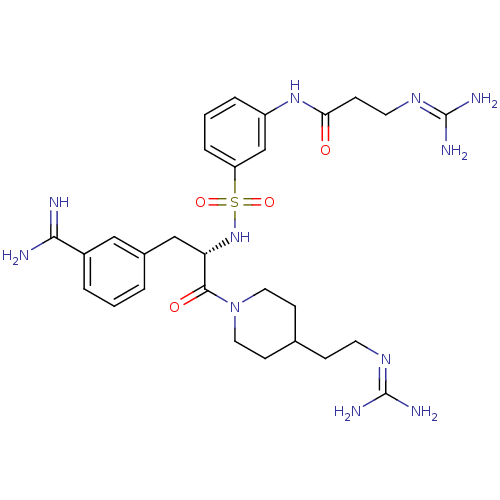 (3-amidinophenylalanine deriv., 58 | 3-carbamimidam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23920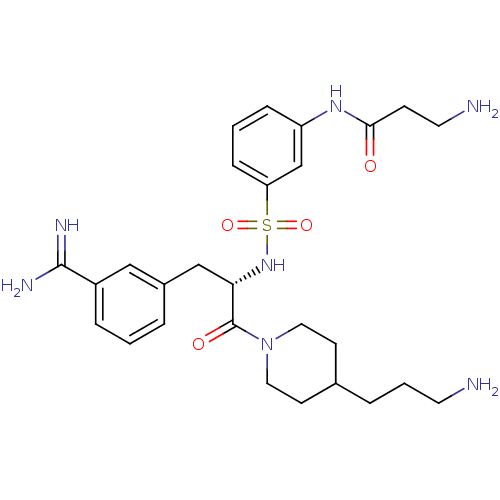 (3-amidinophenylalanine deriv., 62 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072840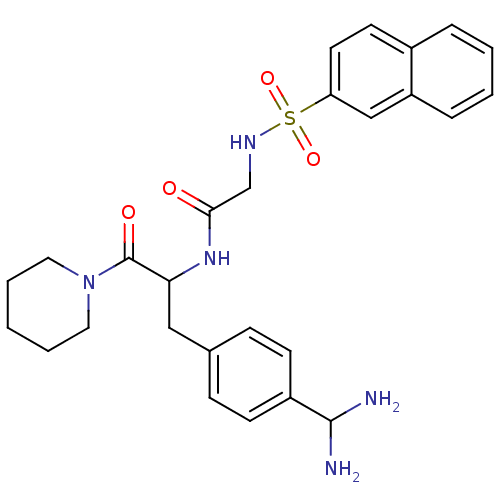 (CHEMBL346607 | N-[1-(4-Diaminomethyl-benzyl)-2-oxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50060033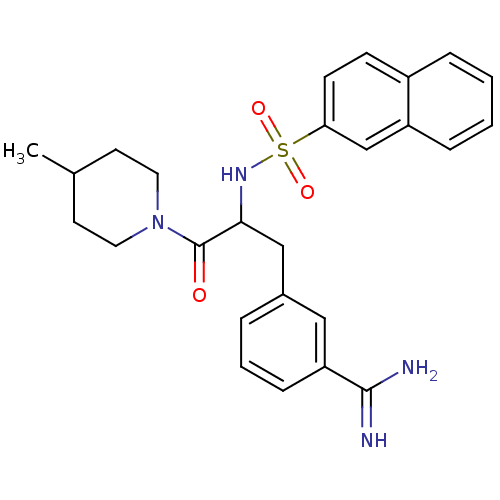 (3-[3-(4-Methyl-piperidin-1-yl)-2-(naphthalene-2-su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23917 (3-amidinophenylalanine deriv., 59 | 3-amino-N-(3-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50041224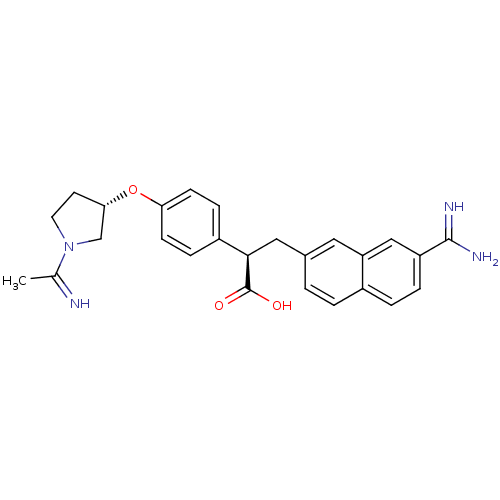 ((R)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Binding affinity of the compound towards Coagulation factor X | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50072851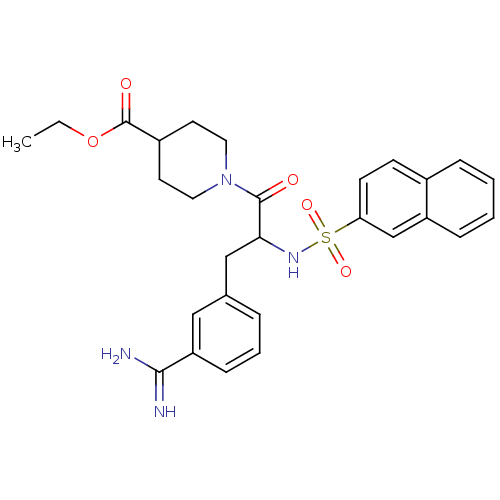 (1-[3-(3-Carbamimidoyl-phenyl)-2-(naphthalene-2-sul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine trypsin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23913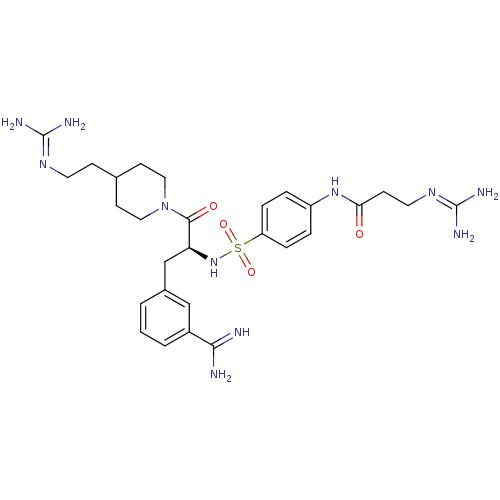 (3-amidinophenylalanine deriv., 56 | 3-carbamimidam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23911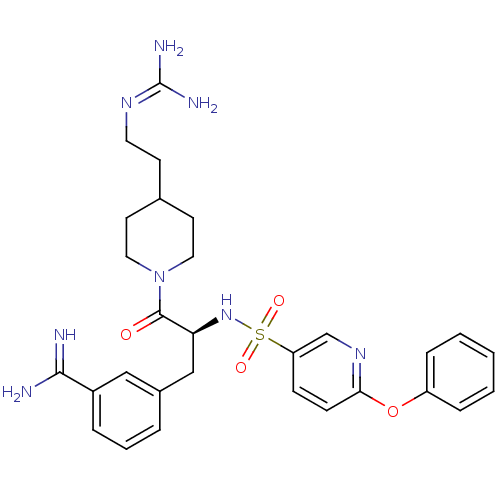 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50072843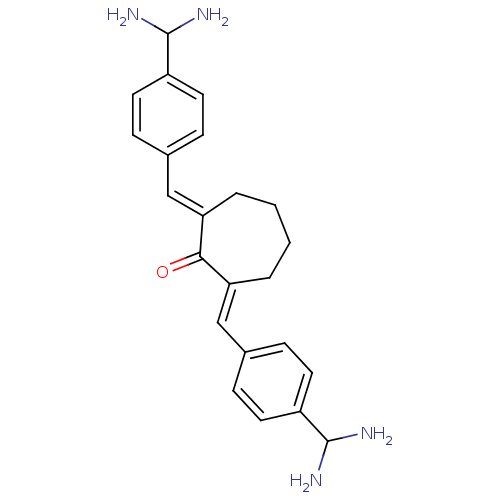 (2,7-Bis-(4-diaminomethyl-benzylidene)-cycloheptano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine coagulation factor X expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23896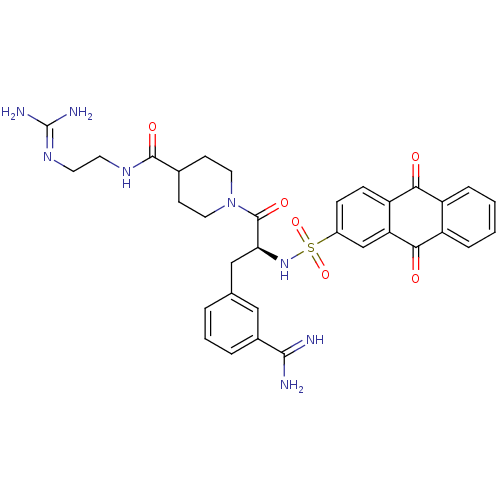 (3-amidinophenylalanine deriv., 40 | N-(2-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23887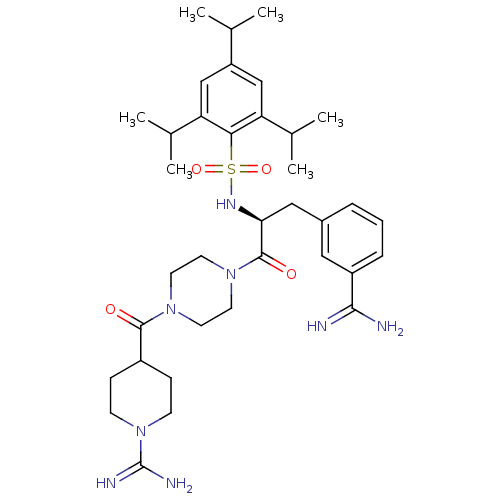 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072846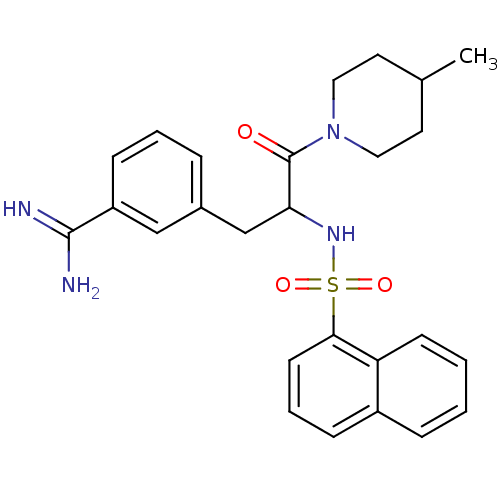 (3-[3-(4-Methyl-piperidin-1-yl)-2-(naphthalene-1-su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072854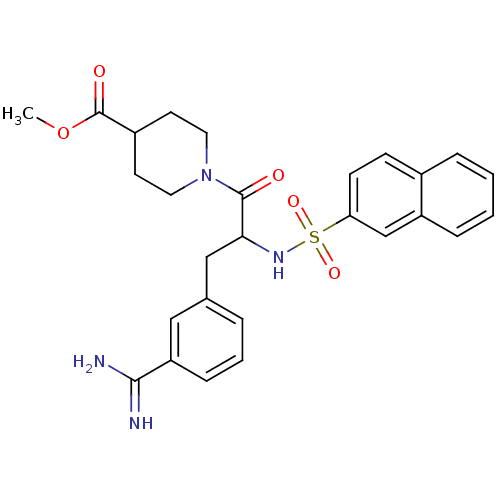 (1-[3-(3-Carbamimidoyl-phenyl)-2-(naphthalene-2-sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23895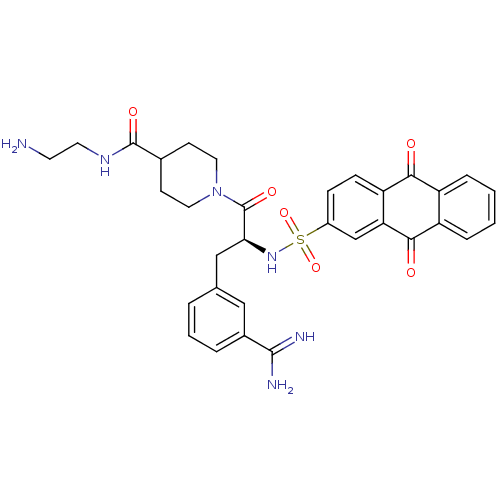 (3-amidinophenylalanine deriv., 39 | N-(2-aminoethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072847 (3-[2-(9,10-Dioxo-9,10-dihydro-anthracene-2-sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23900 (3-amidinophenylalanine deriv., 44 | 4-({1-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072856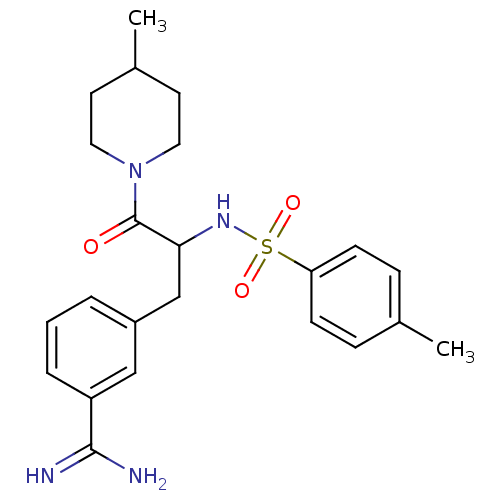 (3-[3-(4-Methyl-piperidin-1-yl)-3-oxo-2-(toluene-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060047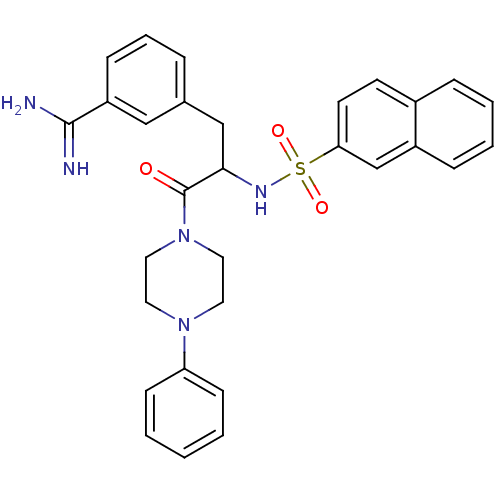 (3-[2-(Naphthalene-2-sulfonylamino)-3-oxo-3-(4-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine trypsin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23914 (3-amidinophenylalanine deriv., 57 | 3-amino-N-(4-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50072843 (2,7-Bis-(4-diaminomethyl-benzylidene)-cycloheptano...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against human tissue-type plasminogen activator expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50072854 (1-[3-(3-Carbamimidoyl-phenyl)-2-(naphthalene-2-sul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine trypsin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50072853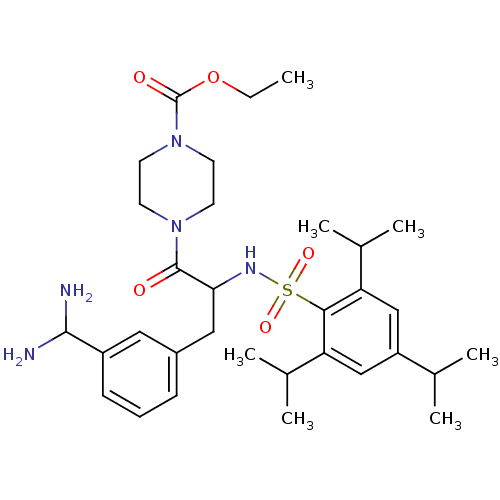 (4-[3-(3-Diaminomethyl-phenyl)-2-(2,4,6-triisopropy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine trypsin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23910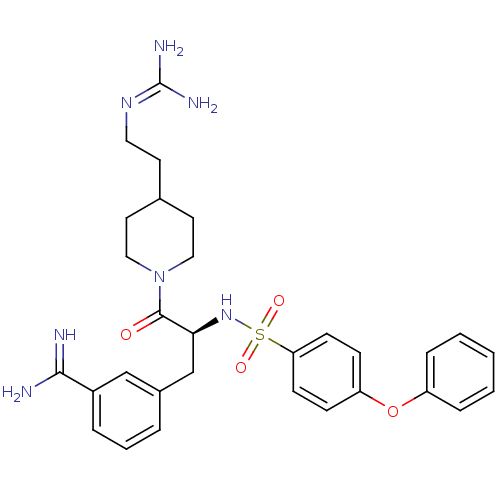 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50041224 ((R)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Binding affinity of the compound towards Coagulation factor X | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23907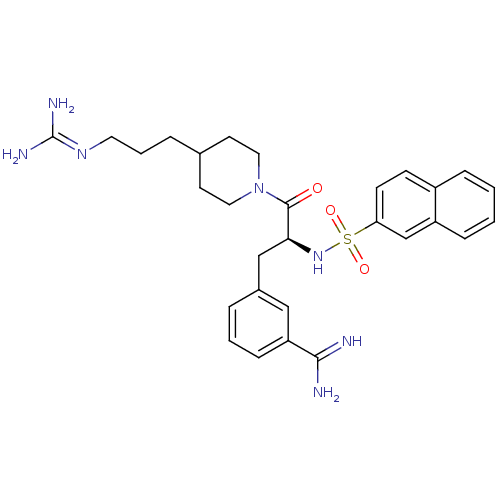 (3-[(2S)-3-[4-(3-carbamimidamidopropyl)piperidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23877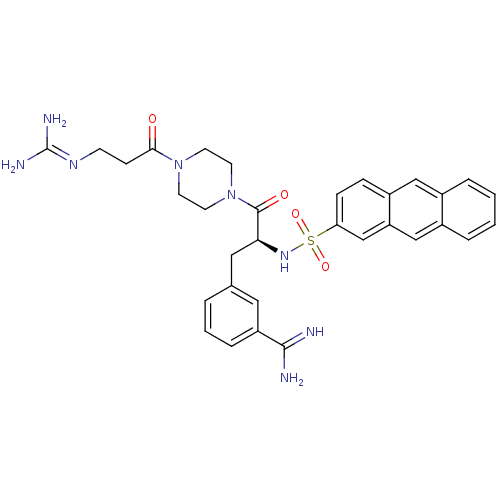 (3-[(2S)-2-(anthracene-2-sulfonamido)-3-[4-(3-carba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | -42.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23909 (3-amidinophenylalanine deriv., 52 | 4-{1-[(2S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23902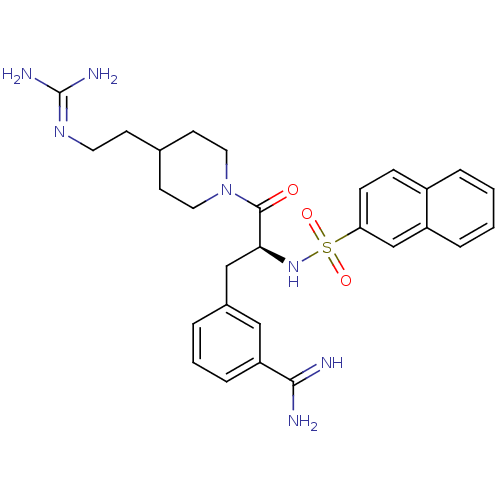 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23890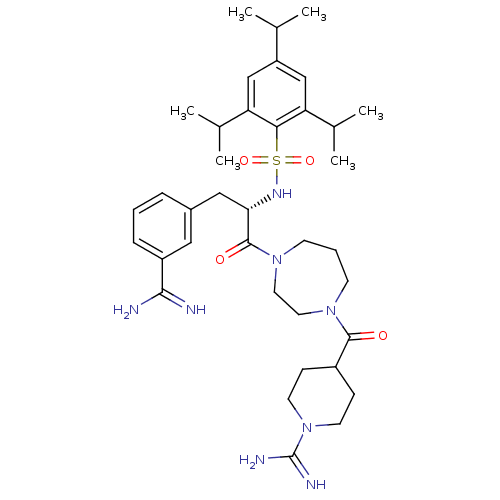 (3-amidinophenylalanine deriv., 34 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM23904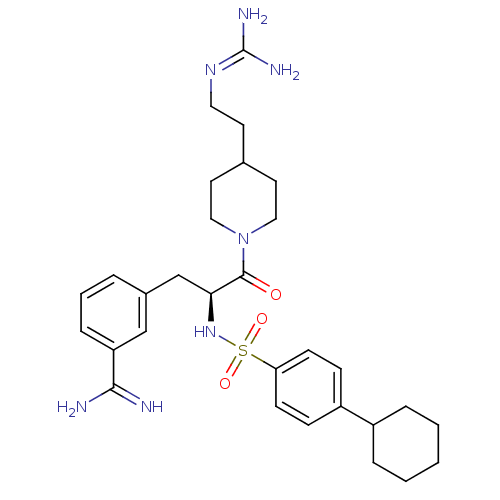 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23912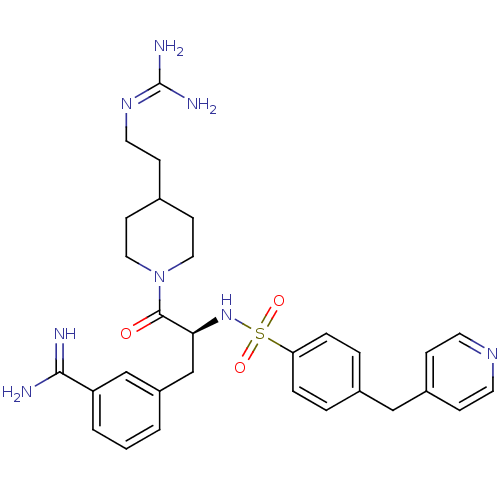 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23876 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)piperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM23892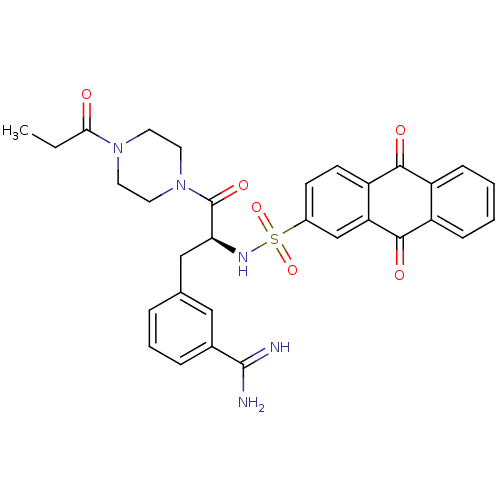 (3-[(2S)-2-[(9,10-dioxo-9,10-dihydroanthracene-2-)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23867 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)piperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072859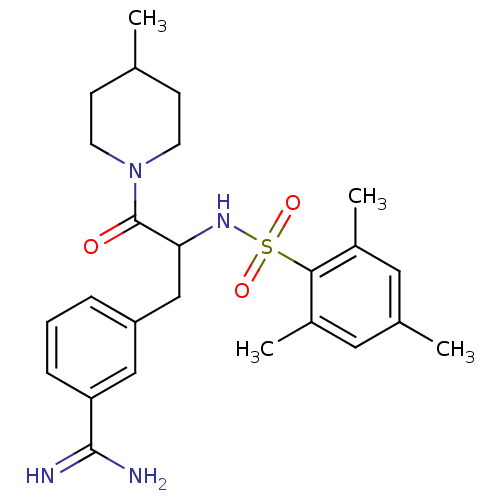 (3-[3-(4-Methyl-piperidin-1-yl)-3-oxo-2-(2,4,6-trim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23888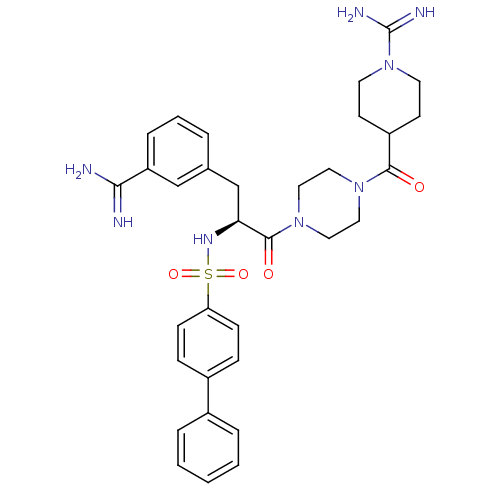 (3-amidinophenylalanine deriv., 32 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23870 (3-[(2S)-3-[4-(4-carbamimidamidobutanoyl)piperazin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23874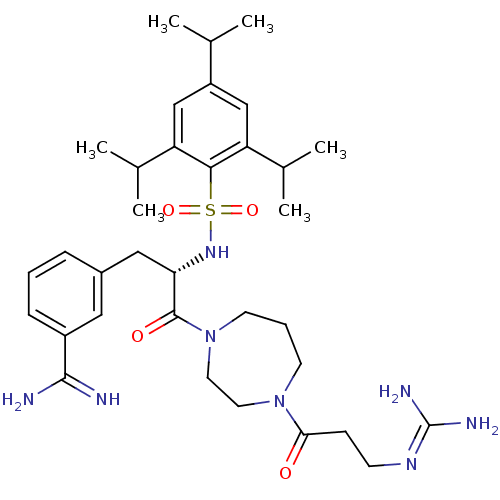 (3-[(2S)-3-[4-(3-carbamimidamidopropanoyl)-1,4-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | -41.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50060049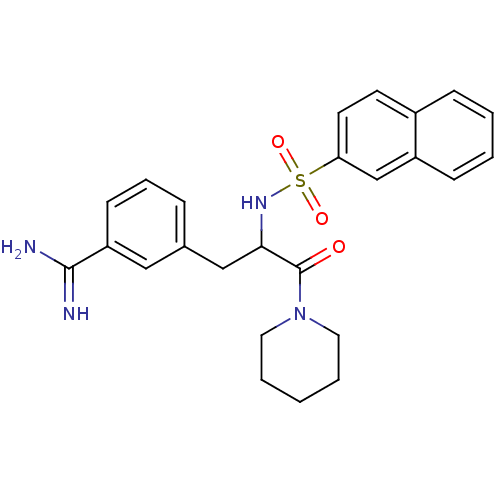 (3-[2-(Naphthalene-2-sulfonylamino)-3-oxo-3-piperid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23903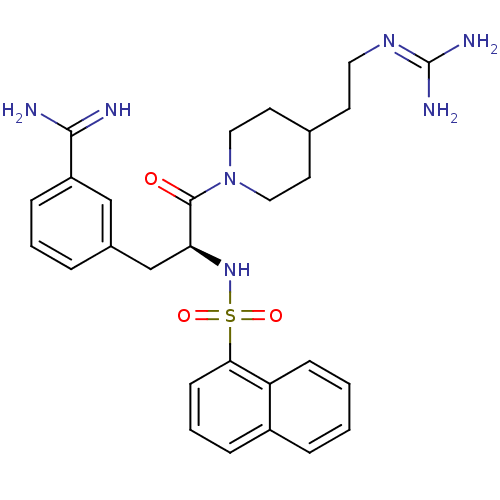 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50072847 (3-[2-(9,10-Dioxo-9,10-dihydro-anthracene-2-sulfony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine trypsin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50072857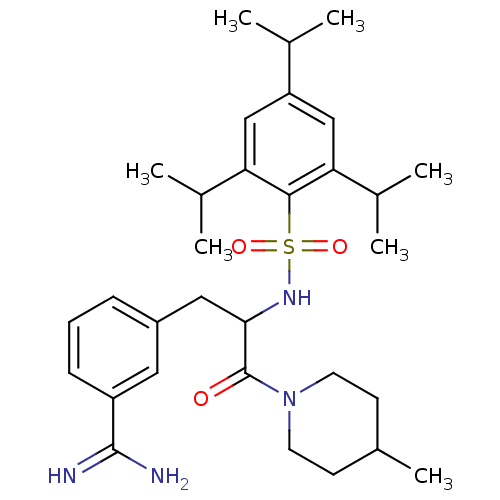 (3-[3-(4-Methyl-piperidin-1-yl)-3-oxo-2-(2,4,6-trii...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin expressed as dissociation constant | J Med Chem 41: 5445-56 (1999) Article DOI: 10.1021/jm981068g BindingDB Entry DOI: 10.7270/Q2KK99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23919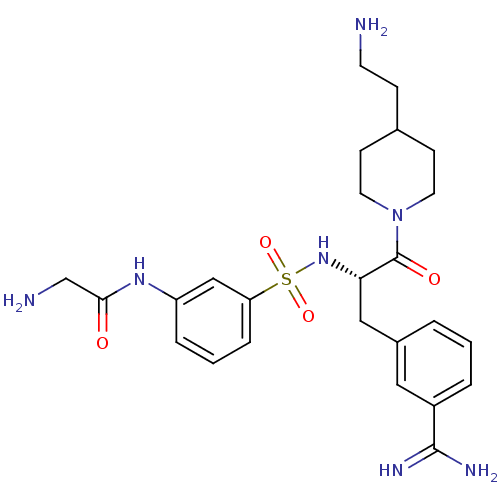 (2-amino-N-(3-{[(2S)-1-[4-(2-aminoethyl)piperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23898 (3-amidinophenylalanine deriv., 42 | 4-({1-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23904 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 482 total ) | Next | Last >> |