

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238227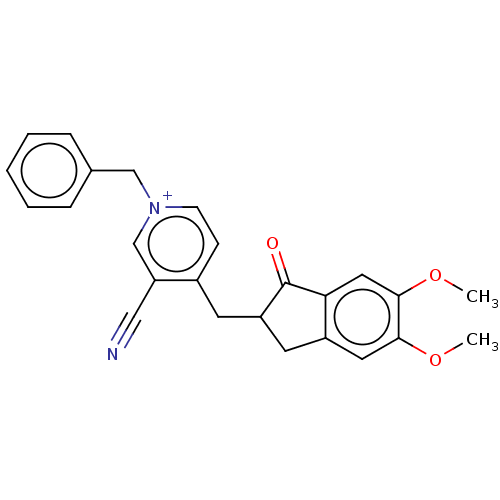 (CHEMBL4071377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238223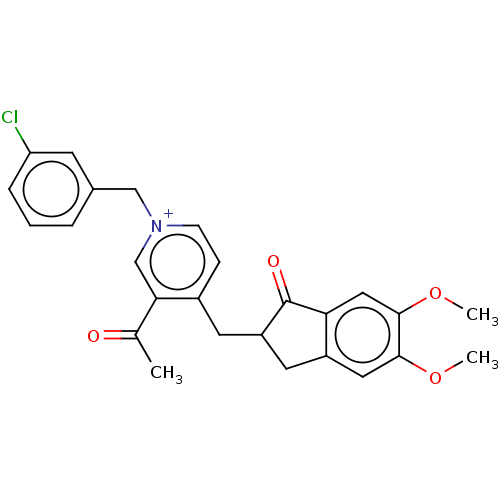 (CHEMBL4102333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238216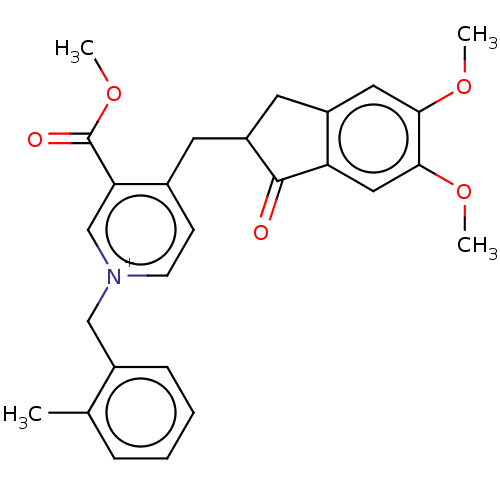 (CHEMBL4095535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238235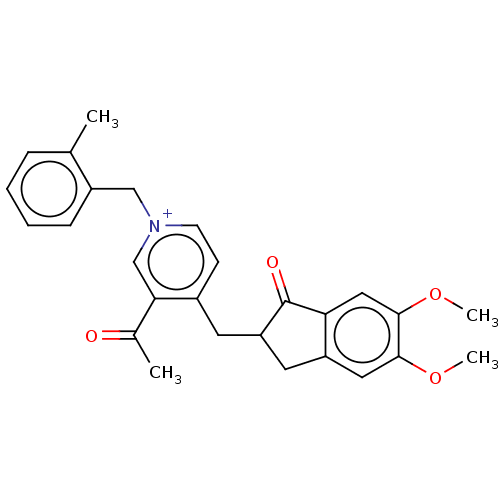 (CHEMBL4081915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238224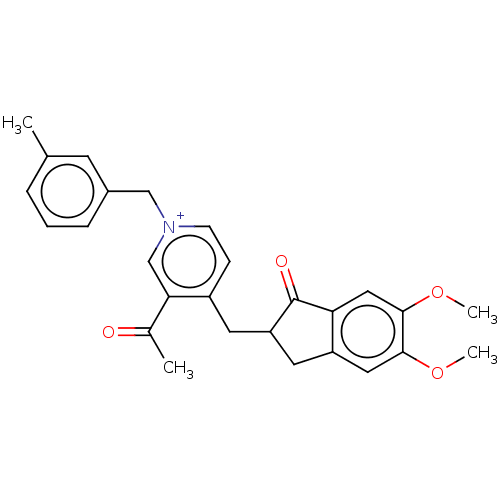 (CHEMBL4094081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238225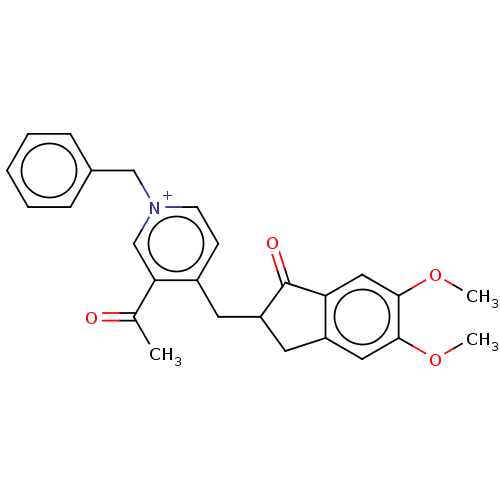 (CHEMBL4060011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238213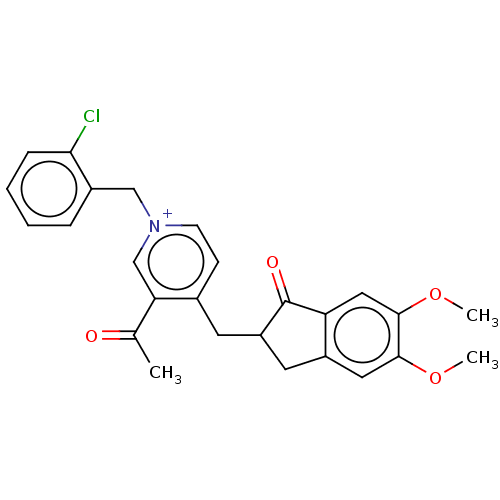 (CHEMBL4066548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238212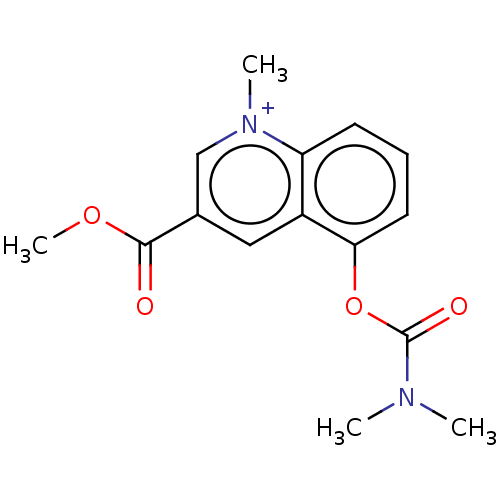 (CHEMBL4077952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238236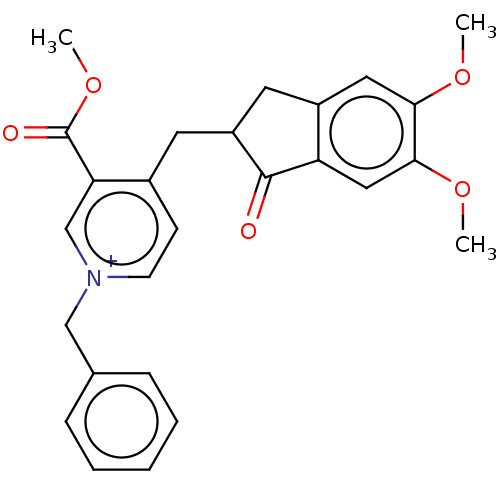 (CHEMBL4068149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238220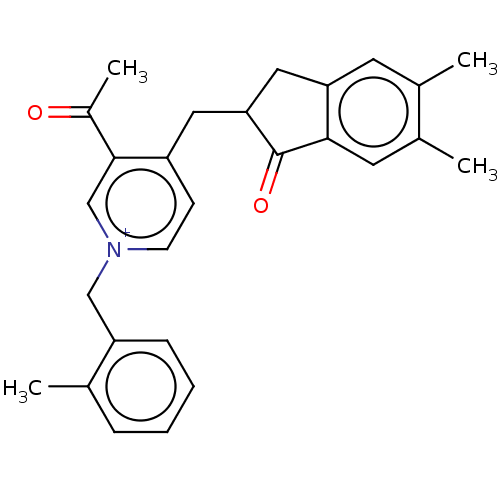 (CHEMBL4074062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238222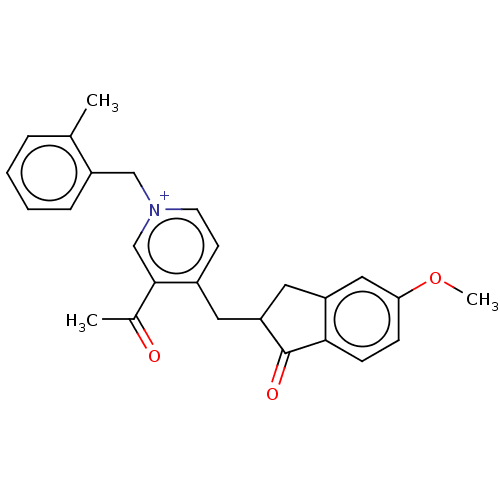 (CHEMBL4094591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117592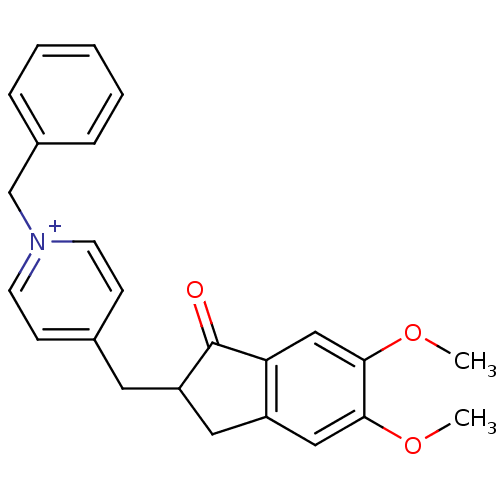 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238228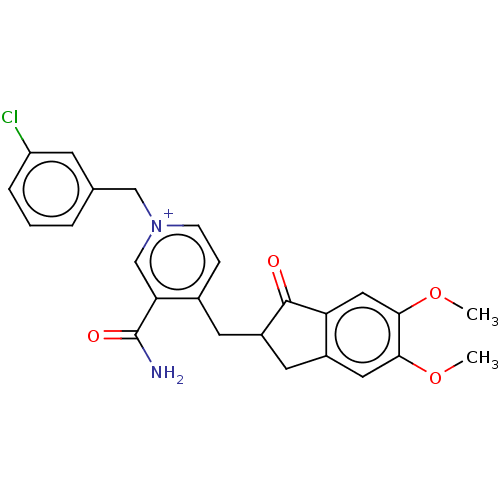 (CHEMBL4092048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238240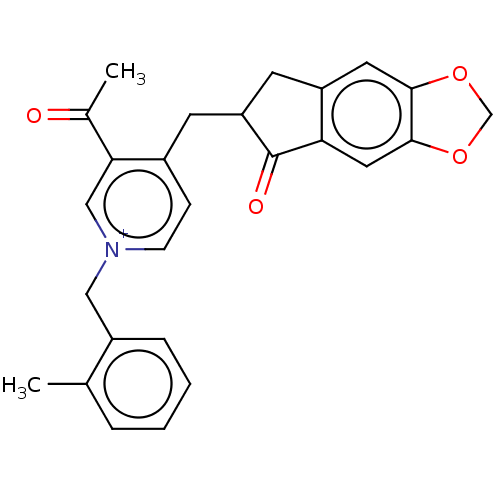 (CHEMBL4084371) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238219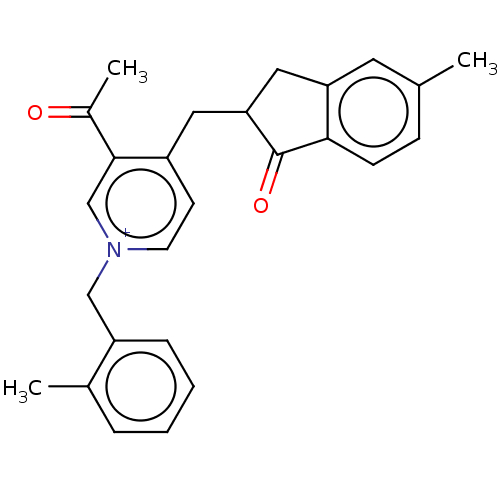 (CHEMBL4072999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238239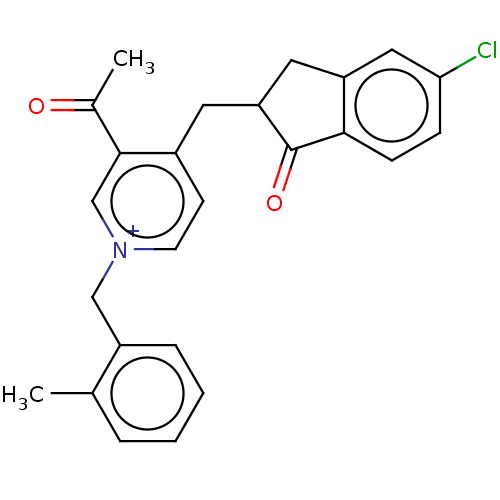 (CHEMBL4099873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238220 (CHEMBL4074062) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238229 (CHEMBL4061527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238231 (CHEMBL4105085) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238215 (CHEMBL4076930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238234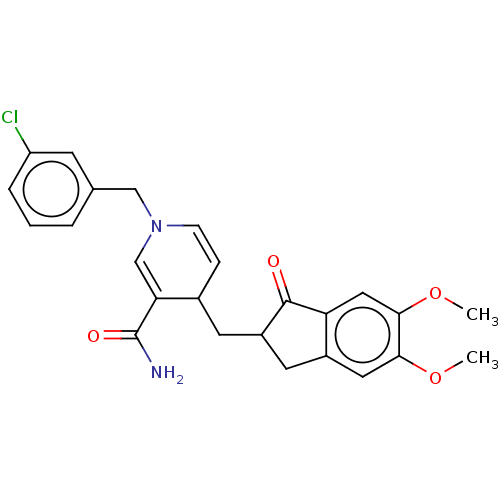 (CHEMBL4101303) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238233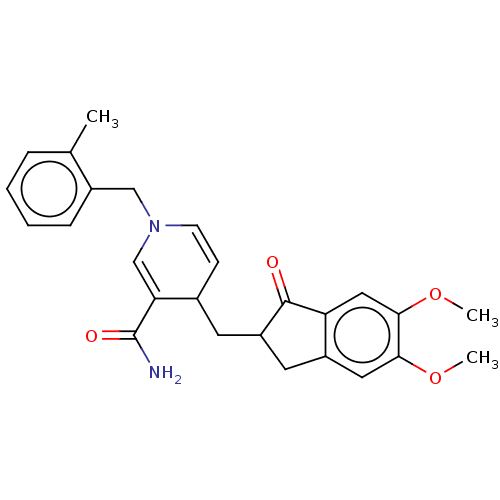 (CHEMBL4072865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238232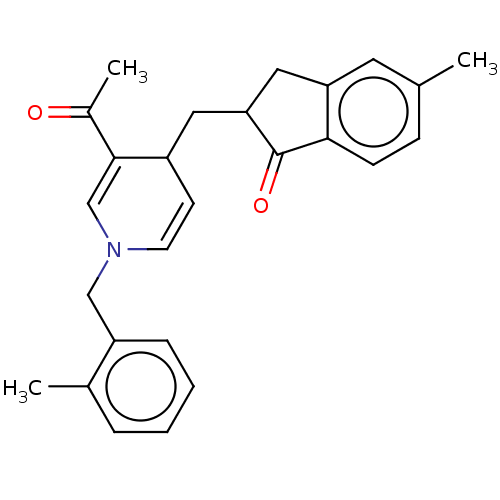 (CHEMBL4083445) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238230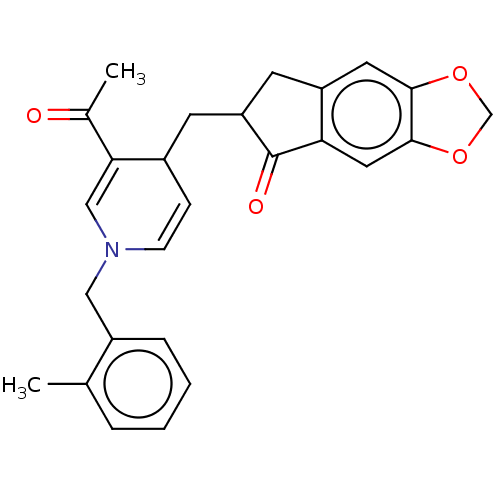 (CHEMBL4081776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238217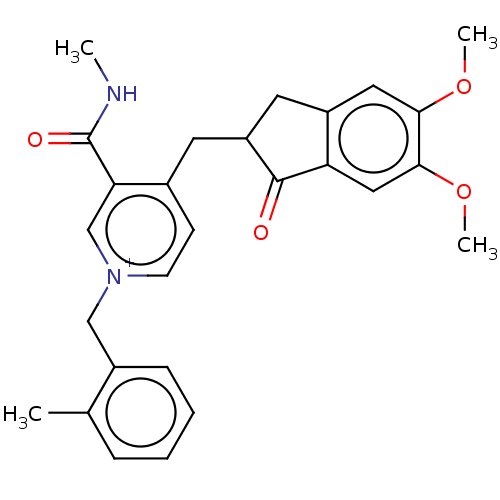 (CHEMBL4092123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238238 (CHEMBL4093567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238237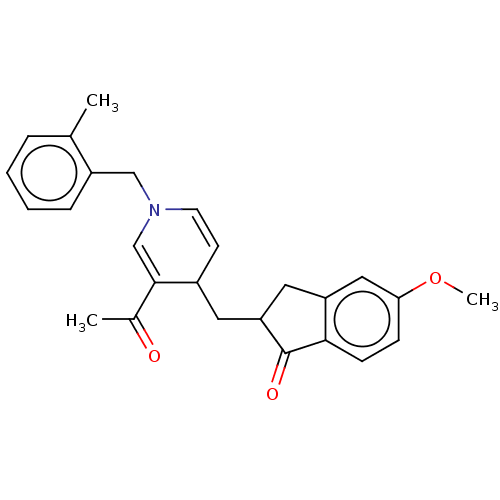 (CHEMBL4099794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238218 (CHEMBL4064413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238240 (CHEMBL4084371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238216 (CHEMBL4095535) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238213 (CHEMBL4066548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238222 (CHEMBL4094591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238236 (CHEMBL4068149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238223 (CHEMBL4102333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238239 (CHEMBL4099873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238219 (CHEMBL4072999) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238218 (CHEMBL4064413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238235 (CHEMBL4081915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238224 (CHEMBL4094081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238228 (CHEMBL4092048) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50238215 (CHEMBL4076930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50238211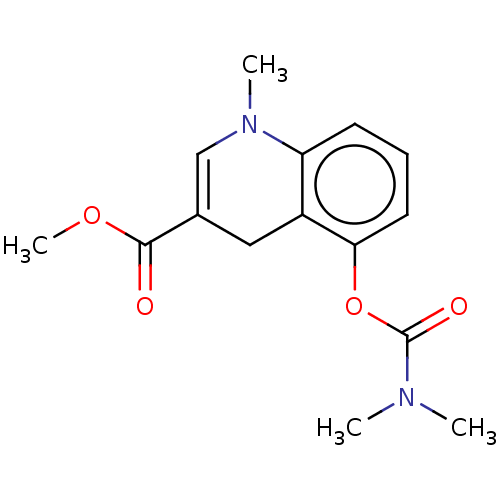 (CHEMBL4083306) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||